Abstract
High-fluorescent p-X-ferrites (XFe2O4; XFO; X = Fe, Cr, Mn, Co, or Ni) embedded in n-hematite (Fe2O3) surfaces were successfully fabricated via a facile bio-approach using Shewanella oneidensis MR-1. The results revealed that the X ions with high/low work functions modify the unpaired spin Fe2+–O2− orbitals in the XFe2O4 lattices to become localized paired spin orbitals at the bottom of conduction band, separating the photovoltage response signals (73.36~455.16/−72.63~−32.43 meV). These (Fe2O3)–O–O–(XFe2O4) interfacial coupling behaviors at two fluorescence emission peaks (785/795 nm) are explained via calculating electron-hole effective masses (Fe2O3–FeFe2O4 17.23 × 10−31 kg; Fe2O3–CoFe2O4 3.93 × 10−31 kg; Fe2O3–NiFe2O4 11.59 × 10−31 kg; Fe2O3–CrFe2O4 −4.2 × 10−31 kg; Fe2O3–MnFe2O4 −11.73 × 10−31 kg). Such a system could open up a new idea in the design of photovoltage response biosensors.
Keywords: Heterostructure, Fluorescence enhancement, Quantum dots, Shewanella oneidensis MR-1
Background
As a n-type semiconductor, hematite (Fe2O3) is an important semiconductor in the fields of photoluminescence and electron paramagnetic imaging due to its chemical stability and band gap (2 eV) [1]. However, poor minority charge carrier mobility (0.2 cm2 V−1 s−1) and ultrafast recombination of photogenerated carriers (~10 ps) limit its application as a high-photostability fluorescent material [2]. Recently, an interesting option is the conjugation of Fe2O3 with a p-type ferrite (band gaps 1.9~2.7 eV) with a lower but similar conduction band level and an appropriate valence band level [3]. It has two advantages, e.g., unpaired-paired spin change and electron-hole recombination [4]. For example, Sun et al. [5] confirmed that the electrons flow through the energy barrier between the Fe2O3 and Fe3O4 phases, according to spin-dependent tunneling mode. Higher void fraction of cubic Fe3O4 provides more transfer channels for tetrahedral ion diffusion and charge transfer. To enhance separation rate of photoinduced charge carriers, Shen et al. [6] used Mg to modify the surface photovoltage of Fe3O4/Fe2O3 heterostructured hollow nanospheres. A remarkable surface photovoltage response in UV and visible spectral region (320~570 nm) was attributed to the 2p(O2−)→3d(Fe3+) charge transfer and Fe3+(3d5) crystal field transitions of the MgFe2O4 and Fe2O3 interface. Therefore, the incorporation of the magnetic nanocrystals associated with heavy metal ion (X)-modified Fe3O4 on the Fe2O3 surface can not only improve fluorescence intensity but also recycle the fluorescence by magnetic separation under modest magnetic fields [7].
In the present work, we designed a facile approach to reduce the effect of lattice mismatch of the components, for coating directly p-ferrite on the n-hematite using Shewanella oneidensis MR-1 [8]. Therein, the cytochromes (OmcA, MtrC, and MtrB) and periplasmic [Fe] hydrogenases of the extracellular matrix represent the bio-reactions as follows: lactate− + 4Fe3+ + 2H2O → acetat− + HCO− + 4Fe2+ + 5H+ [9]; X2+ + 2Fe(OH)3 → XFe2O4 + 2H2O + 2H+ [10]. Therefore, XFe2O4 particles will be directly formed from the Fe2O3 surface, creating a Fe2O3–XFe2O4 interface. The purpose of this paper is to enhance both surface photovoltage response and fluorescence, for designing new multifunctional sensor.
Methods
Here, S. oneidensis MR-1 was cultured in a chemically defined minimal medium as described previously [11]. MR-1 cells were cultured aerobically on TSB (without dextrose) for 16 h at 30 °C with shaking at 100 rpm. Cells were washed twice and centrifuged at 6000 rpm for 10 min in sterile PIPES/AQDS buffer at pH 7, followed by one wash and re-suspension to ~109 cells ml−1 in M1 medium. Anaerobically grown cells of MR-1 were added to the tubes containing the magnetite and M1 medium to obtain a final concentration of 2.3 × 108 cells ml−1. The total volume of medium in each tube, including X(Cr, Mn, Co, or Ni)-modified goethite (FeOOH) and cells, was 250 ml. The X-modified Fe3O4 in the medium had a final concentration of 90 mM [12]. All treatment tubes were incubated in the dark at 30 °C until the end of the experiment. All treatments with anaerobically cultured cells were incubated for 45 days.
