Abstract
Acinetobacter baumannii has emerged as an important opportunistic pathogen due to its ability to acquire resistance to most currently available antibiotics. Colistin is often considered as the last line of therapy for infections caused by multidrug-resistant A. baumannii (MDRAB). However, colistin-resistant A. baumannii strain has recently been reported. To explore how multiple drug-resistant A. baumannii responded to colistin resistance, we compared the genomic, transcriptional and proteomic profile of A. baumannii MDR-ZJ06 to the induced colistin-resistant strain ZJ06-200P5-1. Genomic analysis showed that lpxC was inactivated by ISAba1 insertion, leading to LPS loss. Transcriptional analysis demonstrated that the colistin-resistant strain regulated its metabolism. Proteomic analysis suggested increased expression of the RND efflux pump system and down-regulation of FabZ and β-lactamase. These alterations were believed to be response to LPS loss. In summary, the lpxC mutation not only established colistin resistance but also altered global gene expression.
Keywords: Acinetobacter baumannii, colistin, whole-genome sequencing, transcriptome, proteome
Introduction
Acinetobacter baumannii has emerged as an important opportunistic pathogen due to its ability to acquire resistance to most currently available antibiotics (Peleg et al., 2008; Howard et al., 2012; Antunes et al., 2014). Since current treatment options for multi-drug resistant (MDR) A. baumannii are extremely limited, colistin is often considered as the last line of the therapy for infections caused by MDR A. baumannii (Bae et al., 2016; Cheah et al., 2016b). However, colistin-resistant A. baumannii strain has recently been reported (Cai et al., 2012).
Colistin is a polycationic antimicrobial peptide that targets the polyanionic bacterial lipopolysaccharide (LPS) of Gram-negative bacteria. Two different colistin resistance mechanisms have previously been reported (Beceiro et al., 2014). The first mechanism inactivates the lipid A biosynthesis pathway, leading to the complete loss of surface LPS. Mutations in lpxC, lpxA, or lpxD are involved in the first mechanism. The pmrAB two-component system mediates the second resistance mechanism. Mutations in pmrA and pmrB induce the activity of pmrC, which adds phosphoethanolamine (PEtn) to the hepta-acylated form of lipid A (Beceiro et al., 2011). Further mutations in vacJ, pldA, ttg2C, pheS and a conserved hypothetical protein were reported to involve in reduced colistin susceptibility through novel resistance mechanisms (Thi Khanh Nhu et al., 2016). Four putative colistin resistant genes: A1S_1983, hepA, A1S_3026, and rsfS were also identified in our previous study (Mu et al., 2016).
The response to LPS alteration has been investigated via transcriptional analysis. In response to LPS alteration, A. baumannii alters the expression of critical transport and biosynthesis systems associated with modulating the composition and structure of the bacterial surface (lpxA; Henry et al., 2012) or alters the expression of genes associated with outer membrane structure and biogenesis (pmrB; Cheah et al., 2016a). Moreover, the response to colistin is highly similar to the transcriptional alteration observed in an LPS-deficient strain (Henry et al., 2015). Colistin resistance was also explored using proteomic methods. There were 35 differentially expressed proteins. Most differentially expressed proteins were down-regulated in the colistin resistant strain, including outer membrane proteins, chaperones, protein biosynthesis factors, and metabolic enzymes (Fernandez-Reyes et al., 2009). However, the combination of genomic, transcriptomic, and proteomic methods to examine the colistin resistance mechanism in A. baumannii has rarely been reported. Furthermore, the strain used in this study was an MDR strain, but not laboratory strains (ATCC 19606, ATCC 17978) that do not represent clonal lineages in a clinical environment. Here, we used genome, transcriptome, and proteome to elucidate the colistin resistance mechanism in MDR A. baumannii. There was an ISAba1 insertion in lpxC (ABZJ_03720) in ZJ06-200P5-1 compared with the genome sequence of MDR-ZJ06, where lpxC encoded an UDP-3-O-acyl-N-acetylglucosamine deacetylase.
Materials and methods
Bacterial strains, media, and antibiotics
Restriction enzymes, T4 ligase, and Taq DNA polymerase were purchased from TaKaRa (Otsu, Shiga, Japan). The A. baumannii strain MDR-ZJ06 was isolated from the bloodstream of a patient in Hangzhou, China, in 2006. All A. baumannii cultures were grown at 37 °C in Mueller-Hinton (MH) agar and cation-adjusted MH broth (CAMHB) (Oxoid, Basingstoke, UK). Colistin was purchased from Sigma (Shanghai, China).
Generation of colistin-resistant mutant
A colistin-resistant mutant was generated in A. baumannii MDR-ZJ06 by a previously described method (Li et al., 2006). Briefly, first, MDR-ZJ06 was cultured in CAMHB containing colistin at 8 × minimum inhibitory concentration (MIC). After overnight incubation, the culture was diluted 1:1000 with CAMHB containing colistin at 64 × MIC and then incubated at 37 °C overnight. Finally, the culture was diluted 1:100 with CAMHB containing colistin at 200 × MIC. After overnight incubation, the culture was plated on plates containing 10 μg of colistin at an appropriate dilution, and then one of colistin resistant colonies was collected for further experiments and designated as ZJ06-200P5-1. MICs for colistin and tigecycline were determined by E-test (bioMérieux, France) on MH agar, and the antimicrobial activities of the other antimicrobial agents were detected by disk diffusion. The results were interpreted according to CLSI or EUCAST breakpoints.
Whole genome DNA sequencing and analysis
ZJ06-200P5-1 cells were cultured from a single colony overnight at 37 °C in MH broth. The genomic DNA was extracted via a QIAamp DNA minikit (Qiagen, Valencia, CA) following the manufacturer's protocol. Agarose gel and a NanoDrop spectrophotometer were used to determine the quality and quantity of extracted genomic DNA. The 300 bp library for Illumina paired-end sequencing was constructed from 5 μg of genome DNA of ZJ06-200P5-1 by staff at Zhejiang Tianke (Hangzhou, China). Mapping and SNP detection were performed via Breseq (Deatherage and Barrick, 2014). The regions containing the detected SNPs were amplified by PCR. The PCR products were sent to Biosune (Biosune, Hangzhou, China) for Sanger sequencing.
Transcriptome analysis and real-time quantitative PCR verification
A. baumannii MDR-ZJ06 and ZJ06-200P5-1 were grown overnight at 37 °C in LB broth. Strains were subcultured 1/100 into fresh LB broth and grown at 37 °C for 2 h (OD600: 0.29 ± 0.02 for MDR-ZJ06, 0.26 ± 0.02 for ZJ06-200P5-1). The cells were collected at 4 °C, and the RNA was extracted using TRIZOL Reagent (Invitrogen, Carlsbad, CA, USA) after liquid nitrogen grinding. For RNA sequencing, wild type and mutants were sampled in triplicate. The subsequent RNA extraction, bacteria mRNA sequence library construction, transcriptome analysis and real-time quantitative PCR verification were performed by staff at Zhejiang Tianke (Hangzhou, China) as described previously in reference (Hua et al., 2014). Sequenced reads were mapped to the MDR-ZJ06 genome (CP001937-8) using Rockhopper (McClure et al., 2013). The output data was analyzed by edgeR (McCarthy et al., 2012). Data generated by RNA sequencing were deposited to the NCBI Sequence Read Archive with accession number SRR5234544 (the wild type) and SRR5234545 (the colistin resistant strain).
Proteomic analysis
A. baumannii MDR-ZJ06 and ZJ06-200P5-1 were grown overnight at 37 °C in LB broth. Strains were subcultured 1/100 into fresh LB broth and grown at 37 °C for 2 h (OD600: 0.29 ± 0.02 for MDR-ZJ06, 0.26 ± 0.02 for ZJ06-200P5-1). The cells were collected at 4 °C and sent to Shanghai Applied Protein Technology Co. Ltd. The cell pellets were washed twice with PBS, and 500 μl SDT lysis buffer (4% SDS, 100 mM Tris-HCl, 1 mM DTT, pH 7.6) was added. After being sonicated for 2 mins on ice, the cells were centrifuged at 14,000 × g for 30 min at 4 °C. The protein concentration in the supernatant was determined by the BCA method.
