Abstract
Immune receptors that specifically recognize foreign antigens to activate leukocytes in adaptive immune responses belong to a family of multichain cell surface proteins. All of these contain immunoreceptor tyrosine-based activation motifs in one or more subunits that initiate signaling cascades following stimulated tyrosine phosphorylation by Src-family kinases. As highlighted in this review, lipids participate in this initial activation step, as well as in more downstream signaling steps. We summarize evidence for cholesterol-dependent ordered lipids serving to regulate the store-operated Ca2+ channel, Orai1, and we describe the sensitivity of Orai1 coupling to the ER Ca2+ sensor, STIM1, to inhibition by polyunsaturated fatty acids. Phosphoinositides play key roles in regulating STIM1-Orai1 coupling, as well as in the stimulated Ca2+ oscillations that are a consequence of IgE receptor signaling in mast cells. They also participate in the coupling between the plasma membrane and the actin cytoskeleton, which regulates immune receptor responses in T cells, B cells, and mast cells, both positively and negatively, depending on the cellular context. Recent studies show that other phospholipids with mostly saturated acylation also participate in coupling between receptors and the actin cytoskeleton. Lipid heterogeneity is a central feature of the intimate relationship between the plasma membrane and the actin cytoskeleton. The detailed nature of these interactions and how they are dynamically regulated to initiate and propagate receptor-mediated cell signaling are challenging questions for further investigation.
Keywords: Liquid ordered lipids, phosphoinositides, calcium mobilization, actin cytoskeleton, critical fluctuations, IgE receptors
1. Introduction
Adaptive immune responses utilize a family of multichain immune recognition receptors on B lymphocytes, T lymphocytes, and other hematopoietic-lineage leukocytes, including mast cells. These receptors respond to specific foreign antigens, most commonly due to their crosslinking by multivalent ligands (antigens). Specific ligands are recognized by variable region segments on the T cell receptor (TCR) or B cell receptor (BCR), or by variable region segments on antibodies bound to Fc receptors on these or other leukocytes (1). Consequent receptor clustering results in activation of a cascade of downstream signaling pathways, initiated by tyrosine phosphorylation events, and culminating in Ca2+ mobilization, transcriptional activation, and exocytotic processes that propagate immune responses to the foreign antigens.
A long-standing question has been how immune receptor clustering initiates the tyrosine phosphorylation events that lead to downstream signaling. From biochemical and molecular genetic studies, it is clear that receptor clustering causes phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) in cytoplasmic segments of immune receptor subunits by Src-family tyrosine kinases, which then recruits and activates a second family of tyrosine kinases comprising Zap70 in T cells and Syk in other leukocytes. However, less clear is the mechanism that initiates ITAM phosphorylation. In T cells, the Src family kinase Lck binds to one of two co-receptor proteins, CD4 in helper T cells and CD8 in cytotoxic T cells, and co-clustering of these co-receptor proteins with the T cell receptor (TCR) facilitates this phosphorylation step (2). In B cells and other leukocytes, there are no analogues to CD4 and CD8; yet in these cells, Src family kinases phosphorylate ITAMs in response to receptor clustering in a process that is protected from dephosphorylation for time periods sufficient to activate downstream signaling over many tens of minutes and often hours that are needed for stimulated cytokine gene expression and cell proliferation.
Drawing from accumulated evidence with IgE/receptor complexes (IgE/FcεRI) on mast cells, our laboratory has established a plausible mechanism by which clustering of immune receptors initiates cell activation that can be sustained for extended periods leading to granule exocytosis, as well as cytokine biosynthesis and secretion. This mechanism builds on the preferential association of antigen-crosslinked IgE/FcεRI with liquid order (Lo)-preferring lipids, i.e., cholesterol, sphingolipids, and phospholipids with mostly saturated fatty acyl chains. As extensively characterized in model membrane studies, Lo-type lipids pack together in a two dimensional bilayer more tightly than liquid disorder (Ld)-preferring lipids, while still permitting lateral diffusion of individual molecules that is only several-fold slower than for lipids in disordered membranes. These order-preferring lipids and disorder-preferring lipids can undergo large-scale (micrometer) phase separation in model membranes at certain compositions in a three-component phase diagram that includes cholesterol, together with a saturated phospholipid and a polyunsaturated phospholipid (3). Remarkably, giant plasma membrane vesicles (GPMVs), isolated as detached cell blebs, also undergo large-scale phase separation below their phase transition temperatures, a process that is not detectable in intact cells (4,5). Notably, GPMVs roughly represent the composition of the plasma membrane, including proteins and more than a hundred different lipid species, but they are detached from the actin cytoskeleton. Although some properties of GPMVs and related plasma membrane preparations depend on how they are generated (6), Lo/Ld phase separation observed in GPMVs produced by several different treatments (7) makes it unlikely that this property is the artifact of a particular chemical modification.
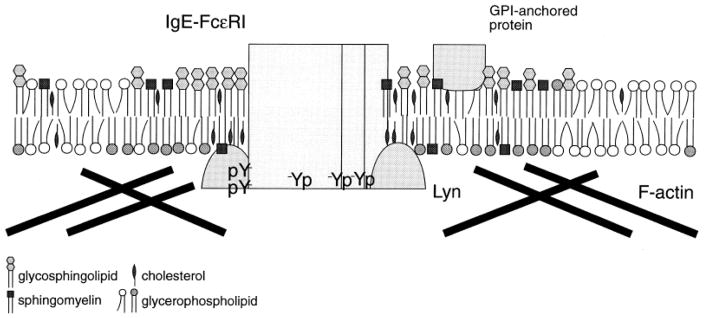
Previous studies with intact cells demonstrated that exclusion of a disorder-preferring transmembrane tyrosine phosphatase from order-preferring crosslinked IgE/FcεRI complexes is sufficient to suppress basal phosphorylation while enabling stimulated tyrosine phosphorylation of receptor ITAMs (8). This occurs in large part because the tyrosine kinase that phosphorylates the ITAMs, the Src-family kinase Lyn, is anchored to the plasma membrane by saturated fatty acid derivatives (palmitoyl and myristoyl), which preferentially associate with ordered lipids, and its specific activity is higher in this ordered lipid environment compared to Lyn in a disordered lipid environment (9). In the steady state of non-stimulated cells, Lyn and IgE/FcεRI diffuse in and out of disordered lipid environment, where they are exposed to transmembrane phosphatases. Antigen-crosslinking of IgE/FcεRI stabilizes their association with ordered lipid regions, resulting in phosphorylation of FcεRI ITAMs by Lyn that is protected from dephosphorylation. This is depicted schematically in Figure 1, along with association of these ordered regions with the actin cytoskeleton as discussed below. The principal Src family kinase in T cells, Lck, is also anchored to the inner leaflet of the plasma membrane by saturated acyl chains, and this has been shown to be important for its role in TCR activation (10), in addition to Lck association with co-receptors CD4 or CD8. Thus, for both mast cells and T cells, there is evidence that lipid segregation plays a role in the earliest biochemical event in immunoreceptor signaling, phosphorylation of receptor ITAMs. This same possibility for B cells is discussed below.