The size of the laser spot was less than 2 μm, and the acquisition time for all spectra was 20 s. Scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS) were performed on a Cu plate with a Hitachi S-4800 field emission machine with an accelerating voltage of 5 KeV [13]. The X-ray diffraction data were collected for all Fe2O3–XFe2O4 heterostructures using an X-ray diffractometer (Bryke D8-Advance, Germany, Cu Kα radiation, λ = 0.154 nm, 40 kV, 40 mA) [14]. Raman scattering measurements (Labram HR evolution, Horiba Scientific, France) were conducted at room temperature under a backscattering geometric configuration using a WITec-Alpha confocal micro-Raman system [15]. The light absorption properties of the heterostructures were tested by UV-Vis diffuse reflectance spectroscopy (DRS) (Evolution 220, USA) [16]. Atomic force microscope (AFM) and Kelvin probe force microscopy (KPFM) measurements were performed on an atomic force microscope (Asylum Research MFP-3D, USA) [17]. Photoluminescence (PL) emission at room temperature was obtained at 420~900 nm with an excitation wavelength at 400 nm. The slit widths for both the excitation and emission were 5.0 nm. A fiber-based fluorescence spectrometer (USB 4000, Ocean Optics, USA) was used to record the in situ PL spectra [18].
Besides, we simulated the effective masses of electron-hole pairs, dielectric functions (ε), and spin-partial densities of states (spin-PDOSs) via Kramers-Kronig transform to give an insight into the electron transfer process at the atomic level and to contribute to the interpretation of experimental results from techniques such as DRS, KPFM-AFM, or PL, based on the generalized gradient-corrected Perdew-Burke-Ernzerhof functional + U (GGA-PBE) (Castep, Materials studio, Accelrys, USA) [19], where the Coulomb and screened exchange parameters (U, J) were set 5 and 1 eV, respectively. A kinetic energy cutoff of 300 eV for the electrons was used, well within the convergence of a total-energy calculation. A 2 × 2 × 2 super cell was introduced for the interstitial plane-wave, and a 5 × 5 × 5 k-point mesh for integration over the Brillouin zone.
Results and Discussion
Structural Characterization
In Burns’ opinion [20], the reduced Fe2+ precipitates tend to accumulate on the extracellular polymeric substance cytochrome and [Fe] hydrogenases of S. oneidensis MR-1, forming the Fe3O4 phase. And H+ ions as the electron donors at pH6 can mediate the electron transfer between Fe2+ and X2+ to modify the Fe3O4 surface. Figure 1a confirms that X-modified Fe3O4 and Fe2O3 are coexisting, because the 500-nm rice-like Fe3O4 particles are attached onto the 3.5-μm Fe2O3 surfaces. And total mass ratios of Fe, O, and X are 26.66~57.98 wt.%, 43.61~46.65 wt.%, and 8.36~11.89 wt.%, respectively.
Fig. 1.
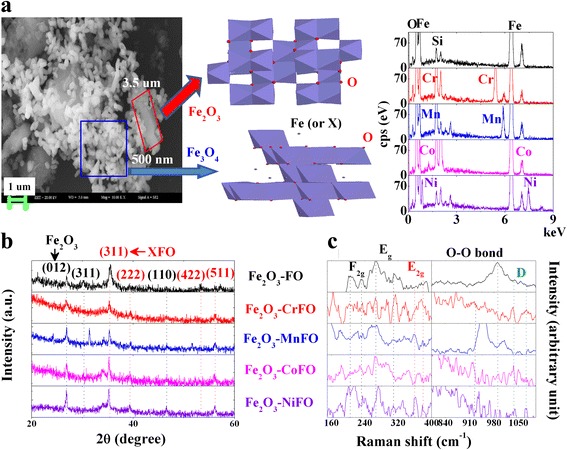
a SEM image of Fe2O3–Fe3O4 and EDS-SEM patterns of Fe2O3–XFe2O4. b, c show the relative XRD and Raman patterns
To verify the crystalline structure of the as-synthesized sample, typical power XRD patterns are shown in Fig. 1b. The clear diffraction peaks at 2θ angles (approximately 27°, 35.5°, 47°, 30.5°, 39°, 53.5°, 57.5°) can be assigned to the (012), (311), and (110) characteristic reflections of Fe2O3 (JCPDS 89-5892) and the (311), (222), (422), and (511) characteristic reflections of the cubic structure of magnetite XFe2O4 (JCPDS 19-0629) [21, 22]. The narrow and sharp peaks suggest that the obtained XFe2O4 and Fe2O3 are highly crystalline in nature, with average crystallite sizes approximately 41.32~44.21 nm and 28.78~32.79 nm at the Fe2O3–(012) and XFO–(311) planes, respectively.