In brief, 300 μg protein was added to 200 μl UA buffer (8 M urea, 150 mM Tris-HCl pH 8.0) and ultrafiltered (Sartorius, 10 kD) with UA buffer. To block reduced cysteine residues, 100 μl iodoacetamide (IAA) buffer (50 mM IAA in UA buffer) was added, centrifuged at 600 rpm for 1 min, and incubated for 30 min in the dark. The filter was washed twice with 100 μl UA buffer and twice with 100 μl Dissolution buffer (50 mM triethylammonium bicarbonate, pH 8.5). Finally, the proteins were digested with 2 μg trypsin (Promega) in 40 μl Dissolution buffer at 37 °C for 16–18 h. The peptides were collected as a filtrate, and its content was estimated at OD280.
For iTRAQ labeling, the peptides were labeled with the 4-plex iTRAQ reagent following the manufacturer's instructions (AB SCIEX). The peptides from MDR-ZJ06 were labeled with 114 and 116 isobaric reagents, and the peptides from ZJ06-200P5-1 were labeled with 115 and 117 isobaric reagents.
RP-HPCL online-coupled to MS/MS (LC-MS/MS) analysis of the iTRAQ-labeled peptides was performed on an EASY-nLC nanoflow LC system (Thermo Fisher Scientific) connected to an Orbitrap Elite hybrid mass spectrometer (Thermo Fisher Scientific). After the samples were reconstituted and acidified with buffer A (0.1% (v/v) formic acid in water), a set-up involving a pre-column and analytical column was used. The pre-column was a 2 cm EASY-column (100, 5 μm C18; Thermo Fisher Scientific), while the analytical column was a 10 cm EASY-column (75, 3 μm, C18; Thermo Fisher Scientific). The 120 min linear gradient from 0 to 100% buffer B (0.1% (v/v) formic acid and 80% acetonitrile) at a constant flow rate of 250 nl/min was as follows: 0–100 min, 0–35% buffer B; 100–108 min, 35–100% buffer B; 108–120 min, 100% buffer B. MS data were acquired using a data-dependent top 10 method, dynamically choosing the most abundant precursor ions from the survey scan (300–180 m/z) for HCD fragmentation. The Dynamic exclusion was set to a repeat count of 1 with a 30 s duration. Survey scans were acquired at a resolution of 30,000 at m/z 200, and the resolution for HCD spectra was set to 15,000 at m/z 200. The normalized collision energy was 35 eV, and the underfill ratio was defined as 0.1%.
The MS/MS spectra were searched using the MASCOT engine (Matrix Science, London, UK; version 2.2) against the A. baumannii MDR-ZJ06 FASTA database. False discovery rates (FDR) were calculated via running all spectra against the FASTA database using the MASCOT software. The following options were used to identify proteins: peptide mass tolerance = 20 ppm, fragment mass tolerance = 0.1 Da, Enzyme = Trypsin, Max missed cleavages = 2, Fixed modification: Carbamidomethyl (C), iTRAQ 4plex (N-term), iTRAQ 4plex (K), Variable modification: Oxidation (M). Quantification was performed based on the peak intensities of the reporter ions in the MS/MS spectra. The proteins were considered overexpressed when the iTRAQ ratio was above 1.5 and underexpressed when the iTRAQ ratio was lower than 0.67 (Wang et al., 2016). Functional classification of differentially expression genes were annotated using the KEGG databases. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE (Vizcaino et al., 2016) partner repository with the dataset identifier PXD005265 and 10.6019/PXD005265. Reviewer account details: Username: reviewer54242@ebi.ac.uk; Password: zR8mE9wu.
Growth rate determination
Four independent cultures per strain were grown overnight, diluted to 1:1000 in MH and aliquots placed into a flat-bottom 100-well plate in four replicates. The plate was incubated at 37 °C with agitation. The OD600 of each culture was determined every 5 min for 16 h using a Bioscreen C MBR machine (Oy Growth Curves Ab Ltd., Finland). The growth rate was estimated based on OD600 curves using an R script (Fang et al., 2016).
Results
Whole genome sequencing, minimum inhibitory concentration and growth rate
The colistin-resistant mutant ZJ06-200P5-1 generated from the culture in CAMHB containing colistin was sent for whole genome sequencing. There was an ISAba1 insertion in lpxC in ZJ06-200P5-1 compared with the genome sequence of MDR-ZJ06 (Figure 1). The MIC of MDR-ZJ06 and ZJ06-200P5-1 were detected and listed in Table 1. The MIC for colistin increased from 0.38 mg/L (MDR-ZJ06) to >256 mg/L (ZJ06-200P5-1). However, ZJ06-200P5-1 showed higher sensitivity to multiple antibiotics: β-lactams, carbapenem, tetracycline, and ciprofloxacin, but not aminoglycosides. Furthermore, ZJ06-200P5-1 showed a lower growth rate (0.81 ± 0.05) than wild type.
Figure 1.
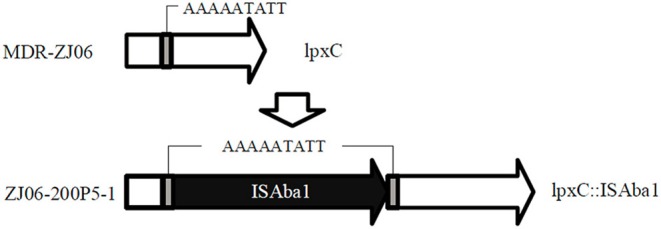
Whole genome sequencing revealed the colistin-resistance mechanism in A. baumannii ZJ06-200P5-1. The gene lpxC was intact in MDR-ZJ06, while in ZJ06-200P5-1, lpxC was inactivated by the insertion sequence ISAba1.
Table 1.
Antibiotic susceptibility of A. baumannii MDR-ZJ06 and its colistin resistant mutant ZJ06-200P5-1.
| Strains | COa | TGCa | IPM | MEM | FEP | CAZ | CTX | ATM | PRL | TZP | SCF | SAM | CN | AK | TE | MH | CIP | CT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MDR-ZJ06 | 0.38 mg/L | 4 mg/L | 8 | 8 | 6 | 6 | 6 | 6 | 6 | 6 | 16 | 10 | 6 | 6 | 6 | 10 | 6 | 14 |
| ZJ06-200P5-1 | >256 mg/L | 0.5 mg/L | 22 | 22 | 20 | 20 | 15 | 22 | 17 | 19 | 30 | 22 | 6 | 6 | 8 | 26 | 9 | 6 |
CO, colistin; TGC, tigecycline; IPM, imipenem; MEM, meropenem; FEP, cefepime; CAZ, ceftazidime; CTX, cefotaxime; ATM, aztreonam; PRL, Piperacillin; TZP, piperacillin/tazobactam; SCF, Cefoperazone/sulbactam; SAM, ampicillin/sulbactam; CN, gentamicin; AK, amikacin; TE, tetracycline; MH, minocycline; CIP, Ciprofloxacin; CT, colistin.
The MIC of colistin and tigecycline were determined by broth dilution method, while antimicrobial sensitivity of other antibiotics were detected by disk diffusion.
Transcriptome analysis
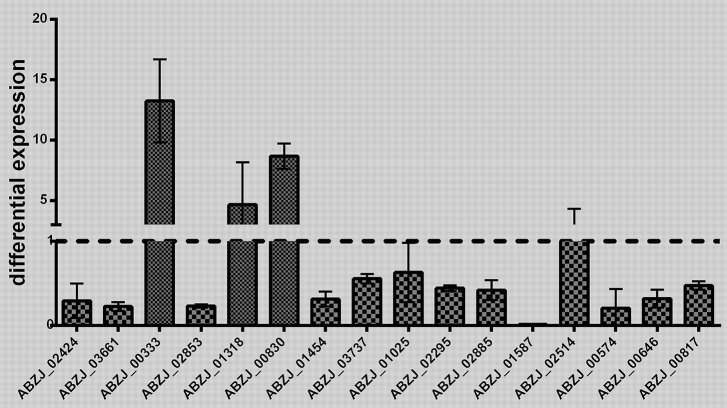
The transcriptome analysis of ZJ06-200P5-1 and MDR-ZJ06 was performed by Illumina RNA deep sequencing technology. Cells of the two strains were collected in the early exponential phase. A total of 137 genes showed significant differential expression [log2(FoldChange) > 1 or log2(FoldChange) < −1], among which 48 genes were upregulated and 89 were downregulated (Table 2). Sixteen selected genes, three up-regulated and thirteen down-regulated genes, were well-validated by RT-qPCR (Figure 2). After mapping the differentially expressed genes into the KEGG pathway, we observed that genes involved in Energy metabolism and Amino acid metabolism were down-regulated, while Carbohydrate metabolism was up-regulated.