Figure 1.
Schematic representation of the coalescence of clustered IgE receptors with Lyn kinase in ordered regions of the plasma membrane that are enriched in GPI-linked proteins, glycosphingolipids, phospholipids with saturated acyl chains, and cholesterol. Also shown is association of F-actin with these regions indicated by several studies, as discussed in Sec. 4. Figure is from (75).
Our current understanding of the role of membrane heterogeneity in immune cell function draws from many laboratories’ investigations of similar issues in a wide range of cell types over the past several decades. Early evidence for functionally relevant phase behavior in cell membranes and the importance of the Lo-like phase came from studies using resistance to detergent solubility (11) or dependence on cholesterol (12). Although by current standards, these simple tools provide crude measures, and their misuse or over-interpretation has caused some confusion in the literature. However, detergent insolubility and cholesterol dependence continue to provide useful correlative information that may point to the possible significance of phase-like behavior in membrane function and suggest models that can then be tested by more sophisticated approaches. As described in this review, a large variety of experimental approaches are essential and continue to be developed with the goal of gaining a clear understanding of complex membrane interactions in dynamic cellular processes.
2. Roles for lipids in Ca2+ signaling
In addition to participating in plasma membrane receptor function, specific types of lipids appear to be involved in regulating more downstream pathways of immunoreceptor signaling. One of these pathways, central to IgE/FcεRI-initiated cellular activation, is mobilization of cytoplasmic Ca2+ in the process known as store-operated Ca2+ entry. Depletion of cellular cholesterol using the hydrophilic lipid chelator, methyl-β-cyclodextrin (MβCD), strongly inhibits this process without affecting basal Ca2+ levels (13,14,15). Under these conditions, we found that depletion of cholesterol interferes with the association of the principal store-operated Ca2+ entry channel protein, Orai1, in the plasma membrane with the Ca2+ sensor protein, STIM1, in the endoplasmic reticulum (ER) (15). We further found that Orai1, which is a tetraspan protein that forms hexameric channels in the plasma membrane (16), is a order-preferring membrane protein, exhibiting resistance to solubilization by non-ionic detergents such as Triton X-100 (D. Holowka, unpublished results). The dependence of store-operated Ca2+ entry on steady-state cholesterol levels provides evidence that the ordered lipid environment of this channel protein is important for its normal function.
In a separate but complementary study, we found that polyunsaturated fatty acids, added acutely in the low micromolar range to mammalian cells, interfere with the coupling between STIM1 and Orai1 that is stimulated by ER store depletion (17). Previous literature indicated that polyunsaturated fatty acids, including linoleic acid, inhibit Ca2+ mobilization in T cells, but the molecular basis for this effect was unclear (18). We found that linoleic acid, but not stearic acid, the saturated fatty acid of the same chain length, interferes with store-operated Ca2+ entry and with the stimulated association of STIM1 with Orai1. Furthermore, we found that linoleic acid inhibits clustering of STIM1 that is normally elicited by ER store depletion, even in the absence of Orai1, suggesting that this is the perturbation underlying linoleic acid-disrupted association with Orai1. Indeed, ER morphology appears to be distorted by linoleic acid addition under these conditions (17). These results imply roles for specific lipids in the structural organization of ER membranes, as well as in their functional coupling to the plasma membrane.
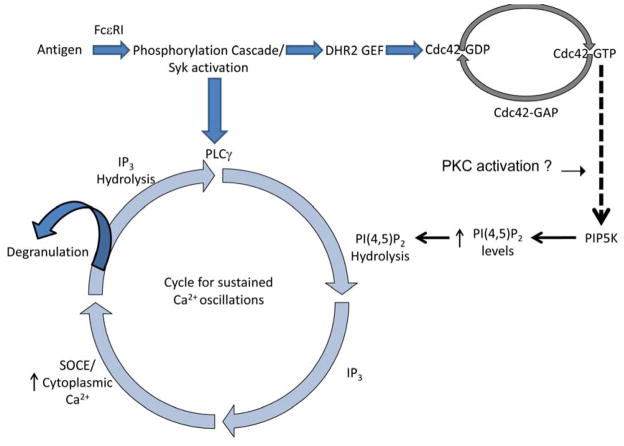
Phosphoinositides, including phosphoinositide 4,5-bisphosphate (PIP2), are found to be important for Ca2+ signaling in response to immunoreceptors, including FcεRI (19). In addition to serving as the substrate for activated phospholipase C to produce inositol 1,4,5-trisphosphate, which activates Ca2+ release from ER stores, PIP2 also regulates the coupling of STIM1 and Orai1 during store-operated Ca2+ entry (15). We recently reviewed these findings, as well as evidence for critical roles for phosphoinositides in immunoreceptor-stimulated exocytosis and other functions (20), and will not further discuss here. Taking advantage of a mutant RBL cell line that is defective in IgE/FcεRI signaling downstream of stimulated tyrosine phosphorylation, we found that the Rho GTPase Cdc42 participates in the activation of sustained Ca2+ oscillations that are necessary for stimulated granule exocytosis (21–23). Recent studies showed that stimulated oscillations in PIP2 levels at the plasma membrane occur synchronously with Ca2+ oscillations (24,25). We found that these antigen-stimulated PIP2 oscillations are absent in the mutant RBL cells, despite evidence for stimulated PIP2 hydrolysis in these cells. We reconstituted stimulated PIP2 oscillations in these mutant cells by expressing a constitutively activated form of Cdc42 that also reconstitutes the stimulated Ca2+ oscillations (23). We hypothesize that this Rho family protein plays a key role in receptor-stimulated activation of PIP2 synthesis, which serves to balance stimulated PIP2 hydrolysis with new synthesis of this phosphoinositide by a mechanism that activates both of these processes synchronously but out of phase. One possible explanation is that Cdc42 activates a protein kinase C isoform causing dissociation of its substrate, MARCKS, from the plasma membrane to expose phosphoinositides, including phosphoinositol 4-phosphate, required for PIP2 synthesis (Figure 2; 26).