To further identify the interactions of iron oxides, we used lines in Fig. 1c to depict the typical Raman spectrum of Fe2O3–XFe2O4 heterostructures. The Raman band at the F2g, Eg, and E2g modes reflect translational movement of the tetrahedron, symmetric bending of oxygen with respect to the metal ion, and asymmetric stretching of Fe(X) and O, respectively. These bands reflect that Fe2O3 comprises hexagonal close-packed layers of O2− and that Fe3+ ions fill two thirds of the octahedral voids, forming a FeO6 octahedral layer. XFe2O4 is a normal spinel, with X2+ ions at tetrahedral sites and Fe3+ ions at octahedral sites. On another side, we can see that a broad asymmetric D peak at 915~970 cm−1 as the phonon scattering near the Brillouin zone boundary is attributed to the long-range (Fe2O3)–O–O–(XFe2O4) interface. These results confirm that the surface Fe2O3 has been successfully reduced as a XFe2O4 and that they are bonded to each other with the shared oxygen atoms of the octahedron-tetrahedron.
Unpaired-Paired Spin Change in the Lattice
In the literature [3], the enhanced photovoltage response performances can be attributed to intra- or inter-electronic transition in the UV and visible region. The origin of electron transfer enhancement of Fe2O3–XFe2O4 can be summarized by (i) unpaired-paired spin change in the lattice and (ii) electron-hole recombination in the interface. Figure 2a verifies that the X ions enhance the average fluorescence intensities by 3.13~6.35 multiples than that of Fe2O3–Fe3O4, where the band gap differences in Fig. 2b are close to the Fermi point, providing high electron transfer ratios. In the octahedral Fe3+–O2− orbital at an intrinsic main fluorescence emission peak of 390 nm, the absorption bands can be attributed to two charge transfers as the oxygen-to-metal 2p(O2−)→3d(Fe3+) (left region: Fe2O3; and middle region: XFe2O4) intra-atomic transitions, reflecting the non-degeneracy (6A1)→three orbital degeneracy (T2g) inter-atomic transition [23]. This corresponds to the T2g–T2g orbital degeneracy, as shown in Fig. 2c.
Fig. 2.
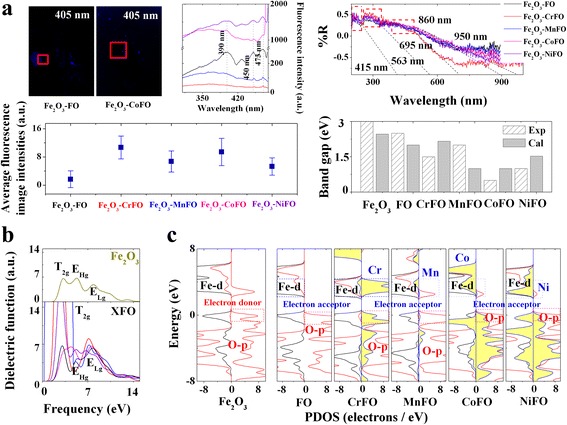
a Fluorescence images, average luminous intensities, and PL patterns of Fe2O3–XFe2O4 by the excitation of 405 nm. b reflects the DRS patterns and confrontation between experimental and calculated band gaps of Fe2O3–XFe2O4. The absorption bands were tested through the slopes of diffuse reflectance curves that the experimental and calculated band gaps are similar with each other. c shows the calculated dielectric functions and spin-PDOSs of Fe2O3–XFe2O4
On another side, the spin parity of the iron pairs can remain the same in the excited state, showing the spin-down PDOSs. To modify the electron transfer behaviors in tetrahedral Fe2+–O2− orbitals at two broad blue fluorescence emission peaks at 450 and 473 nm, we used X2+ as an acceptor to occupy the tetrahedral sites at the bottom of the conduction band, and thus, the electronic transitions implying Fe2+(or X2+)–O2− orbitals are known to be only at the origin of charge transfers between the bottom of the conduction band and the top of the valence band. The unpaired spin orbitals change into paired spin orbitals; therefore, the paired spin orbitals enhance the spin quantum number (S) to 1, changing the multiplicity of the excited state (M = 2S + 1) into 3. This unpaired-paired spin change provides more active electron gas in the Fe2O3–XFe2O4 interface to enhance the average luminous intensities as 3.31~8.18 multiples than that of Fe2O3–Fe3O4 by the excitation of 488 nm, as presented in Fig. 3a. The accelerated electron-hole recombination [24] at the near-infrared photoresponse region is beneficial for transferring long-range electrons to separate the photovoltage response signal.