Table 2.
Genes changed significantly in transcriptome.
| Synonym | Product | logFC | logCPM | P-value | FDR |
|---|---|---|---|---|---|
| ABZJ_00055 | hypothetical protein | 8.308068 | 13.717 | 1.26E-78 | 4.54E-76 |
| ABZJ_00068 | hypothetical protein | 6.4468 | 9.203574 | 2.14E-67 | 4.61E-65 |
| ABZJ_00037 | hypothetical protein | 4.368832 | 9.669037 | 3.48E-68 | 9.36E-66 |
| ABZJ_00056 | hypothetical protein | 4.349519 | 12.2059 | 6.03E-65 | 1.08E-62 |
| ABZJ_00332 | hypothetical protein | 4.264896 | 9.455077 | 2.39E-53 | 2.86E-51 |
| ABZJ_00036 | hypothetical protein | 3.449637 | 9.968726 | 9.61E-27 | 5.17E-25 |
| ABZJ_01879 | hypothetical protein | 2.810666 | 6.769621 | 9.95E-35 | 7.65E-33 |
| ABZJ_01880 | putative transposase | 2.758133 | 6.676606 | 5.52E-27 | 3.13E-25 |
| ABZJ_01079 | hypothetical protein | 2.585295 | 6.001793 | 4.14E-10 | 6.55E-09 |
| ABZJ_03753 | hypothetical protein | 2.318997 | 9.492231 | 2.51E-21 | 1.08E-19 |
| ABZJ_00333 | hypothetical protein | 2.314205 | 5.437541 | 2.36E-11 | 4.53E-10 |
| ABZJ_01881 | transposase component | 2.25458 | 8.338274 | 9.50E-21 | 3.93E-19 |
| ABZJ_01133 | heat shock protein | 2.180889 | 13.35847 | 1.03E-25 | 5.06E-24 |
| ABZJ_01180 | putative phage-like protein | 2.066152 | 3.22126 | 4.47E-06 | 3.56E-05 |
| ABZJ_03752 | PGAP1-like protein | 2.014551 | 10.16569 | 2.49E-27 | 1.49E-25 |
| ABZJ_00060 | Thiol-disulfide isomerase and thioredoxin | 1.894318 | 12.3252 | 7.68E-20 | 2.75E-18 |
| ABZJ_00894 | lactoylglutathione lyase-like protein | 1.797874 | 6.779815 | 5.27E-15 | 1.62E-13 |
| ABZJ_00054 | N-alpha-acetylglutamate synthase (amino-acid acetyltransferase) | 1.77044 | 10.25589 | 3.24E-20 | 1.27E-18 |
| ABZJ_01151 | hypothetical protein | 1.634908 | 3.574211 | 4.88E-06 | 3.84E-05 |
| ABZJ_03714 | hypothetical protein | 1.61859 | 8.500912 | 1.39E-08 | 1.85E-07 |
| ABZJ_01900 | acetoin:2,6-dichlorophenolindophenol oxidoreductase subunit alpha | 1.527437 | 6.102611 | 2.98E-06 | 2.49E-05 |
| ABZJ_01222 | hypothetical protein | 1.515854 | 2.111384 | 0.011897 | 0.034227 |
| ABZJ_01191 | hypothetical protein | 1.46809 | 2.203352 | 0.011349 | 0.032877 |
| ABZJ_01872 | hypothetical protein | 1.423713 | 7.613403 | 1.64E-08 | 2.10E-07 |
| ABZJ_01187 | hypothetical protein | 1.423595 | 5.112417 | 2.82E-07 | 2.81E-06 |
| ABZJ_01857 | hypothetical protein | 1.411761 | 2.566001 | 0.010144 | 0.029905 |
| ABZJ_01829 | Acyl-CoA dehydrogenase | 1.402255 | 6.594396 | 4.45E-06 | 3.56E-05 |
| ABZJ_01150 | hypothetical protein | 1.321675 | 3.205499 | 0.000936 | 0.003799 |
| ABZJ_00028 | lytic murein transglycosylase family protein | 1.296752 | 10.96489 | 3.46E-14 | 9.79E-13 |
| ABZJ_00976 | hypothetical protein | 1.295503 | 5.552053 | 1.46E-07 | 1.57E-06 |
| ABZJ_01855 | hypothetical protein | 1.290522 | 2.587494 | 0.016132 | 0.044395 |
| ABZJ_01186 | hypothetical protein | 1.249298 | 2.481015 | 0.013475 | 0.038054 |
| ABZJ_00978 | hypothetical protein | 1.216859 | 3.038132 | 0.00684 | 0.021395 |
| ABZJ_00977 | hypothetical protein | 1.209422 | 3.887522 | 0.000232 | 0.001118 |
| ABZJ_00102 | D-lactate dehydrogenase FAD-binding protein | 1.170013 | 8.813908 | 1.91E-10 | 3.15E-09 |
| ABZJ_01149 | hypothetical protein | 1.156232 | 3.314522 | 0.003302 | 0.011138 |
| ABZJ_00053 | alkanesulfonate transport protein | 1.143156 | 6.421362 | 5.15E-06 | 3.99E-05 |
| ABZJ_01275 | hypothetical protein | 1.122845 | 8.385252 | 1.31E-08 | 1.76E-07 |
| ABZJ_03838 | membrane-fusion protein | 1.119324 | 7.708838 | 1.84E-08 | 2.33E-07 |
| ABZJ_01901 | acetoin:26-dichlorophenolindophenol oxidoreductase beta subunit | 1.105826 | 6.349341 | 5.58E-05 | 0.000323 |
| ABZJ_01899 | lipoate synthase | 1.08338 | 4.583472 | 0.003397 | 0.011422 |
| ABZJ_00360 | hypothetical protein | 1.076106 | 8.065171 | 1.34E-07 | 1.46E-06 |
| ABZJ_01210 | hypothetical protein | 1.065917 | 3.456549 | 0.011028 | 0.032156 |
| ABZJ_01160 | hypothetical protein | 1.048988 | 3.144467 | 0.012194 | 0.034895 |
| ABZJ_01148 | hypothetical protein | 1.048966 | 5.540519 | 1.77E-05 | 0.000122 |
| ABZJ_00099 | L-lactate permease | 1.044891 | 10.0835 | 8.49E-08 | 9.61E-07 |
| ABZJ_00901 | major facilitator superfamily multidrug resistance protein | 1.016944 | 9.235389 | 1.47E-08 | 1.91E-07 |
| ABZJ_01775 | 6-pyruvoyl-tetrahydropterin synthase | 1.014549 | 10.17374 | 3.05E-12 | 6.84E-11 |
| ABZJ_03786 | VirP protein | −1.0004 | 6.133241 | 3.35E-06 | 2.73E-05 |
| ABZJ_01269 | TPR repeat-containing SEL1 subfamily protein | −1.00222 | 4.702232 | 0.000305 | 0.001408 |
| ABZJ_00120 | hypothetical protein | −1.00591 | 7.042084 | 6.25E-07 | 5.85E-06 |
| ABZJ_00896 | nucleoside-diphosphate sugar epimerase | −1.0079 | 7.57903 | 9.80E-07 | 8.86E-06 |
| ABZJ_01258 | hypothetical protein | −1.01127 | 4.48134 | 0.002855 | 0.009692 |
| ABZJ_01260 | metal ion ABC transporter substrate-binding protein/surface antigen | −1.01249 | 9.488595 | 2.29E-08 | 2.86E-07 |
| ABZJ_01120 | urease accessory protein UreE | −1.01439 | 6.914944 | 6.34E-07 | 5.88E-06 |
| ABZJ_01873 | hypothetical protein | −1.01999 | 5.846082 | 1.89E-05 | 0.000128 |
| ABZJ_03812 | hypothetical protein | −1.02082 | 4.567471 | 0.001409 | 0.005227 |
| ABZJ_01101 | hypothetical protein | −1.03046 | 5.533349 | 0.001752 | 0.006282 |
| ABZJ_01908 | Zn-dependent hydrolase, including glyoxylase | −1.03588 | 9.460654 | 2.53E-10 | 4.12E-09 |
| ABZJ_03819 | hypothetical protein | −1.05745 | 9.905586 | 6.08E-11 | 1.11E-09 |
| ABZJ_03796 | putative acyltransferase | −1.06273 | 6.680253 | 2.34E-07 | 2.42E-06 |
| ABZJ_00947 | hypothetical protein | −1.0641 | 6.738813 | 1.36E-06 | 1.21E-05 |
| ABZJ_01169 | hypothetical protein | −1.06442 | 8.404764 | 8.75E-07 | 7.98E-06 |
| ABZJ_00345 | hypothetical protein | −1.06443 | 6.560939 | 2.47E-07 | 2.53E-06 |
| ABZJ_03828 | hypothetical protein | −1.06567 | 4.05012 | 0.000406 | 0.001813 |
| ABZJ_00922 | hypothetical protein | −1.07121 | 5.599955 | 7.64E-05 | 0.000424 |
| ABZJ_01907 | response regulator | −1.07682 | 6.813752 | 2.94E-07 | 2.90E-06 |
| ABZJ_03790 | gamma-aminobutyrate permease | −1.07931 | 8.18838 | 3.71E-05 | 0.000227 |
| ABZJ_00882 | hypothetical protein | −1.07943 | 9.751157 | 2.22E-11 | 4.34E-10 |
