Abstract
The effect of Zn-doping in CoFe2O4 nanoparticles (NPs) through chemical co-precipitation route was investigated in term of structural, optical, and magnetic properties. Both XRD and FTIR analyses confirm the formation of cubic spinel phase, where the crystallite size changes with Zn content from 46 to 77 nm. The Scherrer method, Williamson-Hall (W-H) analysis, and size-strain plot method (SSPM) were used to study of crystallite sizes. The TEM results were in good agreement with the results of the SSP method. SEM observations reveal agglomeration of fine spherical-like particles. The optical band gap energy determined from diffuse reflectance spectroscopy (DRS) varies increases from 1.17 to 1.3 eV. Magnetization field loops reveal a ferromagnetic behavior with lower hysteresis loop for higher Zn content. The magnetic properties are remarkably influenced with Zn doping; saturation magnetization (Ms) increases then decreases while both coercivity (HC) and remanent magnetization (Mr) decrease continuously, which was associated with preferential site occupancy and the change in particle size.
Keywords: Spinel ferrite, Nanoparticles, Ferromagnetism, Vibrational modes, Energy band gap
Background
Researchers have been interested in studying materials in their nanoscale dimensions due to their high surface area resulting to enhanced properties in comparison with the bulk materials counterpart [1–6]. Spinel ferrite (SF) materials with a general formula of AFe2O4, where A stands for metals as (Mn, Co, Ni, Mg, or Zn), are well known of their remarkable electrical, optical, and magnetic properties, especially in nanometer scale [7–9].
Doping with metal ions as (Zn, Co, Sr, and Gd) was aimed to improve the physicochemical properties of ferrite nanoparticles (NPs) essential for their applications such as photocatalysis [10, 11] in photodegradation of dyes and as antibacterial agents [12, 13], industrial applications [14], and electrochemical energy storage materials [15, 16]. Studies confirmed that doping influences the structural [17], optical [18], electrical [17, 19], infrared radiation properties [20, 21], and magnetic properties [22, 23].
For instance, crystallite size was shown to gradually increase from 12.6 nm for pure ZnFe2O4 to 21.17 nm for Mg-doped one (75%) [22]. The magnetization properties were found to be altered too, as the saturation magnetization (Ms) and remanent magnetization (Mr), at room temperature, increased from 19 to 8 emu/g for pure ZnFe2O4 prepared by combustion method to 45 and 16 emu/g, respectively, for 50% Mg-doped one. However, with further increase of Mg concentration, these values started to drop reaching 16 and 3.5 emu/gm, respectively, for 75% Mg-doped ferrite [22]. Magnetic properties measured at 77 K and were found to follow the same trend as observed at room temperature. The change in magnetic properties with the concentration of dopant was explained by the replacement of Fe ions and the dopant ions in the octahedral and tetrahedral sites according to their site preference. In general, magnetic properties are known to be strongly influenced by annealing [24]. Few reports predicted that oxide nanoparticles tend to undergo nucleation and growth of Fe ions as a result of electron beam annealing [25]. Such Fe nanoclusters are reported to cause large magnetoresistance due a combination of geometric and spin dependent scattering [26]. Moreover, Sr-doped ZnFe2O4 nanoparticles synthesized by microwave combustion were investigated in terms of structural and magnetic properties. Enhancement of coercivity (HC) with the concentration of Sr was linked to the lattice parameter that increased with the Sr-dopant concentration too. This effect was claimed to be caused by the expansion of the unit cell volume caused by doping with Sr ions (Sr has a higher ionic radius (r = 1.44 Å) compared with Zn (r = 0.83 Å)) [18]. Another study demonstrated very similar dependence: the increase of lattice parameter when SrFe12O19 NPs prepared by sol–gel method were doped with Ni and Zr ions [27]. Value of Ms was shown to be enhanced with the concentration of the dopant ions, while value of HC was shown to decrease. This was related to the replacement of ions in spin-down states and to the grain size variations.
Among these ferrite NPs, CoFe2O4 is recognized for its significant chemical stability, high Curie temperature, and high magnetization [7, 8]. Studies on such materials involved analysis of the effect of Gd doping on the structural and magnetic properties of CoFe2O4 synthesized through wet chemical co-precipitation method [7]. X-ray diffraction (XRD) analysis confirmed the decrease of lattice parameter with the concentration of Gd3+, on the other hand, the crystallite size raised from 15 to 17 nm with the increase of doping ion concentration from 0 to 15%. A considerable reduction of both saturation magnetization (Ms) and remanent magnetization (Mr) from 91 to 29 emu/g, respectively, for pure CoFe2O4 to 54 and 15 emu/g, respectively, for CoFe2O4 doped with 15% Gd3+. This was referred to the large ionic radius of the dopant Gd3+ (r = 0.94 Å) compared to that of Co2+ (0.58 Å) leading to a preferential occupation of octahedral sites resulting in the disturbance of the ferromagnetic ordering thereby, leading to a lower magnetization [7]. A recent study showed a similar effect when CoFe2O4 NPs synthesized by sucrose-assisted combustion route were doped with Zn [23]; both Ms and Mr decreased with increasing Zn concentration which was also referred to the occupancy preference of octahedral and tetrahedral sites. Moreover, the coercivity (HC) was noticed to decrease too, from 126.5 to 26.3 kOe, due to the low anisotropy constant of the Zn2+ ions.
It is well known that the preparation technique has a direct influence on the nanoparticle’s shape and size and thus can affect the physical and chemical properties of nanostructures. The magnetic ferrite particles in the nanoscale regime can be synthesized by different methods like soft chemical methods such as co-precipitation, hydrothermal, sol–gel, etc. But the main advantage of co-precipitation method resides in providing particle size in the nanoscale regime with a high crystallinity. Such individual nanoparticles have a large constant magnetic moment and behave like a giant paramagnetic atom with a fast response to applied magnetic field with negligible remanence and coercivity (supermagnetic behavior). Nanoparticles can also result in a low saturation magnetization. These features make superparamagnetic nanoparticles very attractive for a broad range of applications in particular biomedical field. Therefore, the magnetic properties of nanoparticles highly depend upon the synthesis procedure.