Fig. 3.

a Fluorescence images, average luminous intensities, and PL patterns of Fe2O3–XFe2O4 by the excitation of 488 nm. b reflects the surface photovoltages and KPFM phase images. The work function values of Fe2O3 and FeFe2O4 are 5.35 eV [26] and 5.52 eV [27], respectively, and the charges transfer between the different work functions of two materials for their Fermi levels to equilibrate. The corresponding theoretical surface potential images are shown in (c). d means the calculated effective masses of electron-hole pairs and surface potential illustration
Electron-Hole Recombination in the Interface
Ultimately, we found that Fe3O4 has a significant photovoltage response red shifted to 257.7 meV from those of Fe2O3 in Fig. 3b, which allows for the efficient separation of electron-hole pairs at the Fe2O3–Fe3O4 interface, which will be higher than that (200 meV) of the reported Fe2O3–ZnFe2O4 interface [25–27]. The origin of the high/low photovoltage response region (theoretical difference data ~200 meV; experimental difference data 45~160 meV) is attributed to the surface potential difference (270 meV/3~70 meV) of Fe2O3 and XFe2O4, according to the theoretical potential images (see Fig. 3c). The photovoltage response signal in the low photovoltage response region is modified via the X–Fe–O orbital degeneracy.
To our knowledge, the crystal field theory indicates that the Fe–d orbital will split into the doubly degenerate eg orbital (dz2 and dx2–y2) and triple degenerate t2g orbital (dxy, dyz, and dxz) [28]. On the one hand, the high-energy Fe–dx2–y2 orbital (work function 4.5 eV) can be modified by the X–dx2–y2 orbital with the higher work functions (Co 4.7 eV and Ni 4.6 eV) [29]. The X ions reduce both octahedral and tetrahedral surface potentials (~0.7 and 10 meV); because of that, the down-spin Co2+ (or Ni2+)–Fe3+ interfacial coupling degenerates with the down-spin dx2–y2 orbital of Fe2+–3d64s2 (Fig. 2c) at the high-energy eHg orbital. It not only enhances the positive potential direction (polarization angle 5~15° → 60~100°) but also accelerates the electron-hole recombination to create a highly effective mass hole (Fe2O3–FeFe2O4 17.23 × 10−31 kg; Fe2O3–CoFe2O4 3.93 × 10−31 kg; Fe2O3–NiFe2O4 11.59 × 10−31 kg) (see Fig. 3d). A responsibility of the intrinsic main fluorescence emission peak blue shifts to 785 nm.
On the other hand, the Fe–O dz2–pz2 orbital is being modified by the X-dz2 orbital with lower work functions (Cr 4.41 eV and Mn 4.1 eV). The data curve showed that the dz2–dz2–pz2 orbital degeneracy creates a weak quantum well of oxygen vacancy for long-range electronic transition at the low-energy doubly degenerate eLg orbital. A part of O–2p4 electron is trapped in the oxygen vacancy with the deep holes present in the valence band, according to the enhanced electron effective mass (Fe2O3–CrFe2O4 −4.2 × 10−31 kg; Fe2O3–MnFe2O4 −11.73 × 10−31 kg). The down-spin pz2 orbital changes its direction to become an up-spin orbital, showing the negative photovoltage response signal (Cr −32.43 meV; Mn −72.63 meV). Consequently, the different photovoltage response behaviors above can be used for the design of high-mobility electronic devices and fluorescent probes for different heavy metal ion imaging, oxygen evolution catalysts [30], and biomolecules [31], etc.