| ABZJ_01078 | hypothetical protein | −1.08109 | 10.14275 | 5.68E-14 | 1.49E-12 |
| ABZJ_01132 | glutamate dehydrogenase/leucine dehydrogenase | −1.08366 | 7.760303 | 2.14E-07 | 2.24E-06 |
| ABZJ_03802 | putative homogentisate 1,2-dioxygenase | −1.08726 | 6.643847 | 0.000162 | 0.000822 |
| ABZJ_00334 | hypothetical protein | −1.09533 | 6.571739 | 7.17E-08 | 8.25E-07 |
| ABZJ_01250 | outer membrane receptor protein | −1.10965 | 7.442322 | 0.000193 | 0.000956 |
| ABZJ_00367 | hypothetical protein | −1.11395 | 8.476819 | 9.04E-09 | 1.25E-07 |
| ABZJ_00946 | hypothetical protein | −1.12668 | 5.862006 | 7.32E-06 | 5.59E-05 |
| ABZJ_01265 | hypothetical protein | −1.12706 | 10.47521 | 4.03E-13 | 9.42E-12 |
| ABZJ_01257 | Zn-dependent protease with chaperone function | −1.13229 | 6.680195 | 1.30E-05 | 9.11E-05 |
| ABZJ_01110 | putative hemolysin-related protein | −1.13995 | 9.22038 | 1.74E-11 | 3.54E-10 |
| ABZJ_03720 | UDP-3-O-acyl-N-acetylglucosamine deacetylase | −1.14429 | 8.585685 | 1.05E-05 | 7.52E-05 |
| ABZJ_01960 | isochorismate hydrolase | −1.14761 | 5.633402 | 0.000121 | 0.000638 |
| ABZJ_00942 | hypothetical protein | −1.15912 | 8.72549 | 8.38E-09 | 1.17E-07 |
| ABZJ_03859 | putative RND type efflux pump involved in aminoglycoside resistance (AdeT) | −1.17363 | 8.75427 | 3.19E-05 | 0.000202 |
| ABZJ_01874 | hypothetical protein | −1.17434 | 5.206346 | 2.41E-05 | 0.000159 |
| ABZJ_01917 | putative acyl carrier protein phosphodiesterase (ACP phosphodiesterase) | −1.18991 | 7.045816 | 5.55E-08 | 6.50E-07 |
| ABZJ_01861 | membrane-fusion protein | −1.20577 | 6.002924 | 1.77E-07 | 1.87E-06 |
| ABZJ_03742 | hypothetical protein | −1.20817 | 3.772045 | 0.001579 | 0.005748 |
| ABZJ_01262 | hypothetical protein | −1.21556 | 4.167491 | 8.53E-05 | 0.000466 |
| ABZJ_01929 | Aspartate ammonia-lyase (Aspartase) | −1.21837 | 11.63816 | 9.24E-14 | 2.31E-12 |
| ABZJ_00924 | hypothetical protein | −1.2423 | 8.464578 | 1.01E-10 | 1.79E-09 |
| ABZJ_01155 | hypothetical protein | −1.2668 | 10.80427 | 1.20E-16 | 3.79E-15 |
| ABZJ_00388 | 2-polyprenyl-6-methoxyphenol hydroxylase | −1.26695 | 7.901741 | 1.19E-09 | 1.81E-08 |
| ABZJ_01862 | multidrug ABC transporter ATPase | −1.27715 | 6.94382 | 4.78E-09 | 6.86E-08 |
| ABZJ_00944 | hypothetical protein | −1.28276 | 5.658916 | 2.33E-08 | 2.88E-07 |
| ABZJ_01156 | hypothetical protein | −1.28415 | 8.332979 | 5.92E-11 | 1.10E-09 |
| ABZJ_01826 | AraC-type DNA-binding domain-containing protein | −1.29289 | 5.11387 | 5.57E-07 | 5.26E-06 |
| ABZJ_03744 | hypothetical protein | −1.29678 | 8.720807 | 1.08E-08 | 1.47E-07 |
| ABZJ_03737 | hypothetical protein | −1.30269 | 10.28829 | 3.31E-20 | 1.27E-18 |
| ABZJ_00940 | hypothetical protein | −1.30722 | 6.280622 | 2.75E-07 | 2.79E-06 |
| ABZJ_01218 | hypothetical protein | −1.30837 | 4.257169 | 9.06E-06 | 6.63E-05 |
| ABZJ_00061 | putative transcriptional regulator | −1.31564 | 7.634498 | 1.67E-10 | 2.80E-09 |
| ABZJ_01887 | hypothetical protein | −1.3281 | 6.449578 | 1.02E-07 | 1.14E-06 |
| ABZJ_01025 | homocysteine/selenocysteine methylase | −1.33719 | 7.528478 | 3.07E-10 | 4.93E-09 |
| ABZJ_00110 | GNAT family acetyltransferase | −1.33942 | 4.887691 | 1.06E-06 | 9.50E-06 |
| ABZJ_01242 | hypothetical protein | −1.3506 | 7.369014 | 2.45E-09 | 3.61E-08 |
| ABZJ_00895 | hypothetical protein | −1.35351 | 6.693904 | 7.37E-12 | 1.56E-10 |
| ABZJ_03712 | putative flavoprotein | −1.38598 | 6.6067 | 2.04E-09 | 3.04E-08 |
| ABZJ_00048 | transcriptional regulator | −1.40027 | 7.755295 | 9.36E-11 | 1.68E-09 |
| ABZJ_03785 | glutamate racemase | −1.40496 | 7.417511 | 7.08E-12 | 1.52E-10 |
| ABZJ_00938 | hypothetical protein | −1.40799 | 6.629998 | 1.09E-10 | 1.88E-09 |
| ABZJ_01230 | hypothetical protein | −1.41279 | 10.19585 | 3.47E-19 | 1.20E-17 |
| ABZJ_00124 | glycine/D-amino acid oxidase (deaminating) | −1.46015 | 13.3987 | 8.58E-14 | 2.20E-12 |
| ABZJ_03791 | histidine ammonia-lyase (Histidase) | −1.49736 | 9.748038 | 2.37E-08 | 2.90E-07 |
| ABZJ_03739 | hypothetical protein | −1.49749 | 13.98113 | 3.54E-13 | 8.47E-12 |
| ABZJ_00881 | glutamine amidotransferase | −1.51327 | 8.144142 | 5.09E-14 | 1.37E-12 |
| ABZJ_00988 | hypothetical protein | −1.54819 | 6.1324 | 7.44E-09 | 1.05E-07 |
| ABZJ_01840 | putative ferric siderophore receptor protein | −1.55785 | 9.806018 | 9.74E-10 | 1.52E-08 |
| ABZJ_00997 | hypothetical protein | −1.58106 | 5.257799 | 3.12E-08 | 3.77E-07 |
| ABZJ_00339 | HSP90 family molecular chaperone | −1.6168 | 11.15864 | 7.57E-23 | 3.54E-21 |
| ABZJ_00373 | Type II secretory pathway, ATPase PulE/Tfp pilus assembly pathway, ATPase PilB | −1.6419 | 6.706339 | 3.45E-14 | 9.79E-13 |
| ABZJ_01845 | phosphatase/phosphohexomutase | −1.68301 | 7.222507 | 3.67E-12 | 8.06E-11 |
| ABZJ_03793 | urocanate hydratase | −1.69267 | 10.89217 | 1.13E-07 | 1.25E-06 |
| ABZJ_03754 | Rhs element Vgr family protein | −1.69503 | 8.757228 | 5.86E-18 | 1.97E-16 |
| ABZJ_00945 | hypothetical protein | −1.72533 | 5.192791 | 2.02E-11 | 4.03E-10 |
| ABZJ_01002 | putative ABC oligo/dipeptide transport, ATP-binding protein | −1.73182 | 6.449009 | 4.32E-14 | 1.19E-12 |
| ABZJ_01259 | hypothetical protein | −1.75565 | 7.198513 | 1.30E-12 | 2.98E-11 |
| ABZJ_00114 | short chain dehydrogenase family protein | −1.76754 | 7.176594 | 1.03E-13 | 2.52E-12 |
| ABZJ_01177 | hypothetical protein | −1.8053 | 8.135954 | 6.06E-15 | 1.81E-13 |
| ABZJ_03792 | hypothetical protein | −1.82418 | 6.284478 | 3.56E-06 | 2.88E-05 |
| ABZJ_01219 | hypothetical protein | −1.86448 | 9.22858 | 7.68E-22 | 3.45E-20 |
| ABZJ_01088 | carbonic anhydrase | −1.94984 | 9.430551 | 1.08E-27 | 6.83E-26 |
| ABZJ_00346 | hypothetical protein | −2.03948 | 6.219886 | 1.15E-16 | 3.73E-15 |
| ABZJ_01207 | hypothetical protein | −2.1746 | 7.126199 | 6.11E-20 | 2.27E-18 |
| ABZJ_01886 | hypothetical protein | −2.33548 | 5.458495 | 1.05E-11 | 2.18E-10 |
| ABZJ_03766 | putative secretory lipase precursor | −2.38284 | 9.073946 | 1.11E-31 | 7.47E-30 |
| ABZJ_01206 | hypothetical protein | −3.28101 | 9.194837 | 2.48E-45 | 2.42E-43 |
| ABZJ_03736 | thiol:disulfide interchange protein | −3.9361 | 9.872762 | 6.64E-41 | 5.50E-39 |
Figure 2.