Nanocrystalline CoFe2O4 with unique properties has potential applications in high frequency device, memory core, recording media, and in biomedical field. It is known that zinc ions (Zn2+) with diamagnetic nature are known for achieving good control over magnetic parameters in developing technologically important materials. Substitution of magnetic (Co2+) by a nonmagnetic (Zn2+) cations in spinel ferrite phase may induce important changes in their structural, optical, magnetic, and others properties, due to the distribution of cations in between the available A and B sites. However, a detailed study on the structural, elastic, optical, and magnetic properties of Zn2+-doped CoFe2O4 nanoparticles obtained by co-precipitation method in the widely region has not yet been reported so far. The aim of the present work is to synthesize nanoparticles of Co1−xZnxFe2O4 with x varying from 0 to 0.5 from metal salts by co-precipitation of hydroxides. The influence of Zn substitution on the structural, optical, and magnetic properties for this system has also been discussed.
Methods
Synthesis of Zn-Doped Cobalt Ferrites
The detailed description of synthesis route has been presented in our earlier work [28]. For the preparation of Co1−xZnxFe2O4 (x = 0, 0.1, 0.2, 0.3, 0.4, 0.5) samples through co-precipitation method, cobalt, zinc, and iron nitrates were taken in stoichiometric proportions and dissolved separately in distilled water. The as-prepared solutions were mixed and stirred intensely for 1 h to improve homogeneity. The solutions were subjected to constant heating at 80 °C under continuous stirring. Then, 4 M solution of NaOH was added slowly dropwise in required proportion. The black precipitate was formed and then was washed several times with distilled water, then heated at 100 ° C for 72 h for drying. The dried powders were heated at 800 ° C for 2 h and then were left to cooldown slowly to room temperature.
Characterization Techniques
The crystal structure was checked by means of X-ray diffraction (XRD) method using diffractometer equipped with CuKα radiation. The surface morphology was characterized by scanning electron microscopy (SEM) using JEOL JSM-T220A with an accelerating voltage of 20 kV. The particle size of powders was estimated by transmission electron microscope (TEM) operating at 75 kV. Samples were prepared by drop coating from alcohol dispersion on the copper grids using ultrasound. The elemental chemical composition was studied by energy dispersive spectroscopy (EDS) by means of REMMA-102-02 Scanning Electron Microscope-Analyzer (JCS SELMI, Ukraine). Fourier transmission infrared (FTIR) spectra were recorded in the wavenumber range 4000–350 cm−1 using Alpha-P FTIR spectrometer (Bruker) in ATR mode on diamond window with 256 scans at 6 cm−1 resolution. Each spectrum represents the average of six scans. UV–vis diffuse reflectance spectra were recorded using Shimadzu UV-3600 spectrophotometer equipped with an integrating sphere (diameter of 15 cm). BaSO4 was used as a reference. All samples were ground with BaSO4 (1:50) prior to measurements. Magnetic measurements (magnetization, remanence, coercivity) were performed using vibrating sample magnetometer (VSM) at room temperature under an applied field of ±10 kOe.
Results and Discussion
X-ray Diffraction Analysis
X-ray diffraction (XRD) was performed on the powders calcined at 800 °C, and the XRD patterns of all the samples were shown in Fig. 1. The obtained patterns confirm the formation of a homogeneous single phase having cubic spinel structure with the space group Fd3m. The patterns show diffraction peaks of Co1−xZnxFe2O4 (x = 0.0, 0.1, 0.2, 0.3, 0.4, 0.5), corresponding to (111), (220), (311), (222), (400), (422), (511), and (440) reflections. All XRD patterns are analyzed by using the Rietveld method and FullProf program. The results show that the lattice parameter a slightly increases with Zn2+-doping content as shown in Table 1. The increase of a with x can be explained on the basis of the difference in ionic radii of Zn2+ and Co2+. The smaller ionic radius of Co (0.58 Å) was replaced by the larger ionic radius of Zn (0.6 Å) so the lattice parameter increased due to the expansion of the unit cell.
Fig. 1.
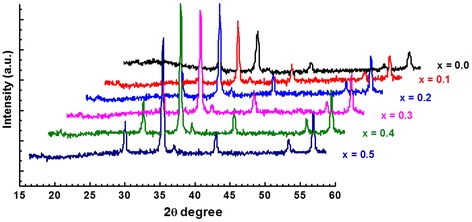
The X-ray diffraction patterns of the Co1−xZnxFe2O4 as function of Zn2+ content
Table 1.
Chemical composition, lattice parameter a exp, crystallite size D, and band gap of the Co1−xZnxFe2O4 spinels
| x (Zn2+) | Chemical composition | a exp (Å) | Crystallite size D (nm) | Band gap (eV) | |||
|---|---|---|---|---|---|---|---|
| Scherrer method | W-H method | SSP method | TEM | ||||
| 0.00 | CoFe2O4 | 8.3536 | 27 | 26 | 25 | 51 | 1.17 |
| 0.10 | Zn0.1Co0.9Fe2O4 | 8.3794 | 36 | 42 | 31 | 46 | 1.30 |
| 0.20 | Zn0.2Co0.8Fe2O4 | 8.3897 | 51 | 49 | 38 | 53 | 1.28 |
| 0.30 | Zn0.3Co0.7Fe2O4 | 8.3982 | 54 | 54 | 43 | 55 | 1.34 |
| 0.40 | Zn0.4Co0.6Fe2O4 | 8.4016 | 55 | 56 | 47 | 69 | 1.32 |
| 0.50 | Zn0.5Co0.5Fe2O4 | 8.4068 | 53 | 58 | 46 | 77 | 1.31 |
By dividing Kα-doublet of each observed peaks, it is found that the peaks would be implicitly described by the Cauchy function. Therefore, while determining the physical broadening, the hardware broadening is subtracted from the integral width of the experimental peaks. Considering that, the forms of mathematical functions that describe different types of physical broadening are unknown, thereby different methods were proposed to determine microstructural parameters (crystallite size and microstrain). The analysis of the crystallite size has been carried out using the broadening of XRD peaks. It is known that peak broadening results from both finite crystallite size and strain effect within the crystal lattice [29]. The crystallite size (D) has been calculated using the Scherrer method (SM) [30], Williamson-Hall method (WHM), and size-strain plot method (SSPM) [29–33].