Conclusions
In summary, a novel bio-induced phase transition method for the growth of XFe2O4 embedded in a Fe2O3 is proposed here using S. oneidensis MR-1. We explained the mechanism of surface photovoltage response and high-photostability fluorescence. The present work demonstrates that the calculated unpaired-paired spin X2+–Fe2+–O2- orbitals verify the enhanced photovoltage response signal (theoretical data ~200 meV; experimental data 45~160 meV) and separate the high/low surface potential regions (270 meV/3~70 meV) based on the calculated electron-hole effective masses. As a consequence, our works provide a reference for designing new photoluminescence and electron paramagnetic imaging biosensors. Further investigation will be focused on the electron transfer process between interfaces and heavy metal ions in an aquatic environment to garner a better understanding of the selective fluorescence probe application.
Acknowledgements
The authors acknowledge the financial supports by the 973 project (2014CB8460003), the One-Thousand-Talents Scheme in Sichuan Province, Xinjiang Science Fund of Outstanding Young Scholars (qn2015yx034), Hebei outstanding young scholars, Science and Technology Program of Hebei Province (D2016403064 and 16044601Z), and Hebei Science and Technology Support Program (15211121).
Authors’ Contributions
LB draft the manuscript and with FD, HD, and MS designed the experimental synthesis and theoretical simulation of XFO-BFO. HL participated in the experimental and theoretical design, FD in the spectrum measurement and analysis. YL and JN directed the experiment on the synthesis of XFO-BFO by microorganism method. LW, TZ, and XZ participated in the microbial synthesis experiment. XL and LX carried out modifying the XFO-BFO by doping the Cr, Mn, Co, or Ni, and the SEM characterization. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Contributor Information
Liang Bian, Email: bianliang555551@126.com.
Hai-long Li, Email: well09131015@126.com.
References
- 1.Teleki A, Suter M, Kidambi PR, Ergeneman O, Krumeich F, Nelson BJ, Pratsinis SE. Hermetically coated superparamagnetic Fe2O3 particles with SiO2 nanofilms. Chem Mater. 2009;21:2094–2100. doi: 10.1021/cm803153m. [DOI] [Google Scholar]
- 2.Sivula K, Zboril R, Formal FL, Robert R, Weidenkaff A, Tucek J, Frydrych J, Grätzel M. Photoelectrochemical water splitting with mesoporous hematite prepared by a solution-based colloidal approach. J Am Chem Soc. 2010;132:7436–7444. doi: 10.1021/ja101564f. [DOI] [PubMed] [Google Scholar]
- 3.Pailhe’ N, Wattiaux A, Gaudon M, Demourgues A. Correlation between structural features and vis–NIR spectra of α-Fe2O3 hematite and AFe2O4 spinel oxides (A = Mg, Zn) J Solid State Chem. 2008;181:1040–1047. doi: 10.1016/j.jssc.2008.02.009. [DOI] [Google Scholar]
- 4.Ren Y, Wang J, Huang X, Ding J. Enhanced lithium-ion storage performance by structural phase transition from two-dimensional rhombohedral Fe2O3 to cubic Fe3O4. Electrochim Acta. 2016;198:22–31. doi: 10.1016/j.electacta.2016.03.076. [DOI] [Google Scholar]
- 5.Sun AC, Kuo PC, Chou CY, Chen SC, Lie CT, Lin MH, Chen JW, Huang HL. Magnetoresistance of sintered (Fe2O3)1.00-x(Fe3O4)x ferrites. J Magnetism Magnetic Mater. 2004;272–276:1776–1777. doi: 10.1016/j.jmmm.2003.12.745. [DOI] [Google Scholar]