Validation of the RNA sequencing results. The transcriptomic results obtained by RNA-seq were validated by quantitative RT-PCR analysis. The differential expression of 16 genes was detected in this study. Three biology replicates were used in this experiment. The results were presented as expression in ZJ06-200P5-1, relative to MDR-ZJ06. The reference gene rpoB was used for inter-sample normalization. Error bars denote standard deviation.
iTRAQ
A total of 1582 proteins were identified in the iTRAQ experiment. A protein ratio >1.5 or <0.67 (p <0.05) was considered to be differentially expressed. After filtration, 82 differentially expressed proteins were identified between ZJ06-200P5-1 and MDR-ZJ06. The detailed information is shown in Table 3.
Table 3.
Genes changed significantly in proteome.
| Protein number | NCBInr acession | Gene tag | Protein description | Pep Count | Unique PepCount | Coverage (%) | MW | pI | log2 of ratio (ZJ06-200P5-1 vs. MDR-ZJ06) | p-value |
|---|---|---|---|---|---|---|---|---|---|---|
| 233 | 384144952 | ABZJ_03706 | hypothetical protein | 75 | 12 | 66.27 | 27649.89 | 4.59 | 1.65184 | 2.90E-20 |
| 1280 | 384143756 | ABZJ_02510 | hypothetical protein | 1 | 1 | 10.18 | 17235.79 | 10.09 | 1.49121 | 8.79E-17 |
| 756 | 384144562 | ABZJ_03316 | hypothetical protein | 27 | 4 | 34.13 | 13935.85 | 9.67 | 1.49075 | 8.99E-17 |
| 1032 | 384144568 | ABZJ_03322 | hypothetical protein | 7 | 2 | 15.75 | 15550.26 | 10.03 | 1.39649 | 6.82E-15 |
| 565 | 384143898 | ABZJ_02652 | hypothetical protein | 23 | 6 | 54.76 | 13282.22 | 8.99 | 1.15312 | 1.36E-10 |
| 594 | 384141430 | ABZJ_00184 | hypothetical protein | 14 | 6 | 32.66 | 22273.87 | 4.56 | 1.131 | 3.05E-10 |
| 1241 | 384141854 | ABZJ_00608 | dehydrogenase | 1 | 1 | 5.13 | 30137.1 | 8.79 | 1.11427 | 5.57E-10 |
| 1188 | 384141579 | ABZJ_00333 | hypothetical protein | 5 | 1 | 10.66 | 11110.55 | 9.66 | 1.09309 | 1.18E-09 |
| 1076 | 384143755 | ABZJ_02509 | hypothetical protein | 4 | 2 | 31.91 | 13701.31 | 10.29 | 1.09014 | 1.31E-09 |
| 147 | 384141823 | ABZJ_00577 | membrane-fusion protein | 59 | 17 | 45.29 | 48231.1 | 9.44 | 0.9855 | 4.28E-08 |
| 1209 | 384142731 | ABZJ_01485 | dihydrodipicolinate synthase | 2 | 1 | 2.89 | 33837.12 | 5.46 | 0.956837 | 1.05E-07 |
| 175 | 384143251 | ABZJ_02005 | membrane-fusion protein | 50 | 15 | 47.22 | 43375.8 | 7.75 | 0.9115 | 4.13E-07 |
| 1281 | 384143760 | ABZJ_02514 | glycosyltransferase | 1 | 1 | 3.37 | 48412.32 | 9.23 | 0.889123 | 7.92E-07 |
| 454 | 384141821 | ABZJ_00575 | putative outer membrane protein | 18 | 8 | 21.57 | 54556.06 | 8.52 | 0.886277 | 8.60E-07 |
| 1009 | 384141578 | ABZJ_00332 | hypothetical protein | 26 | 2 | 32.23 | 11005.53 | 9.93 | 0.859413 | 1.84E-06 |
| 1216 | 384143670 | ABZJ_02424 | hypothetical protein | 2 | 1 | 25.58 | 4520.08 | 5.45 | 0.848157 | 2.51E-06 |
| 201 | 384143250 | ABZJ_02004 | cation/multidrug efflux pump | 26 | 14 | 15.64 | 112744.8 | 7.6 | 0.801366 | 8.82E-06 |
| 885 | 384142076 | ABZJ_00830 | Outer membrane lipoprotein | 12 | 3 | 18.75 | 21087.72 | 6.9 | 0.801241 | 8.85E-06 |
| 323 | 384144243 | ABZJ_02997 | putative porin protein associated with imipenem resistance | 97 | 10 | 50.81 | 26505.22 | 4.8 | 0.770322 | 1.96E-05 |
| 1029 | 384141822 | ABZJ_00576 | peptide ABC transporter permease | 7 | 2 | 3.77 | 71261.81 | 6.24 | 0.753391 | 2.98E-05 |
| 164 | 384144912 | ABZJ_03666 | NAD-dependent aldehyde dehydrogenase | 41 | 16 | 43.15 | 51846.55 | 5.11 | 0.751721 | 3.11E-05 |
| 655 | 384144155 | ABZJ_02909 | hypothetical protein | 27 | 5 | 33.48 | 26172.15 | 7.85 | 0.733875 | 4.80E-05 |
| 812 | 384142146 | ABZJ_00900 | multidrug resistance secretion protein | 8 | 4 | 9.14 | 40956.99 | 6.56 | 0.691132 | 0.000131 |
| 852 | 384145008 | ABZJ_03762 | putative short-chain dehydrogenase | 6 | 4 | 17.24 | 31854.29 | 9.26 | 0.688359 | 0.000139 |
| 539 | 384144680 | ABZJ_03434 | flavoprotein | 10 | 7 | 15.52 | 55720.24 | 9.12 | 0.685088 | 0.00015 |
| 150 | 384144913 | ABZJ_03667 | 4-aminobutyrate aminotransferase | 55 | 17 | 50.23 | 45976.96 | 5.81 | 0.679784 | 0.000169 |
| 1306 | 384144561 | ABZJ_03315 | kinase sensor component of a two component signal transduction system | 1 | 1 | 3.07 | 62690.76 | 6.3 | 0.672652 | 0.000198 |
| 600 | 384144948 | ABZJ_03702 | xenobiotic reductase | 14 | 6 | 21.02 | 38725.16 | 5.08 | 0.608194 | 0.000783 |
| 315 | 384144930 | ABZJ_03684 | hypothetical protein | 322 | 10 | 47.37 | 32732.07 | 4.71 | 0.602647 | 0.000876 |
| 603 | 384143541 | ABZJ_02295 | UDP-glucose 4-epimerase | 13 | 6 | 28.06 | 38064.02 | 5.53 | 0.599175 | 0.000939 |
| 384 | 384142564 | ABZJ_01318 | Zn-dependent protease with chaperone function | 35 | 9 | 48.66 | 27572.18 | 9.44 | 0.592971 | 0.001063 |
| 680 | 384143417 | ABZJ_02171 | hypothetical protein | 14 | 5 | 40.65 | 17046.41 | 8.79 | −0.59205 | 0.000855 |
| 996 | 384143586 | ABZJ_02340 | hypothetical protein | 3 | 3 | 10.61 | 29941.63 | 6.85 | −0.60757 | 0.000626 |
| 1007 | 384145105 | ABZJ_03859 | putative RND type efflux pump involved in aminoglycoside resistance (AdeT) | 3 | 3 | 10.48 | 38641.56 | 9.71 | −0.60779 | 0.000623 |
| 667 | 384144990 | ABZJ_03744 | hypothetical protein | 18 | 5 | 21.99 | 27747.62 | 4.62 | −0.60878 | 0.00061 |
| 820 | 384141318 | ABZJ_00072 | FKBP-type 22KD peptidyl-prolyl cis-trans isomerase | 7 | 4 | 21.65 | 25217.38 | 9.06 | −0.61264 | 0.000564 |
| 767 | 384141553 | ABZJ_00307 | hypothetical protein | 17 | 4 | 48.31 | 10746.92 | 5.3 | −0.61297 | 0.00056 |