The particle size in the Scherrer method was determined by the following equation:
| 1 |
where D is the crystallite size (nm), λ is the wavelength of X-ray radiation source (1.5406 Å for CuKα1), β is the integral width, and θ is the peak position. Plots were drawn with the (1/β) on the x-axis and cosθ on the y-axis (Fig. 2a), and crystallite size D was extracted from the slope of fit line. The estimated values of D are reported in Table 1. It can be seen that experimental data are not in good agreement with approximation line.
Fig. 2.
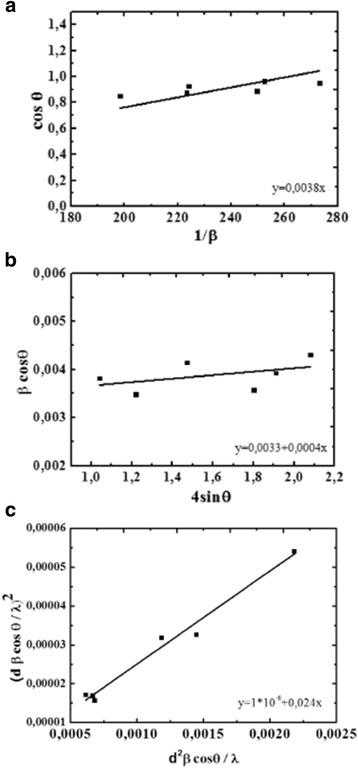
Scherrer plot (a), W-H analysis (b), and SSP plot (c) of Co1−xZnxFe2O4 ferrites
In the Williamson-Hall (W-H plot) method, the XRD peak broadening can be splitted into two parts according to the following expression; β = β size + β strain. Assuming that the particle size and strain contributions to line broadening are independent from each other and both have a Cauchy-like profile, the observed line width is simply the sum of the two contributions leading to the Williamson-Hall equation:
| 2 |
where ε is the strain associated with the nanoparticles. Equation (2) represents a straight line between 4sinθ (x-axis) and βcosθ (y-axis). The slope of the line gives the strain (ε) and the intercept (0.9λ/D) with y-axis gives the crystallite size (D) (Fig. 2b). As can be seen from Fig. 2b, the points are widely spread around the fitted line. This obviously indicates that either some other parameters of the studied powders were not taken into account in the used model or that other methods should be used.
There is another model that can be used also to determine the crystallite size (D)—the size-strain plot method, which has the advantage that less weight is given to data from reflections at height angles, where precision is usually lower. In this approximation, it is assumed that the “crystallite size” profile is described by the Lorentzian function and the “strain profile” by the Gaussian function:
| 3 |
where K is constant that depends on the shape of the particles (for spherical particles, K = 3/4). In Fig. 2c, where (d hkl β hklcosθ/λ)2 and (d hkl)2 β hklcosθ/λ were plotted on x- and y-axes, respectively. In this case, the particle size is calculated from the slope of the linearly fitted data and the root of the y-intercept gives the strain. As can be seen from Fig. 2c, all the experimental points are good enough approximation to a straight line.
The average crystallite sizes obtained from the above methods (Table 1) remain in the nanometer regime by means of Zn doping and are within a close range, even though the values obtained by SSPM are slightly lower than those calculated using SM and WHM methods.
Interestingly, the substitution of Co by Zn results in an increase of crystallite size, almost by half for 50% Zn content. This means that Zn favors grain growth during the preparation process of spinel phase. The as-obtained values are in good agreement with the particle sizes estimated from TEM images as can be seen in Table 1.
The ionic packing coefficients P a and P b at the tetrahedral and octahedral sites, respectively, can be estimated using the following equations [34]:
where r xt and r xo are the interstitial radii whereas R A and R B are the average values of the ionic radii at the tetrahedral and octahedral sites, respectively, u is the anion parameter, a is the lattice parameter, and R O is the oxygen radius (1.38 Å) [28]. It is claimed [34] that the small values of the ionic packing coefficient, P a and P b, (smaller than 1), testify to the smaller ion distances and larger overlapping of the cation and anion orbitals, suggesting the existence of cation or anion vacancies.
The degree of the ionic packing of the spinel structure can be determined using the fulfillment coefficient of the unit cell, α, which can be estimated using the following relation [35]:
The vacancy parameter, β, is defined as a normalized volume of the missing ions at the nodal points of the spinel structure [34]. It is a measure of the total vacancy concentration existing in the material and can be estimated using the following equation [34, 35]:
The calculated values α and β are shown in Table 2 as well as Figs. 3 and 4 as a function of Zn2+ concentration. The small values of the fulfillment coefficient α (α < 1) and the ionic packing coefficient (P a, P b < 1) indicate the presence of vacancies at both tetrahedral and octahedral sites. It can be seen that P b decreases while P a remains almost constant with increasing Zn content. Also, the fulfillment coefficient α decreases to a small extent, while the vacancy parameter β decreases more significantly than α. The increase in β values indicates the presence of cation or anion vacancies.
Table 2.
Ionic packing coefficient P a, P b, fulfillment coefficient α, and vacancy parameter β for the Co1−xZnxFe2O4 ferrites
| x (Zn2+) | r xt (Å) | r xo (Å) | P a | P b | α | β (%) |
|---|---|---|---|---|---|---|
| 0.00 | 0.4367 | 0.7037 | 0.387 | 0.338 | 0.649 | 2.119 |
| 0.10 | 0.4843 | 0.6859 | 0.368 | 0.332 | 0.642 | 1.329 |
| 0.20 | 0.4890 | 0.6870 | 0.368 | 0.332 | 0.640 | 1.090 |
| 0.30 | 0.4848 | 0.6927 | 0.371 | 0.334 | 0.639 | 0.913 |
| 0.40 | 0.4869 | 0.6928 | 0.371 | 0.334 | 0.638 | 0.916 |
| 0.50 | 0.4960 | 0.6894 | 0.367 | 0.333 | 0.637 | 0.853 |
Fig. 3.