- 6.Shen Y, Zhao Q, Li X, Hou Y, Chen G. Surface photovoltage property of magnesium ferrite/hematite heterostructured hollow nanospheres prepared with one-pot strategy. Colloid Sur A: Physicochem Engin Aspects. 2012;403:35–40. doi: 10.1016/j.colsurfa.2012.03.052. [DOI] [Google Scholar]
- 7.Yang L, Jin R, Liang Y, Wang F, Yin P, Yi C. Preparation and magnetic properties of NiFe2O4–Fe2O3@SnO2 heterostructures. Mater Lett. 2015;153:55–58. doi: 10.1016/j.matlet.2015.04.012. [DOI] [Google Scholar]
- 8.Lower SK, Hochella MF, Beveridge TJ. Bacterial recognition of mineral surfaces: nanoscale interactions between shewanella and α-FeOOH. Sci. 2001;292:1360–1363. doi: 10.1126/science.1059567. [DOI] [PubMed] [Google Scholar]
- 9.Ma S, Banfield JF. Micron-scale Fe2+/Fe3+, intermediate sulfur species and O2 gradients across the biofilm-solution-sediment interface control biofilm organization. Geochim Cosmochim Acta. 2011;75:3568–3580. doi: 10.1016/j.gca.2011.03.035. [DOI] [Google Scholar]
- 10.Burgos WD, McDonough JT, Senko JM, Zhang G, Dohnalkova AC, Kelly SD, Gorby YA, Kemner KM. Characterization of uraninite nanoparticles produced by Shewanella oneidensis MR-1. Geochim Cosmochim Acta. 2008;72:4901–4915. doi: 10.1016/j.gca.2008.07.016. [DOI] [Google Scholar]
- 11.Zhang G, Burgos WD, Senko JM, Bishop ME, Dong H, Boyanov MI, Kemner KM. Microbial reduction of chlorite and uranium followed by air oxidation. Chem Geol. 2011;283:242–250. doi: 10.1016/j.chemgeo.2011.01.021. [DOI] [Google Scholar]
- 12.Bian L, Li H, Dong H, Dong F, Song M, Wang L, Zhou T, Li W, Hou W, Zhang X, Lu X, Li X, Xie L. Fluorescent enhancement of bio-synthesized X-Zn-ferrite-bismuth ferrite (X=Mg, Mn or Ni) membranes: experiment and theory. Appl Sur Sci. 2017;396:1177–1186. doi: 10.1016/j.apsusc.2016.11.108. [DOI] [Google Scholar]
- 13.Bamoniri A, Moshtael-Arani N. Nano-Fe3O4 encapsulated-silica supported boron trifluoride as a novel heterogeneous solid acid for solvent-free synthesis of arylazo-1-naphthol derivatives. RSC Adv. 2015;5:16911–16920. doi: 10.1039/C4RA12604A. [DOI] [Google Scholar]
- 14.Li J, Hu Y, Yang J, Wei P, Sun W, Shen M, Zhang G, Shi X. Hyaluronic acid-modified Fe3O4@Au core/shell nanostars for multimodal imaging and photothermal therapy of tumors. Biomater. 2015;38:10–21. doi: 10.1016/j.biomaterials.2014.10.065. [DOI] [PubMed] [Google Scholar]
- 15.Seo SH, Kim BM, Joe A, Han HW, Chen X, Cheng Z, Janga ES. NIR-light-induced surface-enhanced Raman scattering for detection and photothermal/photodynamic therapy of cancer cells using methylene blue-embedded gold nanorod@SiO2 nanocomposites. Biomater. 2014;35:3309–3318. doi: 10.1016/j.biomaterials.2013.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bian L, Dong H, Dong F, Song M, Li H, Hou W, Zhang X, Wang L, Sun G, Li X, Xie L. Mechanism of fluorescence enhancement of bio-synthesized XFe2O4-BiFeO3 (X = Cr, Mn, Co or Ni) membranes. Nanoscale Res Lett. 2016;11:543–550. doi: 10.1186/s11671-016-1747-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao P, Bian L, Wang L, Xu J, Chang A. Enhanced open voltage of BiFeO3 polycrystalline film by surface modification of organolead halide perovskite. Appl Phys Lett. 2014;105:013901–013903. doi: 10.1063/1.4888912. [DOI] [Google Scholar]
- 18.Li J, Huang Z, Wu D, Yin G, Liao X, Gu J, Han D. Preparation and protein detection of Zn-Ferrite film with magnetic and photoluminescence properties. J Phys Chem C. 2010;114:1586–1592. doi: 10.1021/jp907107s. [DOI] [Google Scholar]