| 163 | 384144907 | ABZJ_03661 | hypothetical protein | 47 | 16 | 39.91 | 49757.27 | 8.16 | −0.61374 | 0.000551 |
| 865 | 384144338 | ABZJ_03092 | Zn-dependent hydrolase, including glyoxylase | 5 | 4 | 15.00 | 35333.86 | 8.91 | −0.62839 | 0.000407 |
| 780 | 384141775 | ABZJ_00529 | gluconate kinase | 12 | 4 | 30.59 | 18924.48 | 4.88 | −0.6352 | 0.000353 |
| 1259 | 384142716 | ABZJ_01470 | hypothetical protein | 1 | 1 | 2.52 | 36304.38 | 9.04 | −0.63588 | 0.000348 |
| 424 | 384142064 | ABZJ_00818 | 3-oxoacyl-ACP reductase | 42 | 8 | 45.90 | 26098.39 | 6.1 | −0.64296 | 0.000299 |
| 825 | 384141812 | ABZJ_00566 | hypothetical protein | 7 | 4 | 36.11 | 15329.44 | 9.46 | −0.64431 | 0.00029 |
| 381 | 384141306 | ABZJ_00060 | Thiol-disulfide isomerase and thioredoxin | 37 | 9 | 42.44 | 22825.09 | 9.58 | −0.65529 | 0.000229 |
| 963 | 384142833 | ABZJ_01587 | dehydrogenase | 4 | 3 | 9.93 | 31970.72 | 5.16 | −0.6827 | 0.000125 |
| 645 | 384141583 | ABZJ_00337 | putative outer membrane protein W | 52 | 5 | 28.64 | 22680.64 | 5.9 | −0.69549 | 9.35E-05 |
| 329 | 384142063 | ABZJ_00817 | malonyl-CoA-[acyl-carrier-protein] transacylase | 59 | 10 | 43.15 | 35339.2 | 5.22 | −0.6997 | 8.49E-05 |
| 941 | 384142271 | ABZJ_01025 | homocysteine/selenocysteine methylase | 5 | 3 | 12.33 | 32062.1 | 4.82 | −0.71762 | 5.59E-05 |
| 716 | 384144502 | ABZJ_03256 | protein-disulfide isomerase | 9 | 5 | 23.31 | 26361.06 | 9 | −0.72106 | 5.15E-05 |
| 232 | 384144545 | ABZJ_03299 | acetylCoA carboxylase subunit beta | 76 | 12 | 44.63 | 32971.73 | 5.85 | −0.72297 | 4.93E-05 |
| 836 | 384144135 | ABZJ_02889 | hypothetical protein | 7 | 4 | 38.57 | 15413.52 | 8.43 | −0.72309 | 4.91E-05 |
| 207 | 384141892 | ABZJ_00646 | Acetyl-CoA carboxylase alpha subunit | 87 | 13 | 75.09 | 29640.53 | 5.6 | −0.72798 | 4.37E-05 |
| 1053 | 384144131 | ABZJ_02885 | LysR family transcriptional regulator | 5 | 2 | 6.80 | 34516.26 | 6.26 | −0.74843 | 2.67E-05 |
| 883 | 384142465 | ABZJ_01219 | hypothetical protein | 14 | 3 | 26.54 | 17636.93 | 9.58 | −0.75975 | 2.02E-05 |
| 791 | 384142700 | ABZJ_01454 | hypothetical protein | 10 | 4 | 25.15 | 19116.5 | 5 | −0.77251 | 1.47E-05 |
| 573 | 384144158 | ABZJ_02912 | putative fatty acid desaturase | 20 | 6 | 17.03 | 42202.21 | 9.39 | −0.77608 | 1.34E-05 |
| 663 | 384141673 | ABZJ_00427 | putative type III effector HopPmaJ | 19 | 5 | 37.27 | 12074.21 | 5.41 | −0.78501 | 1.07E-05 |
| 261 | 384141776 | ABZJ_00530 | NAD-dependent aldehyde dehydrogenase | 28 | 12 | 22.69 | 60150.9 | 6.04 | −0.80138 | 7.02E-06 |
| 166 | 384141820 | ABZJ_00574 | NADH-dependent enoyl-ACP reductase | 142 | 15 | 64.24 | 31016.41 | 6 | −0.81807 | 4.53E-06 |
| 280 | 384144728 | ABZJ_03482 | putative toluene tolerance protein (Ttg2D) | 76 | 11 | 61.97 | 23513.33 | 9.83 | −0.82764 | 3.51E-06 |
| 917 | 384142976 | ABZJ_01730 | hypothetical protein | 7 | 3 | 14.80 | 21011.63 | 9.2 | −0.8625 | 1.36E-06 |
| 192 | 384144009 | ABZJ_02763 | hypothetical protein | 63 | 14 | 48.19 | 44493.93 | 8.79 | −0.86539 | 1.25E-06 |
| 635 | 384144826 | ABZJ_03580 | putative penicillin binding protein (PonA) | 8 | 6 | 8.23 | 94767.31 | 9.38 | −0.88231 | 7.77E-07 |
| 292 | 384142962 | ABZJ_01716 | biotin synthetase | 40 | 11 | 34.83 | 37136.95 | 5.45 | −0.89634 | 5.20E-07 |
| 909 | 384142828 | ABZJ_01582 | putative 17 kDa surface antigen | 8 | 3 | 44.76 | 12431.23 | 4.7 | −0.93167 | 1.85E-07 |
| 483 | 384144247 | ABZJ_03001 | hypothetical protein | 43 | 7 | 48.55 | 14704.84 | 9.54 | −0.93498 | 1.67E-07 |
| 188 | 384142100 | ABZJ_00854 | beta-ketoacyl-ACP synthase | 90 | 14 | 46.45 | 43130.17 | 5.2 | −0.94675 | 1.17E-07 |
| 446 | 384144999 | ABZJ_03753 | hypothetical protein | 22 | 8 | 39.09 | 28038.81 | 9.07 | −0.95428 | 9.33E-08 |
| 401 | 384144159 | ABZJ_02913 | flavodoxin reductase (ferredoxin-NADPH reductase) family protein 1 | 23 | 9 | 31.46 | 39570.7 | 6.09 | −0.95498 | 9.13E-08 |
| 489 | 384143515 | ABZJ_02269 | (3R)-hydroxymyristoyl-ACP dehydratase | 39 | 7 | 50.93 | 17988.69 | 6.3 | −0.97767 | 4.53E-08 |
| 459 | 384142835 | ABZJ_01589 | hypothetical protein | 18 | 8 | 13.33 | 43721.35 | 4.96 | −0.97866 | 4.39E-08 |
| 833 | 384143336 | ABZJ_02090 | hypothetical protein | 7 | 4 | 37.91 | 17951.03 | 4.82 | −1.00115 | 2.16E-08 |
| 586 | 384143810 | ABZJ_02564 | hypothetical protein | 16 | 6 | 79.22 | 8718.62 | 5 | −1.02063 | 1.15E-08 |
| 114 | 384143236 | ABZJ_01990 | beta-lactamase OXA-23 | 161 | 18 | 71.38 | 31385.05 | 8.37 | −1.0965 | 8.98E-10 |
| 359 | 384143517 | ABZJ_02271 | putative outer membrane protein (OmpH) | 25 | 10 | 57.49 | 18710.09 | 9.52 | −1.22331 | 8.58E-12 |
| 606 | 384144983 | ABZJ_03737 | hypothetical protein | 13 | 6 | 38.04 | 27580.52 | 4.68 | −1.28419 | 7.75E-13 |
| 95 | 384144431 | ABZJ_03185 | putative DcaP-like protein | 111 | 20 | 50.69 | 47278.17 | 6.37 | −1.36068 | 3.23E-14 |
| 939 | 384141906 | ABZJ_00660 | putative lipoprotein precursor (VacJ) transmembrane | 5 | 3 | 10.67 | 33499.98 | 4.85 | −1.43792 | 1.09E-15 |
| 1289 | 384144099 | ABZJ_02853 | hypothetical protein | 1 | 1 | 8.06 | 14811.21 | 4.39 | −1.58122 | 1.26E-18 |
| 1186 | 384142065 | ABZJ_00819 | acyl carrier protein (ACP) | 21 | 1 | 10.99 | 10132.23 | 4.11 | −1.65109 | 3.70E-20 |
| 412 | 384142699 | ABZJ_01453 | hypothetical protein | 14 | 9 | 46.52 | 25412.88 | 9.89 | −1.66497 | 1.81E-20 |
| 113 | 384144004 | ABZJ_02758 | beta-lactamase | 268 | 18 | 55.05 | 44683.92 | 9.28 | −1.82062 | 3.88E-24 |