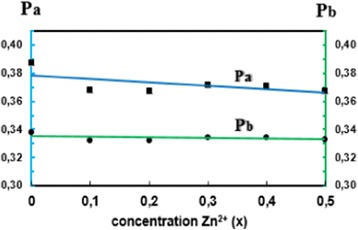
The ionic packing coefficient Pa and Pb versus Zn(x) for the Co1−xZnxFe2O4 ferrites
Fig. 4.
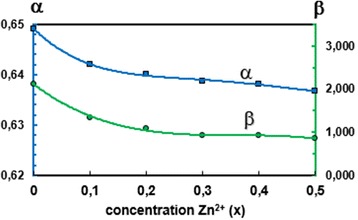
The fulfillment coefficient α and vacancy parameter β versus Zn(x) for the Co1−xZnxFe2O4 ferrites
Surface Morphology of Co-Zn Ferrites
The scanning electron micrographs (SEM) of Zn-doped CoFe2O4 samples (with x = 0.2 and x = 0.5) shown in Fig. 5 indicate the formation of agglomerates of very fine particles with almost spherical shape. It can be seen that the Fig. 5 shows heavily concentrated particles of nanoscale regime for Co0.8Zn0.2Fe2O4 and Co0.5Zn0.5Fe2O4 samples. This is due to its permanent magnetic moment; hence, each particle is permanently magnetized and tends to agglomerate with other particles. The Zn-substituted nanoparticles possess higher magnetic moment leading to more clustering. The average particle size and size distribution obtained from TEM analysis depend on the composition of the ferrite and have a tendency to increase (for example, for x = 0.2, average size is equivalent 53 nm while for x = 0.5 average size is equivalent 77 nm, Fig. 5). It is evident from TEM images that the powders show almost spherical shape. TEM images confirms that the average particle size is in the range of 46–77 nm (Table 1), which is very close to the values obtained by XRD analysis.
Fig. 5.

The SEM images and particle size distribution (obtained from TEM) for the samples with x = 0.2 and x = 0.5 for the Co1−xZnxFe2O4 spinels
The elemental composition of Co1−xZnxFe2O4 spinels (x = 0, 0.1, 0.2, 0.3, 0.4, 0.5) is obtained from energy dispersive X-ray (EDS) analysis (Table 3), only most representative ones are shown in Fig. 6. The peaks corresponding to Zn, Co, Fe, and O elements are observed in all Zn-doped CoFe2O4 samples. The sample compositions are taken to be equal to the nominal ones.
Table 3.
EDS data for the Co1−xZnxFe2O4 spinels
| Zn2+ content x | Elements (at. %) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Theoretical (expected) | Experimental (actual) | |||||||||
| Co | Zn | Fe | O | Total | Co | Zn | Fe | O | Total | |
| 0.00 | 14.29 | – | 28.57 | 57.14 | 100 | 14.15 | – | 28.68 | 57.17 | 100 |
| 0.10 | 12.86 | 1.43 | 28.57 | 57.14 | 100 | 12.71 | 2.01 | 28.22 | 57.06 | 100 |
| 0.20 | 11.43 | 2.86 | 28.57 | 57.14 | 100 | 11.54 | 3.13 | 28.26 | 57.07 | 100 |
| 0.30 | 10.00 | 4.29 | 28.57 | 57.14 | 100 | 10.90 | 4.48 | 27.70 | 56.92 | 100 |
| 0.40 | 8.57 | 5.71 | 28.57 | 57.14 | 100 | 8.80 | 5.79 | 28.33 | 57.08 | 100 |
| 0.50 | 7.14 | 7.14 | 28.57 | 57.14 | 100 | 6.92 | 7.87 | 28.17 | 57.04 | 100 |
Fig. 6.
EDS spectra of Zn0.1Co0.9Fe2O4
FTIR Spectroscopy and Elastic Properties
The FTIR spectra of Co-Zn ferrite samples are shown in Fig. 7. All spectra consist of two main peaks located at about 542–529 cm−1 and 360–365 cm−1, which confirm the formation of spinel ferrite structure [28]. The ~535 cm−1 peak is due to vibration mode of tetrahedral sublattice, while ~363 cm−1 peak is due to vibration mode of octahedral sublattice in the spinel structure.
Fig. 7.
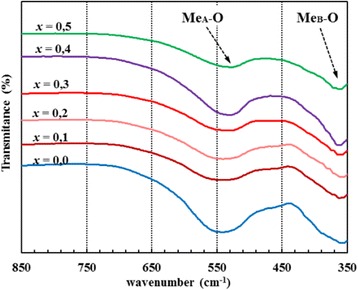
FTIR spectra of Co1−xZnxFe2O4 ferrites
Structural and FTIR data of spinel ferrite are used for the estimation of elastic moduli and the Debye temperature. The Debye temperature of all samples is calculated using the wavenumber of IR bands [36]:
where  , k is the Boltzmann constant, C is velocity of light (c = 3 × 108 cm/s), and υ
av is the average wavenumber of bands. The values of the Debye temperature for Co1-xZnxFe2O4 samples are shown in Table 4. It is observed that the Debye temperature decreases with increasing Zn2+ content and can be associated to the decrease in wavenumber of the peak usually attributed to Me-O bond vibration in the tetrahedral site.
, k is the Boltzmann constant, C is velocity of light (c = 3 × 108 cm/s), and υ
av is the average wavenumber of bands. The values of the Debye temperature for Co1-xZnxFe2O4 samples are shown in Table 4. It is observed that the Debye temperature decreases with increasing Zn2+ content and can be associated to the decrease in wavenumber of the peak usually attributed to Me-O bond vibration in the tetrahedral site.
Table 4.