- 19.Bian L, Xu J, Song M, Dong H, Dong F. Effects of halogen substitutes on the electronic and magnetic properties of BiFeO3. RSC Adv. 2013;3:25129–25135. doi: 10.1039/c3ra45156a. [DOI] [Google Scholar]
- 20.Burns JL, Ginn BR, Bates DJ, Dublin SN, Taylor JV, Apkarian RP, Amaro-Garcia S, Neal AL, DiChristina TJ. Outer membrane-associated serine protease involved in adhesion of Shewanella oneidensis to Fe(III) oxides. Envir Sci Techn. 2010;44:68–73. doi: 10.1021/es9018699. [DOI] [PubMed] [Google Scholar]
- 21.Sa T, Wu G, Qin N, Bao D. Solution processed highly sensitive visible-light photodetectors based on α-Fe2O3/p-Si heterojunctions. Sen Act B: Chem. 2012;173:414–418. doi: 10.1016/j.snb.2012.07.027. [DOI] [Google Scholar]
- 22.Horák D, Shagotova T, Mitina N, Trchová M, Boiko N, Babič M, Stoika R, Kovářová J, Hevus O, Beneš MJ, Klyuchivska O, Holler P, Zaichenko A. Surface-initiated polymerization of 2-hydroxyethyl methacrylate from heterotelechelic oligoperoxide-coated γ-Fe2O3 nanoparticles and their engulfment by mammalian cells. Chem Mater. 2011;23:2637–2649. doi: 10.1021/cm2004215. [DOI] [Google Scholar]
- 23.Todol JL, Mermet JM. Sample introduction systems for the analysis of liquid microsamples by ICP-AES and ICP-MS. Spectrochim Acta B. 2006;61:239–283. doi: 10.1016/j.sab.2005.12.010. [DOI] [Google Scholar]
- 24.Chen YZ, Bovet N, Trier F, Christensen DV, Qu FM, Andersen NH, Kasama T, Zhang W, Giraud R, Dufouleur J, Jespersen TS, Sun JR, Smith A, Nygård J, Lu L, Büchner B, Shen BG, Linderoth S, Pryds N. A high-mobility two-dimensional electron gas at the spinel/perovskite interface of γ-Al2O3/SrTiO3. Nat Comm. 2013;4:1371–1375. doi: 10.1038/ncomms2394. [DOI] [PubMed] [Google Scholar]
- 25.McDonald KJ, Choi KS. Synthesis and photoelectrochemical properties of Fe2O3/ZnFe2O4 composite photoanodes for use in solar water oxidation. Chem Mater. 2011;23:4863–4869. doi: 10.1021/cm202399g. [DOI] [Google Scholar]
- 26.Fan Z, Wen X, Yang S, Lu JG. Controlled p- and n-type doping of Fe2O3 nanobelt field effect transistors. Appl Phys Lett. 2005;87:013113–013115. doi: 10.1063/1.1977203. [DOI] [Google Scholar]
- 27.Wang C, Daimon H, Sun S. Dumbbell-like Pt–Fe3O4 nanoparticles and their enhanced catalysis for oxygen reduction reaction. Nano Lett. 2009;9:1493–1496. doi: 10.1021/nl8034724. [DOI] [PubMed] [Google Scholar]
- 28.Mi WB, Jiang EY, Bai HL. Current-perpendicular-to-plane transport properties of polycrystalline Fe3O4/α-Fe2O3 heterostructures. Appl Phys Lett. 2009;93:132504–132507. doi: 10.1063/1.2993223. [DOI] [Google Scholar]
- 29.Muñoz AG, Staikov G. Electrodeposition of Co on oxide modified p-Si surfaces. Electrochim Acta. 2006;51:2836–2844. doi: 10.1016/j.electacta.2005.08.015. [DOI] [Google Scholar]
- 30.Peng X, Du J, Fan J, Wang J, Wu Y, Zhao J, Sun S, Xu T. A selective fluorescent sensor for imaging Cd2+ in living cells. J Am Chem Soc. 2007;129:1500–1501. doi: 10.1021/ja0643319. [DOI] [PubMed] [Google Scholar]
- 31.Bian L, Dong F, Song M, Xu J, Zhang X. Computational study of the cation-modified GSH peptide interactions with perovskite-type BFO-(111) membranes under aqueous conditions. Nanoscale Res Lett. 2015;10:261–267. doi: 10.1186/s11671-015-0967-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