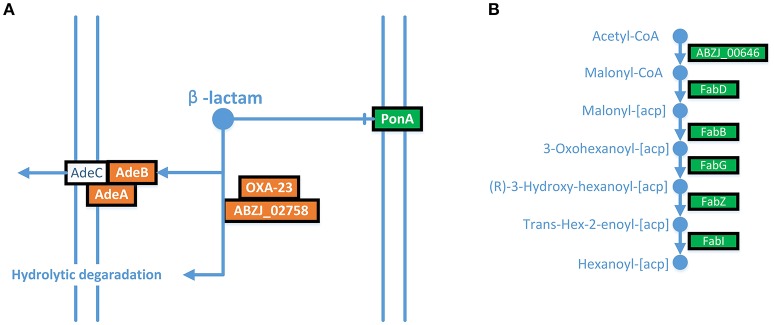
The expression of AdeABC was up-regulated in the LPS-loss ZJ06-200P5-1 strain. The AdeABC efflux pump confers resistance to various antibiotics classes. The expression of AdeABC genes was increased approximately two-fold in ZJ06-200P5-1 (Figure 3A). However, ZJ06-200P5-1 showed higher susceptibility to multiple antibiotics than MDR-ZJ06 (Table 1).
Figure 3.
ITRAQ analysis showed that AdeABC were up-regulated, and the fatty acid biosynthesis pathway was down-regulated in ZJ06-200P5-1. (A) AdeABC efflux pump, (B) fatty acid biosynthesis pathway. Green shows genes with significantly reduced expression levels, and red shows genes with significantly increased expression levels.
The fatty acid biosynthesis pathway was down-regulated in the ZJ06-200P5-1 strain (Figure 3B). The expression of FabZ was decreased by approximately two-fold in ZJ06-200P5-1. The β-lactamases blaOXA−23 and blaADC−25 were down-regulated in ZJ06-200P5-1 strain. The expression levels of blaOXA−23 and blaADC−25 were decreased two- to four-fold in ZJ06-200P5-1.
Common genes altered expression in both transcriptome and proteome
A total of 15 differentially expressed genes (or proteins) were identified in both transcriptome and proteome (Table 4). Among them, three genes were both up-regulated, and nine genes were both down-regulated. Although there was correlation between transcriptome and proteome data, the absolute expression difference values in transcriptome data was higher than those in proteome data. In addition, the result of three gene/proteins were contradictory (highlighted in red letters in Table 4). The contradictory result might be caused by post-transcriptional regulation.
Table 4.
Common genes altered expression both in transcriptome and proteome.
| Synonym | Product | Fold change (log2, Transcriptome) | Fold change (log2, Proteome) |
|---|---|---|---|
| ABZJ_00332 | hypothetical protein | 4.26489563 | 0.859413 |
| ABZJ_03753 | hypothetical protein | 2.318997325a | −0.95428 |
| ABZJ_00333 | hypothetical protein | 2.314204886 | 1.09309 |
| ABZJ_01133 | heat shock protein | 2.180888936 | 0.532117 |
| ABZJ_00060 | Thiol-disulfide isomerase and thioredoxin | 1.894317881a | −0.65529 |
| ABZJ_00028 | lytic murein transglycosylase family protein | 1.296751692a | −0.57293 |
| ABZJ_01078 | hypothetical protein | −1.081092562 | −0.44448 |
| ABZJ_03720 | UDP-3-O-acyl-N-acetylglucosamine deacetylase | −1.144287283 | −0.48378 |
| ABZJ_03859 | putative RND type efflux pump involved in aminoglycoside resistance (AdeT) | −1.173634714 | −0.60779 |
| ABZJ_03744 | hypothetical protein | −1.296782077 | −0.60878 |
| ABZJ_03737 | hypothetical protein | −1.302692756 | −1.28419 |
| ABZJ_01025 | homocysteine/selenocysteine methylase | −1.337189269 | −0.71762 |
| ABZJ_01219 | hypothetical protein | −1.864476303 | −0.75975 |
| ABZJ_01088 | carbonic anhydrase | −1.949843631 | −0.56001 |
| ABZJ_01206 | hypothetical protein | −3.281014801 | −0.4346 |
The result of three gene/proteins were contradictory.
Discussion
Due to the limitation of antimicrobial agents in clinical use, it is urgent to extend our understanding of the emergence of colistin resistance in A. baumannii. A. baumannii MDR-ZJ06, a multidrug-resistant clinical strain isolated from bloodstream, has been sequenced and was considered an ideal strain for examining the colistin-resistant mechanism in A. baumannii (Zhou et al., 2011). In this study, colistin-resistant strain was rapidly obtained, and its resistance mechanism was LPS loss caused by ISAba1 insertion in lpxC. This result confirmed a previous finding (Moffatt et al., 2010). The rapid isolation of colistin-resistant mutant from multiple drug-resistant A. baumannii indicated a high risk of A. baumannii evolving resistance to colistin in clinical use.
We successfully detected the whole transcriptional profile of A. baumannii strain MDR-ZJ06 and its colistin-resistant mutant ZJ06-200P5-1 via Illumina RNA-sequencing. In another transcriptome study (Henry et al., 2012), A. baumannii ATCC 19606 and its lpxA mutant were used. Although both the lpxC and lpxA mutation lead to LPS loss, the different transcriptional response may be due to differences in the strain genetic background and the resistant mutation. In transcriptional analysis, we observed that genes involved in Energy metabolism and Amino acid metabolism were down-regulated, while Carbohydrate metabolism was up-regulated.
The expression of AdeABC was up-regulated in the LPS-loss ZJ06-200P5-1 strain. Similar results were also observed in all polymyxin-treated samples (Cheah et al., 2016a). In addition, the expression levels of adeIJK and macAB-tolC were up-regulated in the LPS loss mutant (Henry et al., 2012). Increased expression of the RND efflux pump system (AdeABC) was a common finding across all experiments in colistin exposure. The up-regulation of AdeABC indicated the diminished integrity and barrier function of the outer membrane in colistin-resistant A. baumannii (Henry et al., 2015; Cheah et al., 2016a). However, ZJ06-200P5-1 showed higher susceptibility to multiple antibiotics than MDR-ZJ06. The higher susceptibility might result from the higher outer membrane permeability of ZJ06-200P5-1 due to LPS-loss. The increased expression of the efflux pump was thought to be a response to toxic substances that accumulated in the cells due to the increased membrane permeability (Henry et al., 2012).