FTIR parameters, the Debye temperature θ D, elastic moduli for the Co1−xZnxFe2O4 spinels
| Zn2+ content (x) | υ av (cm−1) | θ D (K) | E (GPa) | G (GPa) | B (GPa) | σ |
|---|---|---|---|---|---|---|
| 0.00 | 154.59 | 649.2 | 149.11 | 58.85 | 106.59 | 0.27 |
| 0.10 | 155.10 | 650.3 | 149.79 | 59.21 | 106.15 | 0.26 |
| 0.20 | 153.35 | 646.1 | 153.78 | 61.77 | 100.43 | 0.24 |
| 0.30 | 153.32 | 646.1 | 149.71 | 59.49 | 103.25 | 0.26 |
| 0.40 | 153.43 | 646.1 | 160.37 | 65.82 | 94.86 | 0.22 |
| 0.50 | 152.58 | 644.0 | 165.47 | 69.57 | 88.73 | 0.19 |
The different elastic moduli for cubic structure are calculated using the standard relations discussed elsewhere [36–38]: Young’s modulus (E), rigidity modulus (G), bulk modulus (B), and Poisson’s ratio (σ). The values of these moduli are shown in Fig. 8 and Table 4. Figure 8 shows that with increasing Zn content, all elastic moduli increase except B. This behavior of elastic moduli is attributed to the interatomic bonding between various cation within spinel ferrites. The values of Poisson’s ratio for all samples remain almost constant, i.e., in the range 0.19–0.27. It has been reported that a value that lies within the range between −1 and 00.5 implies a good elastic behavior and is in accordance with the theory of isotropic elasticity [36, 38]. This value of the Poisson ratio is in good agreement with Al-substituted Mn0.5Zn0.5Fe2O4 ferrite [38].
Fig. 8.
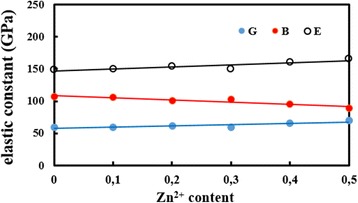
Variation of Young’s modulus (E), rigidity modulus (G), and bulk modulus (B) with Zn content (x) in the Co1−xZnxFe2O4 system
Optical Properties (Diffuse Reflectance Spectroscopy)
Diffuse reflectance spectra have been recorded and transformed to the Kubelka-Munk function (Fig. 9). The Kubelka-Munk function is the conversion of the sample reflectance, defined as:
Fig. 9.
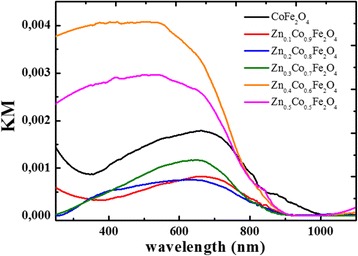
UV–vis spectra presented as the Kubelka-Munk function for ZnxCo1−xFe2O4 system as a function of Zn content
where R is absolute reflectance. In order to determine the band gap energy of the material, it is necessary to use the Tauc plot. The band gap can be estimated using the following equation:
where α, h, v, E BG, and n are absorption coefficient, the Planck constant, oscillation frequency, band gap energy, and constant relating to a mode of transition, respectively. The constant n is for allowed direct transition and 2 for indirect transition.
All materials absorb up to ca. 900 nm, except CoFe2O4 which absorbs up to 1000 nm. For higher Zn content (x = 0.4–0.5), a stronger absorbance within the UV–vis range is observed. The Tauc transformation of spectra enables the determination of optical band gap energies (E bg). An example of the Tauc plot, derived from UV–vis DRS spectrum, is presented in Fig. 10 for CoFe2O4 (indirect semiconductor). By checking the linearity of the plot of vs. hv, it is possible to determine the band gap energy as the x-intercept of the extrapolated linear fits. This procedure is commonly used for semiconductors characterization and is well described in literature [39–42]. It can be noticed that the substitution of Co by Zn results in an increase of E bg from 1.17 eV for CoFe2O4 to 1.30 ± 0.03 eV for Zn0.1Co0.9Fe2O4, then it becomes almost constant (Fig. 11).
Fig. 10.
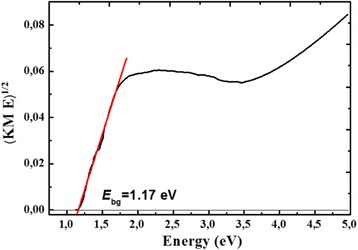
Tauc plot for indirect band gap CoFe2O4
Fig. 11.

Band gap energy of ZnxCo1−xFe2O4 system as a function of Zn content (error ±0.03 eV)
VSM Measurements
CoFe2O4 shows a ferromagnetic behavior with a large hysteresis loop (Fig. 12). Doping with Zn ions reveals also a ferromagnetic behavior while induces important modifications in the magnetic properties; the hysteresis loop decreases drastically with Zn content (Table 5). Ms was found to increase with Zn to reach an optimum value of 114 emu/g for 20% Zn content and then decrease to 82 emu/g for 50% Zn content (Fig. 13). At lower concentrations, Zn ions occupy preferentially tetrahedral A sites of CoFe2O4 whereas for higher concentration, Zn ions have the tendency to move to octahedral B sites (Fe). It is surprisingly interesting that the substitution of magnetic Co (μ B = 3) with a nonmagnetic Zn (μ B = 0) results in a 25% increase in Ms value.
Fig. 12.
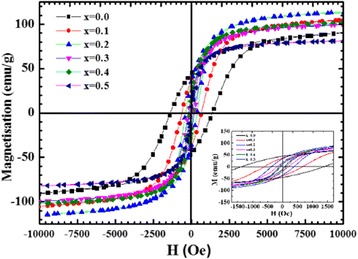
Saturation magnetization (Ms) versus applied magnetic field (H) of the Co1−xZnxFe2O4 samples at room temperature (Inset low field region of the loops)
Table 5.
Magnetic parameters (saturation magnetization Ms, remanent magnetization Mr, coercitivity HC) at room temperature of Co1−xZnxFe2O4 system as function of Zn content (x)
| x (Zn2+) | D (nm) | Ms (emu/g) | Mr (emu/g) | HC (Oe) |
|---|---|---|---|---|
| 0 | 51 | 91 | 44 | 1382 |
| 0.1 | 46 | 105 | 38 | 628 |
| 0.2 | 53 | 114 | 36 | 377 |
| 0.3 | 55 | 100 | 19 | 188 |
| 0.4 | 69 | 102 | 18 | 119 |
| 0.5 | 77 | 82 | 10 | 75 |
Fig. 13.