Figure 2.
Antigen crosslinks IgE/FcεRI complexes to initiate a tyrosine phosphorylation cascade that results in the activation of both PLCγ and Cdc42. Hydrolysis of PIP2 by PLCγ produces IP3, which mediates Ca2+ release from ER stores to stimulate SOCE that is necessary for degranulation. Activation of Cdc42 is important for sustained Ca2+ responses, including sustained Ca2+ oscillations, possibly because it promotes PIP5-kinase-mediated synthesis of PIP2 via a mechanism that involves activation of protein kinase C. Modified from (23).
3. Roles for the actin cytoskeleton in regulating immunoreceptor signaling
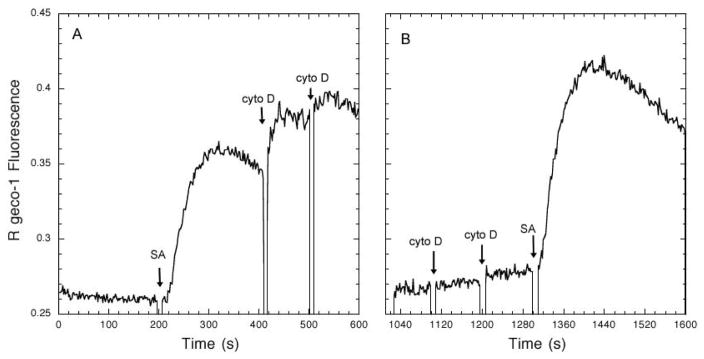
It is well-established that actin polymerization positively regulates TCR signaling in T cells, such that, for example, inhibition is observed in the presence of cytochalasin D or latrunculin. This positive regulation is manifested both for stimulation by peptide/MHC ligands on either antigen-presenting cells (27) or supported lipid bilayers (28), and for stimulation caused by anti-TCR/CD3 when these antibodies are adsorbed to surfaces (28). In contrast, F-actin negatively regulates FcεRI signaling in mast cells, beginning at the earliest signaling events, i.e., antigen-stimulated tyrosine phosphorylation of FcεRI ITAMs (29,30). To investigate whether this differential regulation is a receptor-specific or a cell type-specific property, we expressed a single chain chimeric IgE receptor in Jurkat T cells and in RBL mast cells, together with a cytoplasmic Ca2+ reporter, R geco-1, and we compared the consequences of inhibition of actin polymerization on stimulated Ca2+ mobilization. This single chain receptor (αζζ) has an extracellular segment from the α subunit of human FcεRI that binds both human and rodent IgE with high affinity, and transmembrane and cytoplasmic segments from the TCR ζ subunit, including three ITAM sequences (31). This receptor can be sensitized with biotinylated human IgE (which does not bind to endogenous rodent FcεRI on RBL mast cells) and crosslinked with streptavidin. Robust Ca2+ responses are stimulated in this manner, and these are enhanced 30–50% by inhibition of F-actin polymerization by cytochalasin D (Figure 3). This level of enhancement by cytochalasin D is consistent with that observed for responses to multivalent antigen by IgE bound to endogenous FcεRI (D. Holowka, unpublished observations).
Figure 3.
Activation of Ca2+ responses by streptavidin (SA) crosslinking of biotinylated huIgE bound to the chimeric IgE receptor αζζ expressed in RBL-2H3 mast cells is enhanced ~30–50% by cytochalasin D (cyto D) added after (A) or before (B) 3 nM SA. Ca2+ responses were monitored by steady-state fluorimetry at 37°C in a basal salt solution. Each addition of cyto D is 1 μM.
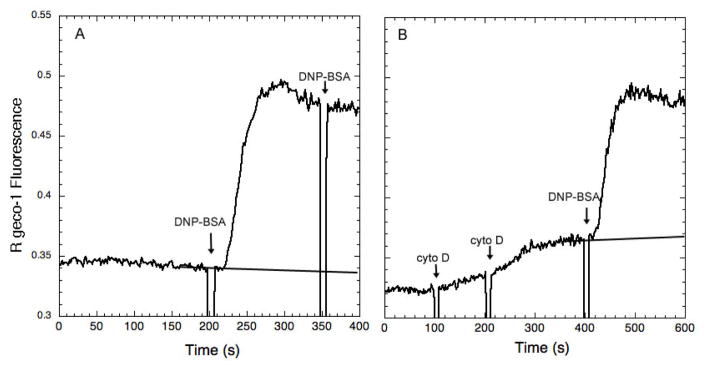
This single chain receptor αζζ also mediates a robust Ca2+ response to multivalent antigen in Jurkat T cells when sensitized with antigen-specific IgE. However, in this case, the response to antigen is inhibited ~20% by cytochalasin D (Figure 4). Similar results were obtained for both RBL mast cells and Jurkat T cells when a related chimeric IgE receptor containing the transmembrane sequence from the IL-1 receptor was used (αIζ; D. Holowka, unpublished observations). When multivalent antigen was adsorbed to a coverslip and presented to the Jurkat T cells transfected with αIζ, the Ca2+ response was prevented by latrunculin B, an alternative inhibitor of actin polymerization. In contrast, the Ca2+ response to adsorbed streptavidin by RBL cells transfected with αIζ was not detectably inhibited by latrunculin B, consistent with the results from stimulation by soluble streptavidin in Figure 2 (D. Holowka, unpublished observations). These findings support the possibility that the characteristics of the cell type, rather than the characteristics of the stimulating receptor, or the means of ligand presentation, determine the role that actin polymerization plays in regulating downstream signaling responses. For T cells, the signaling networks that emanate from TCR activation by peptide/MHC complexes (their natural ligand) need to be tuned to respond to very limited receptor engagement, often only a few ligand-receptor complexes per cell (32), with surprisingly long occupancy times per receptor despite the low affinity TCR/peptide/MHC association (33). Thus, the actin cytoskeleton may drive ligand-receptor microcluster formation that more efficiently couples to the downstream signaling complexes, including phosphorylated LAT, Grb2, and SOS (34), as well as other interacting adaptor proteins (35). In the case of mast cells, antigen binding to IgE to crosslink IgE/FcεRI is often of high affinity due to the affinity maturation of this class of immunoglobulins (36), and IgE also binds to FcεRI with very high affinity (37). In this case the responses appear to be regulated by the actin cytoskeleton to limit spontaneous activation or over-activation downstream of the initial crosslinking events.
Figure 4.
Activation of Ca2+ responses by multivalent DNP-BSA crosslinking of anti-DNP-IgE bound to the chimeric IgE receptor αζζ expressed in Jurkat T cells (A) is inhibited ~20% by pre-addition of 2 μM cyto D, which stimulates a small response itself in these cells (B).