The fatty acid biosynthesis pathway was down-regulated in the ZJ06-200P5-1 strain. In E. coli, it is important to balance LPS and fatty acid biosynthesis to maintain cell integrity. FabZ, which dehydrates R-3-hydroxymyristoyl-acyl carrier protein in fatty acid biosynthesis, plays an important role in rebalancing lipid A and fatty acid homeostasis (Bojkovic et al., 2016). The decrease in FabZ was considered to be a response to LPS-loss in ZJ06-200P5-1. The β-lactamases blaOXA−23 and blaADC−25 were down-regulated in the ZJ06-200P5-1 strain. Decreased expression levels of blaOXA−23 and blaADC−25 were also observed in A. baumannii MDR-ZJ06 under a subinhibitory concentration of tigecycline (Hua et al., 2014). Meanwhile, the strain under tigecycline stress showed a lower MIC of ceftazidime (Hua et al., 2014). The decrease in blaOXA−23 and blaADC−25 might contribute to the increased sensitivity to β-lactam antimicrobial agents.
A multi-omics approach was adopted to obtain a more global view of colistin-resistant A. baumannii. Genomic analysis showed that lpxC was inactivated by ISAba1 insertion, leading to LPS loss. Transcriptional analysis demonstrated that the colistin-resistant strain regulated its metabolism. Metabolic change and LPS loss were concomitant. Proteomic analysis suggested increased expression of the RND efflux pump system and the down-regulation of FabZ and β-lactamase. These alterations are believed to be responses to LPS loss. Together, the lpxC mutation not only confirmed colistin resistance but also altered global gene expression.
Nucleotide sequence accession numbers
The whole-genome shotgun sequencing results for A. baumannii ZJ06-200P5-1 have been deposited at DDBJ/EMBL/GenBank under the accession number MIFW00000000.
Author contributions
XH and YY conceived and designed the study. XH, LL, YF, QS, XL, QC, KS, YJ, and HZ performed the experiments. XH and YY performed data analysis and drafted the manuscript. All authors reviewed and approved the final manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81230039, 31670135, 81378158), the 973 Preliminary Research Program (2014CB560707), the Natural Science Foundation of Zhejiang province, China (LY15H190004, Y16H190013) and the Zhejiang Province Medical Platform Backbone Talent Plan (2016DTA003).
References
- Antunes L. C., Visca P., Towner K. J. (2014). Acinetobacter baumannii: evolution of a global pathogen. Pathog. Dis. 71, 292–301. 10.1111/2049-632X.12125 [DOI] [PubMed] [Google Scholar]
- Bae S., Kim M. C., Park S. J., Kim H. S., Sung H., Kim M. N., et al. (2016). In vitro synergistic activity of antimicrobial agents in combination against clinical isolates of colistin-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 60, 6774–6779. 10.1128/AAC.00839-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beceiro A., Llobet E., Aranda J., Bengoechea J. A., Doumith M., Hornsey M., et al. (2011). Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob. Agents Chemother. 55, 3370–3379. 10.1128/AAC.00079-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beceiro A., Moreno A., Fernandez N., Vallejo J. A., Aranda J., Adler B., et al. (2014). Biological cost of different mechanisms of colistin resistance and their impact on virulence in Acinetobacter baumannii. Antimicrob. Agents Chemother. 58, 518–526. 10.1128/AAC.01597-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojkovic J., Richie D. L., Six D. A., Rath C. M., Sawyer W. S., Hu Q., et al. (2016). Characterization of an Acinetobacter baumannii lptD deletion strain: permeability defects and response to inhibition of lipopolysaccharide and fatty acid biosynthesis. J. Bacteriol. 198, 731–741. 10.1128/JB.00639-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Chai D., Wang R., Liang B., Bai N. (2012). Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. J. Antimicrob. Chemother. 67, 1607–1615. 10.1093/jac/dks084 [DOI] [PubMed] [Google Scholar]
- Cheah S. E., Johnson M. D., Zhu Y., Tsuji B. T., Forrest A., Bulitta J. B., et al. (2016a). Polymyxin resistance in Acinetobacter baumannii: genetic mutations and transcriptomic changes in response to clinically relevant dosage regimens. Sci. Rep. 6:26233. 10.1038/srep26233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah S. E., Li J., Tsuji B. T., Forrest A., Bulitta J. B., Nation R. L. (2016b). Colistin and polymyxin B dosage regimens against Acinetobacter baumannii: differences in activity and the emergence of resistance. Antimicrob. Agents Chemother. 60, 3921–3933. 10.1128/AAC.02927-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deatherage D. E., Barrick J. E. (2014). Identification of mutations in laboratory-evolved microbes from next-generation sequencing data using breseq. Methods Mol. Biol. 1151, 165–188. 10.1007/978-1-4939-0554-6_12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L., Chen Q., Shi K., Li X., Shi Q., He F., et al. (2016). Step-Wise increase in tigecycline resistance in klebsiella pneumoniae associated with Mutations in ramR, lon and rpsJ. PLoS ONE 11:e0165019. 10.1371/journal.pone.0165019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Reyes M., Rodriguez-Falcon M., Chiva C., Pachon J., Andreu D., Rivas L. (2009). The cost of resistance to colistin in Acinetobacter baumannii: a proteomic perspective. Proteomics 9, 1632–1645. 10.1002/pmic.200800434 [DOI] [PubMed] [Google Scholar]
- Henry R., Crane B., Powell D., Deveson Lucas D., Li Z., Aranda J., et al. (2015). The transcriptomic response of Acinetobacter baumannii to colistin and doripenem alone and in combination in an in vitro pharmacokinetics/pharmacodynamics model. J. Antimicrob. Chemother. 70, 1303–1313. 10.1093/jac/dku536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry R., Vithanage N., Harrison P., Seemann T., Coutts S., Moffatt J. H., et al. (2012). Colistin-resistant, lipopolysaccharide-deficient Acinetobacter baumannii responds to lipopolysaccharide loss through increased expression of genes involved in the synthesis and transport of lipoproteins, phospholipids, and poly-beta-1,6-N-acetylglucosamine. Antimicrob. Agents Chemother. 56, 59–69. 10.1128/AAC.05191-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A., O'Donoghue M., Feeney A., Sleator R. D. (2012). Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence 3, 243–250. 10.4161/viru.19700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X., Chen Q., Li X., Yu Y. (2014). Global transcriptional response of Acinetobacter baumannii to a subinhibitory concentration of tigecycline. Int. J. Antimicrob. Agents 44, 337–344. 10.1016/j.ijantimicag.2014.06.015 [DOI] [PubMed] [Google Scholar]
- Li J., Rayner C. R., Nation R. L., Owen R. J., Spelman D., Tan K. E., et al. (2006). Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 50, 2946–2950. 10.1128/AAC.00103-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D. J., Chen Y., Smyth G. K. (2012). Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 40, 4288–4297. 10.1093/nar/gks042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure R., Balasubramanian D., Sun Y., Bobrovskyy M., Sumby P., Genco C. A., et al. (2013). Computational analysis of bacterial RNA-Seq data. Nucleic Acids Res. 41:e140. 10.1093/nar/gkt444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt J. H., Harper M., Harrison P., Hale J. D., Vinogradov E., Seemann T., et al. (2010). Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob. Agents Chemother. 54, 4971–4977. 10.1128/AAC.00834-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X., Wang N., Li X., Shi K., Zhou Z., Yu Y., et al. (2016). The Effect of Colistin Resistance-Associated Mutations on the Fitness of Acinetobacter baumannii. Front. Microbiol. 7:1715. 10.3389/fmicb.2016.01715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg A. Y., Seifert H., Paterson D. L. (2008). Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 21, 538–582. 10.1128/CMR.00058-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thi Khanh Nhu N., Riordan D. W., Do Hoang Nhu T., Thanh D. P., Thwaites G., Huong Lan N. P., et al. (2016). The induction and identification of novel Colistin resistance mutations in Acinetobacter baumannii and their implications. Sci. Rep. 6:28291. 10.1038/srep28291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizcaino J. A., Csordas A., Del-Toro N., Dianes J. A., Griss J., Lavidas I., et al. (2016). 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 44, D447–D456. 10.1093/nar/gkw880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Yang Y., Zhao Y., Zhao H., Bai J., Chen J., et al. (2016). Sub-MIC tylosin inhibits Streptococcus suis biofilm formation and results in differential protein expression. Front. Microbiol. 7:384. 10.3389/fmicb.2016.00384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Zhang T., Yu D., Pi B., Yang Q., Zhou J., et al. (2011). Genomic analysis of the multidrug-resistant Acinetobacter baumannii strain MDR-ZJ06 widely spread in China. Antimicrob. Agents Chemother. 55, 4506–4512. 10.1128/AAC.01134-10 [DOI] [PMC free article] [PubMed] [Google Scholar]