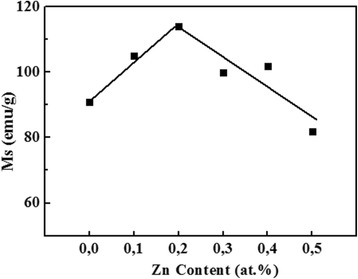
Variation of the saturation magnetization (Ms) of the Co1−xZnxFe2O4 (x = 0.0, 0.1, 0.2, 0.3, 0.4, and 0.5) system versus Zn content
HC is found to decrease with increasing the concentration of Zn. HC decreases drastically by more than 50% with only 10% of Zn, while Mr decreases linearly and gradually with Zn content reaching a reduction of 77% for 50% Zn substitution in comparison with pure CoFe2O4 (Fig. 14).
Fig. 14.
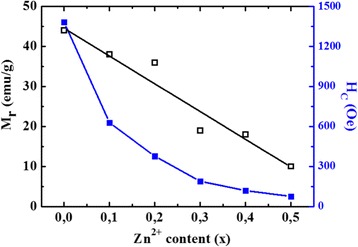
Variation of the remanent magnetization (Mr) and coercivity (HC) of the Co1−xZnxFe2O4 (x = 0.0, 0.1, 0.2, 0.3, 0.4, and 0.5) system versus Zn content
It is reported that at particular range of grain size, HC and Mr become highly sensitive to the change in grain size [43], which is consistent with the presented results. At smaller ranges of crystallite size (D), HC and Mr showed a rapid decrease as D increases, while a gradual decrease is noticed as D gets larger. This relation is significant for the Zn-doped CoFe2O4 (Fig. 15).
Fig. 15.
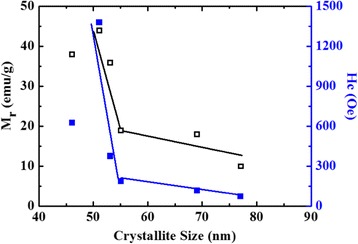
Variation of the remanent magnetization (Mr) and coercivity (HC) of the Co1−xZnxFe2O4 (x = 0.0, 0.1, 0.2, 0.3, 0.4, and 0.5) system versus crystallite size (from TEM)
Conclusions
Zn-doped CoFe2O4 NPs have been successfully synthesized via chemical co-precipitation route. XRD and FTIR confirmed the formation of single cubic spinel phase. Doping with Zn showed a considerable effect on structural, spectral, and magnetic properties. The crystallite size and lattice parameter increase gradually while increasing Zn content. This can be associated with ionic radii (Zn is larger than Co) and that Zn favors grain growth. The line broadening was analyzed by the Scherrer formula, W-H analysis, and the SSP method. The TEM results were in good agreement with the results of the SSP method. SEM analysis showed spherical-shaped particles forming agglomerates. The energy gap (Eg) is found to increase for 10% Zn and then remains constant for higher doping level. Magnetic measurements reveal a ferromagnetic behavior while the hysteresis loop tends to decrease with Zn concentration. Ms is found to be sensitive to Zn concentration, while Ms and HC decrease dramatically with increasing the amount of Zn.
Acknowledgements
None.
Funding
None.
Authors’ Contributions
TT and NP have synthesized the ferrite samples and has participated in the SEM and EDS studies of the characteristic of the ferrite surface. NP has participated in the VSM studies. OS carried out the FTIR analysis of the ferrite samples. IY analyzed the XRD data. WM and MP carried out the DRS analysis of the ferrite samples. BAN and MB analyzed the VSM data. MB and WM assisted in the preparation and proof reading of the manuscript. TT initiated the research, designed the experimental strategy, drafted the manuscript, and supervised all the work. All the authors have read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Ethics Approval and Consent to Participate
This study has nothing to do with human participants or health-related outcomes.
Abbreviations
- DRS
Diffuse reflectance spectroscopy
- EDS
Energy dispersive spectroscopy
- FTIR
Fourier transform infrared spectroscopy
- HC
Coercivity
- Mr
Remanent magnetization
- Ms
Saturation magnetization
- NPs
Nanoparticles
- SEM
Scanning electron microscopy
- SF
Spinel ferrite
- SM
Scherrer method
- SSPM
Size-strain plot method
- TEM
Transmission electron microscopy
- VSM
Vibrating sample magnetometer
- WHM
Williamson-Hall method
- XRD
X-ray diffraction
Contributor Information
Tetiana Tatarchuk, Email: tatarchuk.tetyana@gmail.com.
Mohamed Bououdina, Email: mboudina@gmail.com.
Wojciech Macyk, Email: macyk@chemia.uj.edu.pl.
Olexander Shyichuk, Email: szyjczuk@utp.edu.pl.
Natalia Paliychuk, Email: nsbu@ukr.net.
Ivan Yaremiy, Email: yaremiyip@gmail.com.
Basma Al-Najar, Email: balnajar85@gmail.com.
Michał Pacia, Email: pacia@chemia.uj.edu.pl.