4. Roles for lipids in actin cytoskeleton coupling to the plasma membrane
In mammalian cells, F-actin polymers are largely cortical, i.e., concentrated at the cytoplasmic surface of the plasma membrane, and polymerization/depolymerization of some portion of this network is dynamic as regulated by cellular activities (38). A number of proteins have been identified as participants in association of F-actin with the plasma membrane, including the ERM family of ezrin, radixin, and moesin, which are cytoplasmic proteins containing an N-terminal segment (FERM domain) that binds to PIP2 and multiple proteins, as well as an actin-binding C-terminal segment (ERAD) that bridges the plasma membrane to F-actin (39). These proteins are often in an auto-inhibited conformation that requires phosphorylation at specific threonine residues to adopt a conformation that can simultaneously bind to the plasma membrane and F-actin. Receptor-mediated stimulation of phosphorylation events is frequently a mechanism to activate this plasma membrane-cytoskeletal coupling, which can lead to morphological changes at the plasma membrane such as cell ruffling (40) or immune synapse formation (41), among other examples.
Integrins are another family of proteins that mediate F-actin-plasma membrane association. These are heterodimeric transmembrane proteins, substantially targeted to the plasma membrane, that bind to cytoplasmic proteins, such as talin, which bridge integrins with F-actin (42). In many cell types, integrins mediate the association of large bundles of F-actin, known as actin stress fibers, with plasma membrane within surface attachments known as focal adhesion contacts (43). Much is known about the identity of proteins that participate and the details of these protein-protein interactions from high-resolution structural data and mutagenesis studies (44). Less is known about roles for specific lipids in the structural organization of plasma membrane-actin cytoskeletal association. For integrin-mediated coupling of F-actin to the plasma membrane, these integral membrane proteins must adopt an activated conformation in which the cytoplasmic segments of the α and β integrin heterodimer become separated from each other by structural transitions in the extracellular segments (45). This “activated conformation” can be driven by binding of an extracellular ligand such as fibronectin, or by an “inside-out” mediated structural transition caused by intracellular phosphorylation events (46). As for other plasma membrane – F-actin interactions, there is evidence that PIP2 participates in integrin-mediated coupling (47).
Other proteins also mediate coupling between the plasma membrane and F-actin, including the transmembrane protein CBP, also known as PAG, which participates in the coupling of the GPI-linked protein Thy-1 to the actin cytoskeleton (48). CBP preferentially associates with detergent-resistant membrane domains (49), suggesting that F-actin association with the plasma membrane occurs preferentially with order-preferring components to make this connection. In fact, crosslinking of IgE/FcεRI or other order-preferring proteins and lipids, including GPI-linked proteins and gangliosides, results in large-scale co-redistribution with F-actin in micron-scale clusters at the plasma membrane (30,50). Roles for specific lipids in these ordered membrane-actin cytoskeleton connections are largely undefined, except for the frequent demonstration that cholesterol is important for the association of particular components with ordered membranes (51–53), One case in which this connection is better defined is that for the B cell receptor (BCR), which associates with detergent-resistant membranes when crosslinked by antigen or by anti-BCR antibodies (54). For this receptor, crosslink-dependent, large-scale clustering in actin-dependent caps (55) has been shown to depend on the transient dissociation and reassociation of ezrin (56), suggesting that PIP2 in ordered membrane domains couples crosslinked BCR to F-actin via ezrin. A model for the association of ordered membrane domains with the actin cytoskeleton via PIP2 that is consistent with this view was recently described (74).
PIP2 has also been implicated in regulating lateral diffusion of transmembrane proteins, including Class I MHC. Edidin and colleagues showed that depletion of cellular cholesterol by several different methods results in reduction in the lateral diffusion of this protein, but subsequent addition of cytochalasin D restored this diffusion to normal levels (57). In parallel with the loss of Class I MHC lateral diffusion, cholesterol depletion also reduced the capacity of the PLCδ PH domain to bind to PIP2 at the plasma membrane, suggesting that cholesterol depletion sequesters a pool of PIP2 that is normally involved in plasma membrane-F-actin association. Other studies have found that cholesterol depletion reduces the lateral diffusion of plasma membrane proteins, and they have even suggested that this could result from the conversion of a Lo-like phase to a gel phase in the plasma membrane (58).
In recent studies, we found that cholesterol depletion by 10 mM MβCD for 30 minutes at 37°C causes the concentration of order-preferring proteins into micron-sized domains at the ventral surface of the plasma membrane of RBL mast cells. These domains exhibit co-localization of F-actin, and pretreatment with cytochalasin D prevents the formation of these ventral domains, suggesting that their formation depends on actin polymerization. Interestingly, the proteins in these ventral domains exhibit much reduced lateral diffusion, consistent with their residency in largely gel-like lipid domains formed by cholesterol depletion. Interestingly, addition of cytochalasin D after cholesterol depletion does not reverse the ventral domain formation, despite the depletion of F-actin from these domains (D. Holowka, unpublished results). Lateral diffusion of a membrane protein in these domains remains substantially reduced under these conditions, suggesting that immobilization at this stage does not depend on anchoring to the actin cytoskeleton. Although the depletion of cholesterol by MβCD under these conditions results in substantial inhibition of early steps in FcεRI signaling, more downstream processes such as stimulated granule exocytosis are not significantly affected, indicating that cell physiology is not grossly disrupted (12). Our recent findings are consistent with the coalescence of order-preferring membrane components into a gel-like phase in the ventral plasma membrane upon depletion of cholesterol, driven by the actin cytoskeleton that is associated with this ordered membrane phase.
Tandem mass spectrometry analysis yields composition of membrane lipids in terms of both head groups and degree of saturation of the fatty acyl chains, enabling investigation of the structural basis for ordered lipid membranes. In collaborative studies with the McLafferty group, we evaluated membranes from RBL cells and compared the lipid composition of detergent-resistant membranes with GPMVs and whole cell lysates (including internal membranes) (59). A key feature of the ordered, detergent-resistant membranes from unstimulated cells is the high percentage of phospholipids with saturated acyl chains or acyl chains with one double bond (>60%) compared to phospholipids with multiple double bonds, i.e., polyunsaturated phospholipids. Phase partitioning of a monounsaturated phospholipid together with a saturated phospholipid and cholesterol in Lo domains was previously demonstrated in model membrane studies (60). The high percentage of saturated and monounsaturated phospholipids in detergent-resistant membranes compares to ~50% for GPMVs and <30% for whole cell lysates. Interestingly, we found this percentage to be much lower in detergent-resistant membranes from RBL cells that had been stimulated by crosslinking IgE/FcεRI (~40%; (59)).