References
- 1.Kaviyarasu K, Manikandan E, Paulraj P, et al. One dimensional well-aligned CdO nanocrystal by solvothermal method. J Alloys Comp. 2014;593:67–70. doi: 10.1016/j.jallcom.2014.01.071. [DOI] [Google Scholar]
- 2.Kaviyarasu K, Manikandan E, Kennedy J, Jayachandran M. Quantum confinement and photoluminescence of well-aligned CdO nanofibers by a solvothermal route. Mater Lett. 2014;120:243–245. doi: 10.1016/j.matlet.2014.01.048. [DOI] [Google Scholar]
- 3.Kennedy J, Leveneur J, Williams GVM, et al. Fabrication of surface magnetic nanoclusters using low energy ion implantation and electron beam annealing. Nanotechnology. 2011;22:115602. doi: 10.1088/0957-4484/22/11/115602. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy J, Williams GVM, Murmu PP, Ruck BJ. Intrinsic magnetic order and inhomogeneous transport in Gd-implanted zinc oxide. Phys Rev B. 2013;88:214423. doi: 10.1103/PhysRevB.88.214423. [DOI] [Google Scholar]
- 5.Kaviyarasu K, Manikandan E, Kennedy J, Maaza M. A comparative study on the morphological features of highly ordered MgO:AgO nanocube arrays prepared via a hydrothermal method. RSC Adv. 2015;5:82421–82428. doi: 10.1039/C5RA15132E. [DOI] [Google Scholar]
- 6.Kasinathan K, Kennedy J, Elayaperumal M, Henini M, Malik M. Photodegradation of organic pollutants RhB dye using UV simulated sunlight on ceria based TiO2 nanomaterials for antibacterial applications. Sci Rep. 2016;6:38064–38076. doi: 10.1038/srep38064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joshi S, Kumar M, Chhoker S, et al. Effect of Gd3+ substitution on structural, magnetic, dielectric and optical properties of nanocrystalline CoFe2O4. J Magn Mag Mater. 2017;426:252–263. doi: 10.1016/j.jmmm.2016.11.090. [DOI] [Google Scholar]
- 8.Vadivela M, Babua RR, Ramamurthib K, Arivanandhanc M. CTAB cationic surfactant assisted synthesis of CoFe2O4 magnetic nanoparticles. Ceram Inter. 2016;42:19320–19328. doi: 10.1016/j.ceramint.2016.09.101. [DOI] [Google Scholar]
- 9.Franco A, Jr, Pessoni HVS, Neto FO. Enhanced high temperature magnetic properties of ZnO-CoFe2O4 ceramic composite. J Alloys Compd. 2016;680:198–205. doi: 10.1016/j.jallcom.2016.04.110. [DOI] [Google Scholar]
- 10.Huang S, Xu Y, Xie M, et al. Synthesis of magnetic CoFe2O4/g-C3N4 composite and its enhancement of photocatalytic ability under visible-light. Colloids Surf A. 2015;478:71–80. doi: 10.1016/j.colsurfa.2015.03.035. [DOI] [Google Scholar]
- 11.Kaviyarasu K, Geetha N, Kanimozhi K, et al. In vitro cytotoxicity effect and antibacterial performance of human lung epithelial cells A549 activity of zinc oxide doped TiO2 nanocrystals: investigation of bio-medical application by chemical method. Mat Sci Eng: C. doi:10.1016/j.msec.2016.12.024. [DOI] [PubMed]
- 12.Jesudoss SK, Vijaya JJ, Kennedy LJ, et al. Studies on the efficient dual performance of Mn1–xNixFe2O4 spinel nanoparticles in photodegradation and antibacterial activity. J Photochem Photobiol B. 2016;165:121–132. doi: 10.1016/j.jphotobiol.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Kaviyarasu K, Ayeshamariam A, Manikandan E, et al. Solution processing of CuSe quantum dots: photocatalytic activity under RhB for UV and visible-light solar irradiation. Mat Sci Eng: B. 2016;210:1–9. doi: 10.1016/j.mseb.2016.05.002. [DOI] [Google Scholar]
- 14.Dantas J, Leal E, Mapossa AB, et al. Magnetic nanocatalysts of Ni0.5Zn0.5Fe2O4 doped with Cu and performance evaluation in transesterification reaction for biodiesel production. Fuel. 2017;191:463–471. doi: 10.1016/j.fuel.2016.11.107. [DOI] [Google Scholar]
- 15.Wang M, Huang Y, Chen X, et al. Synthesis of nitrogen and sulfur co-doped graphene supported hollow ZnFe2O4 nanosphere composites for application in lithium-ion batteries. J Alloys Compd. 2017;691:407–415. doi: 10.1016/j.jallcom.2016.08.285. [DOI] [Google Scholar]
- 16.Kaviyarasu K, Manikandan E, Kennedy J, et al. Synthesis and characterization studies of NiO nanorods for enhancing solar cell efficiency using photon upconversion materials. Ceram Int. 2016;42:8385–8394. doi: 10.1016/j.ceramint.2016.02.054. [DOI] [Google Scholar]
- 17.Agrawal S, Parveen A, Azam A. Structural, electrical, and optomagnetic tweaking of Zn doped CoFe2-xZnxO4-δ nanoparticles. J Magn Mag Mater. 2016;414:144–152. doi: 10.1016/j.jmmm.2016.04.059. [DOI] [Google Scholar]
- 18.Manikandana A, Vijaya JJ, Kennedy LJ, Bououdina M. Microwave combustion synthesis, structural, optical and magnetic properties of Zn1−xSrxFe2O4 nanoparticles. Ceram Inter. 2013;39:5909–5917. doi: 10.1016/j.ceramint.2013.01.012. [DOI] [Google Scholar]
- 19.Rathi R, Neogi R. Structural, Electric and magnetic properties of Titanium doped Ni-Cu-Zn Ferrite. Mater Today. 2016;3:2437–2442. doi: 10.1016/j.matpr.2016.04.159. [DOI] [Google Scholar]
- 20.Wu X, Yun H, Dongn H. Enhanced infrared radiation properties of CoFe2O4 by doping with Y3+ via sol–gel auto-combustion. Ceram Inter. 2014;40:12883–12889. doi: 10.1016/j.ceramint.2014.04.147. [DOI] [Google Scholar]
- 21.Wu X, Yun H, Dongn H, Geng L. Enhanced infrared radiation properties of CoFe2O4 by single Ce3+-doping with energy-efficient preparation. Ceram Inter. 2014;40:5905–5911. doi: 10.1016/j.ceramint.2013.11.035. [DOI] [Google Scholar]
- 22.Choodamani C, Nagabhushana GP, Ashoka S, et al. Structural and magnetic studies of Mg(1−x)ZnxFe2O4 nanoparticles prepared by a solution combustion method. J Alloys Compd. 2013;578:103–109. doi: 10.1016/j.jallcom.2013.04.152. [DOI] [Google Scholar]
- 23.Gabal MA, Al-Juaid AA, Al-Rashed SM, et al. Synthesis, characterization and electromagnetic properties of Zn-substituted CoFe2O4 via sucrose assisted combustion route. J Magn Mag Mater. 2016; doi.org/10.1016/j.jmmm.2016.10.147.
- 24.Murmu PP, Kennedy J, Ruck BJ, Williams GVM, Markwitz A, Rubanov S, et al. Effect of annealing on the structural, electrical and magnetic properties of Gd-implanted ZnO thin films. J Mater Sci. 2012;47(3):1119–1126. doi: 10.1007/s10853-011-5883-z. [DOI] [Google Scholar]
- 25.Leveneur J, Waterhouse GIN, Kennedy J, et al. Nucleation and growth of Fe nanoparticles in SiO2: a TEM, XPS, and Fe L-edge XANES investigation. J Phys Chem C. 2011;115:20978–20985. doi: 10.1021/jp206357c. [DOI] [Google Scholar]
- 26.Leveneur J, Kennedy J, Williams GVM, et al. Large room temperature magnetoresistance in ion beam synthesized surface Fe nanoclusters on SiO2. Appl Phys Lett. 2011;98:5. doi: 10.1063/1.3553274. [DOI] [Google Scholar]
- 27.Kuruva P, Matli PR, Bououdina M, et al. Effect of Ni–Zr codoping on dielectric and magnetic properties of SrFe12O19 via sol–gel route. J Magn Mag Mater. 2015;382:172–178. doi: 10.1016/j.jmmm.2015.01.050. [DOI] [Google Scholar]
- 28.Tatarchuk TR, Bououdina M, Paliychuk ND, et al. Structural characterization and antistructure modeling of cobalt-substituted zinc ferrites. J Alloys Compd. 2017;694:777–791. doi: 10.1016/j.jallcom.2016.10.067. [DOI] [Google Scholar]
- 29.Kumar L, Kumar P, Narayan A, Kar M. Rietveld analysis of XRD patterns of different sizes of nanocrystalline cobalt ferrite. Int Nano Lett. 2013;3:8. doi: 10.1186/2228-5326-3-8. [DOI] [Google Scholar]
- 30.Zak AK, Majid AWH, Abrishami ME, Yousefi R. X-ray analysis of ZnO nanoparticles by Williamson–Hall and size–strain plot methods. Solid State Sci. 2011;13:251–256. doi: 10.1016/j.solidstatesciences.2010.11.024. [DOI] [Google Scholar]
- 31.Prabhu YT, Rao KV, Kumar VSS, Kumari BS. X-Ray analysis by Williamson-Hall and size-strain plot methods of ZnO nanoparticles with fuel variation. World J Nano Sci Eng. 2014;4:21–28. doi: 10.4236/wjnse.2014.41004. [DOI] [Google Scholar]
- 32.Bushkova VS, Mudry SI, Yaremiy IP, Kravets VI. X-ray analysis of nickel-cobalt ferrite nanoparticles by using Debye-Scherrer, Williamson-Hall and Ssp methods. J Phys Stud. 2016;20:1702–1708. [Google Scholar]
- 33.Esparza I, Paredes M, Martinez R, et al. Solid state reactions in Cr2O3-ZnO nanoparticles synthesized by triethanolamine chemical precipitation. Mater Sci Appl. 2011;2:1584–1592. [Google Scholar]
- 34.Mohammed KA, Al-Rawas AD, Gismelseed AM, et al. Infrared and structural studies of Mg1–xZnxFe2O4 ferrites. Physica B: Condens Matter. 2012;407:795–804. doi: 10.1016/j.physb.2011.12.097. [DOI] [Google Scholar]
- 35.Khalaf KAM, Al-Rawas AD, Widatallah HM, et al. Influence of Zn2+ ions on the structural and electrical properties of Mg1−xZnxFeCrO4 spinels. J Alloys Compd. 2016;657:733–747. doi: 10.1016/j.jallcom.2015.10.157. [DOI] [Google Scholar]
- 36.Patange SM, Shirsath SE, Jadhav SP, et al. Elastic properties of nanocrystalline aluminum substituted nickel ferrites prepared by co-precipitation method. J Mol Struct. 2013;1038:40–44. doi: 10.1016/j.molstruc.2012.12.053. [DOI] [Google Scholar]
- 37.Modi KB, Raval PY, Shah SJ, et al. Raman and Mossbauer spectroscopy and X-ray diffractometry studies on quenched Copper–Ferri–Aluminates. Inorg Chem. 2015;54(4):1543–1555. doi: 10.1021/ic502497a. [DOI] [PubMed] [Google Scholar]
- 38.Mohamed MB, Wahba AM. Structural, magnetic, and elastic properties of nanocrystalline Al-substituted Mn0.5Zn0.5Fe2O4 ferrite. Ceram Inter. 2014;40:11773–11780. doi: 10.1016/j.ceramint.2014.04.006. [DOI] [Google Scholar]
- 39.Ohtani B. Photocatalysis A, to Z—what we know and what we do not know in a scientific sense. J Photochem Photobiol C: Photochemistry Reviews. 2010;11(4):157–178. doi: 10.1016/j.jphotochemrev.2011.02.001. [DOI] [Google Scholar]
- 40.Kisch H (ed) (2014) Semiconductor photocatalysis, in Semiconductor Photocatalysis: Principles and Applications. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. doi:10.1002/9783527673315.ch5
- 41.Güner S, Baykal A, Amir M, Güngüneş H, et al. Synthesis and characterization of oleylamine capped MnxFe1−xFe2O4 nanocomposite: magneto-optical properties, cation distribution and hyperfine interactions. J Alloys Comp. 2016;688(Part A):675–686. doi: 10.1016/j.jallcom.2016.07.033. [DOI] [Google Scholar]
- 42.Carmona-Carmona AJ, Palomino-Ovando MA, Hernández-Cristobal O, Sánchez-Mora E, Toledo-Solano M. Synthesis and characterization of magnetic opal/Fe3O4 colloidal crystal. J Cryst Growth. 2016. doi:10.1016/j.jcrysgro.2016.12.105.
- 43.Vollath D. Nanomaterials: an introduction to synthesis, properties and applications. Germany: WILEY-VCH Verlag GmbH & Co.KGaA; 2008. [Google Scholar]