In a follow-up study, we found that the increase in the ratio of polyunsaturated to mostly saturated phospholipids caused by IgE/FcεRI crosslinking observed for detergent-resistant membranes (59) is also caused by inhibition of actin polymerization by cytochalasin D, or by ionophore-mediated elevation in cytoplasmic Ca2+ (61). Consistent with implied roles for net actin depolymerization and Ca2+ signaling in causing these phospholipid changes, stabilization of the actin cytoskeleton with jasplakinolide or removal of extracellular Ca2+ both reduced this structural response to IgE/FcεRI crosslinking. Processes associated with cell signaling evidently contribute to this stimulated shift in phospholipid composition of detergent-resistant membranes. These may include changes in the interactions of F-actin with the plasma membrane that regulate availability of order-preferring vs. disorder-preferring lipids to partition into regions that fractionate with detergent-resistant membranes. Actin interactions with proteins that associate with ordered lipids may also play a role, as suggested from ESR studies described below. Another possibility is that trafficking of intracellular membranes to the plasma membrane is altered: Crosslinking of IgE/FcεRI stimulates outward trafficking of recycling endosomes (62), in addition to granule exocytosis, and this may alter the composition of the plasma membrane and consequently the phospholipid grouping that resists solubilization by detergents.
Electron Spin Resonance (ESR) offers a distinctive and detailed view of dynamic lipid order in membranes, and our collaborative studies on RBL cell preparations with the Freed group evaluated detergent-resistant membranes (63), GPMVs (64), and live cells (65). Consistent with our mass spectrometry results, we found that detergent-resistant membranes contain a substantial amount of a Lo-like phase as measured by the rotational diffusion rates and order parameters of spin labeled lipids and compared to model membranes. The GPMV preparation yielded two components, and detailed analysis indicated coexistence of a Lo-like phase (the more abundant component) and a Ld-like phase. Interestingly, membranes reconstituted from lipids extracted from the GPMV exhibit the more ordered phase only, suggesting that membrane-associated proteins are involved in the observed phase coexistence. Subsequently, ESR studies on live cells provided direct evidence for the presence in the plasma membrane of two types of environments, Lo-like and Ld-like, with distinct order parameters and rotational diffusion coefficients. As for GPMVs, the Lo region is found to be the major component. However, a relatively larger Ld spectral component was observed in live RBL cells, consistent with a somewhat higher protein concentration in the cell membrane compared to the GPMV derived from these cells. Also, the actin cytoskeleton that attaches to the plasma membrane in cells but not in GPMVs likely plays a significant role in the differences between GPMVs and live cells measured by ESR parameters of order.
Collaborative studies from the laboratories of Mayor and Rao provide evidence that GPI-linked proteins, including the folate receptor, are anchored to the actin cytoskeleton by interdigitation with long-chain phosphatidylserine species containing less than two double bonds at the inner leaflet of the plasma membrane. Genetic and biochemical evidence support this coupling, which also involves a cholesterol-dependent, ordered lipid environment (52,66). Identification of phosphatidylserine as a key phospholipid in this environment is novel, and it remains to be determined whether this negatively charged phospholipid has a more general role in coupling between ordered lipid-preferring components and the actin cytoskeleton. Although phosphatidylserine has a lower charge density than most phosphoinositides, it is typically more abundant in plasma membranes (59) and is concentrated at the inner leaflet (67). In a different study, Harder and colleagues analyzed the phospholipid composition of anti-TCR-attached fragments of T cell plasma membrane and compared it to an anti-transferrin receptor control. They found that the anti-TCR-enriched membrane fragments contained a higher proportion of sphingomyelin and saturated phosphatidylcholine species than the control membrane fragments (68). Interestingly, they also found enrichment of phosphatidylserine species with one double bond relative to polyunsaturated phosphatidylserine in the anti-TCR membrane fragments, consistent with a preference for a more ordered lipid environment.
5. Model membrane studies and conclusions
A recent model membrane study utilized the pinning of F-actin to either Lo or Ld membrane phases in a supported lipid bilayer containing a ternary lipid mixture of dioleylphosphatidylcholine, dipalmitoylphosphatidylcholine, and cholesterol, selected to be near a critical point. In this system, biotinylated phalloidin binds to F-actin and mediates its pinning via streptavidin bridging to biotinylated phospholipids at low densities in either the Lo or the Ld phase. This pinning suppresses macroscopic phase separation in the supported lipid bilayers, and results were consistent with the formation of nanoscopic phase separation under these conditions (69). These findings are remarkably consistent with the earlier predictions of Machta and colleagues, who simulated the effects of F-actin pinning and critical behavior on phase separation in model membranes using an Ising model and Monte Carlo methods (70). Additionally, indications that membrane curvature can alter the partitioning of Lo or Ld-preferring lipids emerged from related Monte Carlo simulations (69). These findings are also consistent with those examining phase like properties of model membrane vesicles or GPMVs anchored to cytoskeletal preparations (71) or to supported lipid bilayers by Ld vs Lo probes (72). They support the hypothesis that critical behavior is a property of the plasma membrane and is relevant to its biological functions. The surprising robustness of critical behavior in GPMVs of varying compositions (73) suggests a physiological conservation of this property. A definitive test for its importance in plasma membrane function for intact cells will be challenging but worthy of the creative efforts that will be required.
In summary, we have been fortunate to witness during the past two decades an extremely rich period of both experimental and theoretical progress toward elucidating plasma membrane structure as related to its multifaceted functions. This has been true not only for the roles of plasma membranes in cell signaling by hematopoietic cells in immune responses, but more generally for polarized cells in the endothelium and epithelium, as well as other specialized cells in specific functional organs of eukaryotic multicellular organisms. It is now established that plasma membranes are heterogeneous in structure, and that redistributions in proteins and lipids that accompany receptor-mediated signaling and other functions are regulated, in part by the actin cytoskeleton. Our own ongoing studies are using super resolution fluorescence localization microscopy to evaluate this regulation in the first minutes of antigen-crosslinked IgE/FcεRI coupling with Lyn kinase in an ordered lipid environment (Shelby, Veatch, Holowka and Baird, unpublished), which may be interpreted in terms of current models described above.
Our emerging view of the dynamic structure and function of the plasma membrane is inevitablly limited by technical shortcomings of current experimental approaches, including the imperfect plasma membrane model provided by GPMVs, and the perturbation of cell homeostatis that is incurred by cholesterol depletion. Never-the-less, the community has gained an appreciation of the evolved complexity of the plasma membrane, which drives ongoing investigations with evermore sophisticated approaches. Our understanding of lipid-protein interactions involved in intracellular membrane function is even less complete than for plasma membranes. Structural heterogeneity of ER, mitochondria, and other intracellular membranes, and how these membranes communicate with each other and with the plasma membrane remain as intriguing challenges for the future.
Acknowledgments
Our work was supported by grants R01 AI018306, R01 GM117552, and R01 AI022499 from the National Institutes of Health. Some unpublished results were obtained with support from a HHMI Collaborative Innovation Award.
References
- 1.Parham P. The Immune System. 4. Garland Science; New York, New York: 2014. [Google Scholar]
- 2.van der Merwe PA, Davis SJ. Molecular interactions mediating T cell antigen recognition. Annual Review of Immunology. 2003;21:659–684. doi: 10.1146/annurev.immunol.21.120601.141036. [DOI] [PubMed] [Google Scholar]
- 3.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nature Reviews. Molecular Cell Biology. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumgart T, Hammond AT, Sengupta P, Hess ST, Holowka DA, Baird BA, Webb WW. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3165–3170. doi: 10.1073/pnas.0611357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sengupta P, Hammond A, Holowka D, Baird B. Structural determinants for partitioning of lipids and proteins between coexisting fluid phases in giant plasma membrane vesicles. Biochimica et Biophysica Acta. 2008;1778:20–32. doi: 10.1016/j.bbamem.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaiser HJ, Lingwood D, Levental I, Sampaio JL, Kalvodova L, Rajendarn L, Simons K. Order of lipid phases in model and plasma membranes. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:16645–16650. doi: 10.1073/pnas.0908987106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sezgin E, Kaiser HJ, Baumgart T, Schwille P, Simons K, Levental I. Elucidating membrane structure and protein behavior using giant plasma membrane vesicles. Nature Protocols. 2012;7:1042–1051. doi: 10.1038/nprot.2012.059. [DOI] [PubMed] [Google Scholar]
- 8.Young RM, Zheng X, Holowka D, Baird B. Reconstitution of regulated phosphorylation of FcepsilonRI by a lipid raft-excluded protein-tyrosine phosphatase. The Journal of Biological Chemistry. 2005;280:1230–1235. doi: 10.1074/jbc.M408339200. [DOI] [PubMed] [Google Scholar]
- 9.Young RM, Holowka D, Baird B. A lipid raft environment enhances Lyn kinase activity by protecting the active site tyrosine from dephosphorylation. The Journal of Biological Chemistry. 2003;278:20746–20752. doi: 10.1074/jbc.M211402200. [DOI] [PubMed] [Google Scholar]
- 10.Janes PW, Ley SC, Magee AI. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. The Journal of Cell Biology. 1999;147:447–461. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown DA, London E. Structure and origin of ordered lipid domains in biological membranes. J Membrane Biology. 1998;164:1-3-114. doi: 10.1007/s002329900397. [DOI] [PubMed] [Google Scholar]
- 12.Sheets ED, Holowka D, Baird B. Critical role for cholesterol in Lyn-mediated tyrosine phosphorylation of FceRI and their association with detergent-resistant membranes. Journal of Cell Biology. 1999;145:877–887. doi: 10.1083/jcb.145.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galan C, Woodard GE, Dionisio N, Salido GM, Rosado JA. Lipid rafts modulate the activation but not the maintenance of store-operated Ca(2+) entry. Biochimica et Biophysica Acta. 2010;1803:1083–1093. doi: 10.1016/j.bbamcr.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Pani B, Ong HL, Liu X, Rauser K, Ambudkar IS, Singh BB. Lipid rafts determine clustering of STIM1 in endoplasmic reticulum-plasma membrane junctions and regulation of store-operated Ca2+ entry (SOCE) The Journal of Biological Chemistry. 2008;283:17333–17340. doi: 10.1074/jbc.M800107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calloway N, Owens T, Corwith K, Rodgers W, Holowka D, Baird B. Stimulated association of STIM1 and Orai1 is regulated by the balance of PtdIns(4,5)P(2) between distinct membrane pools. Journal of Cell Science. 2011;124:2602–2610. doi: 10.1242/jcs.084178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou X, Pedi L, Diver MM, Long SB. Crystal structure of the calcium release-activated calcium channel Orai. Science. 2012;338:1308–1313. doi: 10.1126/science.1228757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holowka D, Korzeniowski MK, Bryant KL, Baird B. Polyunsaturated fatty acids inhibit stimulated coupling between the ER Ca(2+) sensor STIM1 and the Ca(2+) channel protein Orai1 in a process that correlates with inhibition of stimulated STIM1 oligomerization. Biochimica et Biophysica Acta. 2014;1841:1210–1216. doi: 10.1016/j.bbalip.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richieri GV, Mescher MF, Kleinfeld AM. Short term exposure to cis unsaturated free fatty acids inhibits degranulation of cytotoxic T lymphocytes. Journal of Immunology. 1990;144:671–677. [PubMed] [Google Scholar]
- 19.de Santos MS, Naal RM, Baird B, Holowka D. Inhibitors of PI(4,5)P2 synthesis reveal dynamic regulation of IgE receptor signaling by phosphoinositides in RBL mast cells. Mol Pharmacol. 2013;83:793–804. doi: 10.1124/mol.112.082834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holowka D, Baird B. Nanodomains in early and later phases of FcepsilonRI signalling. Essays Biochem. 2015;57:147–163. doi: 10.1042/bse0570147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Field KA, Apgar JR, Hong-Geller E, Siraganian RP, Baird B, Holowka D. Mutant RBL mast cells defective in Fc epsilon RI signaling and lipid raft biosynthesis are reconstituted by activated Rho-family GTPases. Mol Biol Cell. 2000;11:3661–3673. doi: 10.1091/mbc.11.10.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong-Geller E, Holowka D, Siraganian RP, Baird B, Cerione RA. Activated Cdc42/Rac reconstitutes Fcepsilon RI-mediated Ca2+ mobilization and degranulation in mutant RBL mast cells. Proceedings of the National Academy of Sciences USA. 2001;98:1154–1159. doi: 10.1073/pnas.98.3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkes MM, Wilson JD, Baird B, Holowka D. Activation of Cdc42 is necessary for sustained oscillations of Ca2+ and PIP2 stimulated by antigen in RBL mast cells. Biology Open. 2014;3:700–710. doi: 10.1242/bio.20148862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wollman R, Meyer T. Coordinated oscillations in cortical actin and Ca2+ correlate with cycles of vesicle secretion. Nat Cell Biol. 2012;14:1261–1269. doi: 10.1038/ncb2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu M, Wu X, De Camilli P. Calcium oscillations-coupled conversion of actin travelling waves to standing oscillations. Proceedings of the National Academy of Sciences USA. 2013;110:1339–1344. doi: 10.1073/pnas.1221538110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gadi D, Wagenknecht Wiesner A, Holowka D, Baird B. Sequestration of phosphoinositides by mutated MARCKS effector domain inhibits stimulated Ca2+ mobilization and degranulation in mast cells. Mol Biol Cell. 2011;22:4908–4917. doi: 10.1091/mbc.E11-07-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valitutti S, Dessing M, Aktories K, Gallati H, Lanzavecchia A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. The Journal of Experimental Medicine. 1995;181:577–584. doi: 10.1084/jem.181.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumari S, Curado S, Mayya V, Dustin ML. T cell antigen receptor activation and actin cytoskeleton remodeling. Biochimica et Biophysica Acta. 2014;1838:546–556. doi: 10.1016/j.bbamem.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frigeri L, Apgar JR. The role of actin microfilaments in the down-regulation of the degranulation response in RBL-2H3 mast cells. Journal of Immunology. 1999;162:2243–2250. [PubMed] [Google Scholar]
- 30.Holowka D, Sheets ED, Baird B. Interactions between Fc(epsilon)RI and lipid raft components are regulated by the actin cytoskeleton. Journal of Cell Science. 2000;113:1009–1019. doi: 10.1242/jcs.113.6.1009. [DOI] [PubMed] [Google Scholar]
- 31.Gosse JA, Wagenknecht-Wiesner A, Holowka D, Baird B. Transmembrane sequences are determinants of immunoreceptor signaling. Journal of Immunology. 2005;175:2123–2131. doi: 10.4049/jimmunol.175.4.2123. [DOI] [PubMed] [Google Scholar]
- 32.Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–849. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 33.O’Donoghue GP, Pielak RM, Smoligovets AA, Lin JJ, Groves JT. Direct single molecule measurement of TCR triggering by agonist pMHC in living primary T cells. eLife. 2013;2:e00778. doi: 10.7554/eLife.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Houtman JC, Yamaguchi H, Barda-Saad M, Braiman A, Bowden B, Appella E, Schuck P, Samelson LE. Oligomerization of signaling complexes by the multipoint binding of GRB2 to both LAT and SOS1. Nature Structural & Molecular Biology. 2006;13:798–805. doi: 10.1038/nsmb1133. [DOI] [PubMed] [Google Scholar]
- 35.Banjade S, Rosen MK. Phase transitions of multivalent proteins can promote clustering of membrane receptors. eLife. 2014:3. doi: 10.7554/eLife.04123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong H, Dolpady J, Wabl M, Curotto de Lafaille MA, Lafaille JJ. Sequential class switching is required for the generation of high affinity IgE antibodies. The Journal of Experimental Medicine. 2012;209:353–364. doi: 10.1084/jem.20111941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metzger H. The IgE-mast cell system as a paradigm for the study of antibody mechanisms. Immunol Rev. 1978;41:186–199. doi: 10.1111/j.1600-065x.1978.tb01465.x. [DOI] [PubMed] [Google Scholar]
- 38.Cooper GM, Hausman RE. The Cell: A Molecular Approach. 3. ASM Press and Sinauer Associates; Sunderland, MA: 2004. [Google Scholar]
- 39.Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nature reviews. Molecular Cell Biology. 2010;11:276–287. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bretscher A. Rapid phosphorylation and reorganization of ezrin and spectrin accompany morphological changes induced in A-431 cells by epidermal growth factor. The Journal of Cell Biology. 1989;108:921–930. doi: 10.1083/jcb.108.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ilani T, Khanna C, Zhou M, Veenstra TD, Bretscher A. Immune synapse formation requires ZAP-70 recruitment by ezrin and CD43 removal by moesin. The Journal of Cell Biology. 2007;179:733–746. doi: 10.1083/jcb.200707199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Das M, Subbayya Ithychanda S, Qin J, Plow EF. Mechanisms of talin-dependent integrin signaling and crosstalk. Biochimica et Biophysica Acta. 2014;1838:579–588. doi: 10.1016/j.bbamem.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nature reviews. Molecular Cell Biology. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 44.Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, Waterman CM. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010;468:580–584. doi: 10.1038/nature09621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo BH, Springer TA. Integrin structures and conformational signaling. Current Opinion in Cell Biology. 2006;18:579–586. doi: 10.1016/j.ceb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Springer TA, Dustin ML. Integrin inside-out signaling and the immunological synapse. Current Opinion in Cell Biology. 2012;24:107–115. doi: 10.1016/j.ceb.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orlowski A, Kukkurainen S, Poyry A, Rissanen S, Vattulainen I, Hytonen VP, Rog T. PIP2 and Talin Join Forces to Activate Integrin. The Journal of Physical Chemistry. B. 2015;119:12381–12389. doi: 10.1021/acs.jpcb.5b06457. [DOI] [PubMed] [Google Scholar]
- 48.Chen Y, Veracini L, Benistant C, Jacobson K. The transmembrane protein CBP plays a role in transiently anchoring small clusters of Thy-1, a GPI-anchored protein, to the cytoskeleton. Journal of Cell Science. 2009;122:3966–3972. doi: 10.1242/jcs.049346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brdicka T, Pavlistova D, Leo A, Bruyns E, Korinek V, Angelisova P, Scherer J, Shevchenko A, Hilgert I, Cerny J, Drbal K, Kuramitsu Y, Kornacker B, Horejsi V, Schraven B. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. The Journal of Experimental Medicine. 2000;191:1591–1604. doi: 10.1084/jem.191.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harder T, Simons K. Clusters of glycolipid and glycosylphosphatidylinositol-anchored proteins in lymphoid cells: accumulation of actin regulated by local tyrosine phosphorylation. European Journal of Immunology. 1999;29:556–562. doi: 10.1002/(SICI)1521-4141(199902)29:02<556::AID-IMMU556>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 51.Dykstra M, Cherukuri A, Sohn HW, Tzeng SJ, Pierce SK. Location is everything: lipid rafts and immune cell signaling. Annual review of immunology. 2003;21:457–481. doi: 10.1146/annurev.immunol.21.120601.141021. [DOI] [PubMed] [Google Scholar]
- 52.Raghupathy R, Anilkumar AA, Polley A, Singh PP, Yadav M, Johnson C, Suryawanshi S, Saikam V, Sawant SD, Panda A, Guo Z, Vishwakarma RA, Rao M, Mayor S. Transbilayer lipid interactions mediate nanoclustering of lipid-anchored proteins. Cell. 2015;161:581–594. doi: 10.1016/j.cell.2015.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheets ED, Holowka D, Baird B. Critical role for cholesterol in Lyn-mediated tyrosine phosphorylation of FcepsilonRI and their association with detergent-resistant membranes. The Journal of cell biology. 1999;145:877–887. doi: 10.1083/jcb.145.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng PC, Dykstra ML, Mitchell RN, Pierce SK. A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. The Journal of Experimental Medicine. 1999;190:1549–1560. doi: 10.1084/jem.190.11.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor RB, Duffus WP, Raff MC, de Petris S. Redistribution and pinocytosis of lymphocyte surface immunoglobulin molecules induced by anti-immunoglobulin antibody. Nature: New Biology. 1971;233:225–229. doi: 10.1038/newbio233225a0. [DOI] [PubMed] [Google Scholar]
- 56.Gupta N, Wollscheid B, Watts JD, Scheer B, Aebersold R, DeFranco AL. Quantitative proteomic analysis of B cell lipid rafts reveals that ezrin regulates antigen receptor-mediated lipid raft dynamics. Nature Immunology. 2006;7:625–633. doi: 10.1038/ni1337. [DOI] [PubMed] [Google Scholar]
- 57.Kwik J, Boyle S, Fooksman D, Margolis L, Sheetz MP, Edidin M. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proceedings of the National Academy of Sciences USA. 2003;100:13964–13969. doi: 10.1073/pnas.2336102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nishimura SY, Vrljic M, Klein LO, McConnell HM, Moerner WE. Cholesterol depletion induces solid-like regions in the plasma membrane. Biophysical Journal. 2006;90:927–938. doi: 10.1529/biophysj.105.070524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fridriksson EK, Shipkova PA, Sheets ED, Holowka D, Baird B, McLafferty FW. Quantitative analysis of phospholipids in functionally important membrane domains from RBL-2H3 mast cells using tandem high-resolution mass spectrometry. Biochemistry. 1999;38:8056–8063. doi: 10.1021/bi9828324. [DOI] [PubMed] [Google Scholar]
- 60.de Almeida RF, Redorov A, Prieto M. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: boundaries and composition of lipid rafts. Biophysical Journal. 2003;85:2406–2416. doi: 10.1016/s0006-3495(03)74664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han X, Smith NL, Sil D, Holowka DA, McLafferty FW, Baird BA. IgE Receptor-Mediated Alteration of Membrane-Cytoskeleton Interactions Revealed by Mass Spectrometric Analysis of Detergent-Resistant Membranes. Biochemistry. 2009;48:6540–6550. doi: 10.1021/bi900181w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Naal R, Holowka E, Baird B, Holowka D. Antigen-stimulated trafficking from the recycling compartment to the plasma membrane in RBL mast cells. Traffic. 2003;4:190–200. doi: 10.1034/j.1600-0854.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- 63.Ge M, Field KA, Aneja R, Holowka D, Baird B, Freed JH. Electron spin resonance characterization of liquid ordered phase of detergent-resistant membranes from RBL-2H3 cells. Biophysical Journal. 1999;77:925–933. doi: 10.1016/S0006-3495(99)76943-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ge M, Gidwani A, Brown HA, Holowka D, Baird B, Freed JH. Ordered and disordered phases coexist in plasma membrane vesicles of RBL-2H3 mast cells. An ESR study. Biophysical Journal. 2003;85:1278–1288. doi: 10.1016/S0006-3495(03)74563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swamy MJ, Ciani L, Ge M, Smith AK, Holowka D, Baird B, Freed JH. Coexisting domains in the plasma membranes of live cells characterized by spin-label ESR spectroscopy. Biophysical Journal. 2006;90:4452–4465. doi: 10.1529/biophysj.105.070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goswami D, Gowrishankar K, Bilgrami S, Ghosh S, Raghupathy R, Chadda R, Vishwakarma R, Rao M, Mayor S. Nanoclusters of GPI-anchored proteins are formed by cortical actin-driven activity. Cell. 2008;135:1085–1097. doi: 10.1016/j.cell.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leventis PA, Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Annual Review of Biophysics. 2010;39:407–427. doi: 10.1146/annurev.biophys.093008.131234. [DOI] [PubMed] [Google Scholar]
- 68.Zech T, Ejsing CS, Gaus K, de Wet B, Shevchenko A, Simons K, Harder T. Accumulation of raft lipids in T-cell plasma membrane domains engaged in TCR signalling. The EMBO Journal. 2009;28:466–476. doi: 10.1038/emboj.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Honigmann A, Sadeghi S, Keller J, Hell SW, Eggeling C, Vink R. A lipid bound actin meshwork organizes liquid phase separation in model membranes. eLife. 2014;3:e01671. doi: 10.7554/eLife.01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Machta BB, Papanikolaou S, Sethna JP, Veatch SL. Minimal model of plasma membrane heterogeneity requires coupling cortical actin to criticality. Biophysical Journal. 2011;100:1668–1677. doi: 10.1016/j.bpj.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu AP, Fletcher DA. Photopatterning of actin filament structures. Nano Letters. 2005;5:625–628. doi: 10.1021/nl0478878. [DOI] [PubMed] [Google Scholar]
- 72.Zhao J, Wu J, Veatch SL. Adhesion stabilizes robust lipid heterogeneity in supercritical membranes at physiological temperature. Biophysical Journal. 2013;104:825–834. doi: 10.1016/j.bpj.2012.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Veatch SL, Cicuta P, Sengupta P, Honerkamp-Smith A, Holowka D, Baird B. Critical fluctuations in plasma membrane vesicles. ACS Chem Biol. 2008;3:287–293. doi: 10.1021/cb800012x. [DOI] [PubMed] [Google Scholar]
- 74.Byrum JN, Rodgers W. Membrane-cytoskeleton interactions in cholesterol-dependent domain formation. Essays Biochem. 2015;57:177–187. doi: 10.1042/bse0570177. [DOI] [PubMed] [Google Scholar]
- 75.Baird B, Sheets ED, Holowka D. How does the plasma membrane participate in cellular signaling by receptors for immunoglobulin E? Biophysical Chemistry. 1999;82:109–199. doi: 10.1016/s0301-4622(99)00110-6. [DOI] [PubMed] [Google Scholar]