Abstract
There are three mammalian SUMO paralogues: SUMO-1 is ∼45% identical to SUMO-2 and SUMO-3, which are 96% identical to each other. It is currently unclear whether SUMO-1, -2, and -3 function in ways that are unique, redundant, or antagonistic. To address this question, we examined the dynamics of individual SUMO paralogues by using cell lines that stably express each of the mammalian SUMO proteins fused to the yellow fluorescent protein (YFP). Whereas SUMO-2 and -3 showed very similar distributions throughout the nucleoplasm, SUMO-1 was uniquely distributed to the nuclear envelope and to the nucleolus. Photobleaching experiments revealed that SUMO-1 dynamics was much slower than SUMO-2 and -3 dynamics. Additionally, the mobility of SUMO paralogues differed between subnuclear structures. Finally, the timing and distributions were dissimilar between paralogues as cells exited from mitosis. SUMO-1 was recruited to nuclear membrane as nuclear envelopes reformed in late anaphase, and accumulated rapidly into the nucleus. SUMO-2 and SUMO-3 localized to chromosome earlier and accumulated gradually during telophase. Together, these findings demonstrate that mammalian SUMO-1 shows patterns of utilization that are clearly discrete from the patterns of SUMO-2 and -3 throughout the cell cycle, arguing that it is functionally distinct and specifically regulated in vivo.
INTRODUCTION
SUMO family proteins are small ubiquitin-related proteins that become covalently conjugated to cellular substrates. The SUMO conjugation pathway is biochemically similar to the ubiquitin conjugation pathway (Melchior et al., 2003). SUMO proteins are first posttranslationally processed, to expose a C-terminal diglycine motif. The processed forms are linked through a thioester bond to the SUMO activating (E1) enzyme Aos1/Uba2. This SUMO thioester linkage is transferred to the SUMO conjugating (E2) enzyme Ubc9. In the last step, an isopeptide bond is formed between SUMO proteins and substrates. Typically, this transfer occurs through the cooperative action of Ubc9 and SUMO protein ligases (E3) (Melchior et al., 2003). The linkage of SUMO proteins to their substrates can be severed by SUMO proteases (Melchior et al., 2003) and may therefore be dynamic in vivo.
Fission and budding yeast each contain a single SUMO protein, pmt3 and Smt3p, respectively. Pmt3p has been implicated in cell cycle control and DNA damage responses. pmt3– mutants undergo aberrant mitosis and display high-frequency loss of minichromosomes (Tanaka et al., 1999). They are also sensitive to DNA-damaging agents, replication inhibitors, UV light, and increased temperature. Smt3p has been implicated in a number of nuclear functions, including nuclear transport (Stade et al., 2002), chromosome segregation (Bachant et al., 2002; Stead et al., 2003), and control of mitotic progression (Dieckhoff et al., 2004). In mammals, there are three SUMO paralogues. Two of these paralogues, SUMO-2 and -3, are 96% identical in their processed forms. (In contexts where it is not possible to differentiate between them, they will be referred to collectively as SUMO-2/3.) SUMO-1 is more distinct, roughly 45% identical with the other two paralogues. SUMO modification in vertebrates has been implicated in a variety of processes, including nuclear transport, gene expression, signal transduction, and cell cycle control (Azuma et al., 2003; reviewed in Seeler and Dejean, 2003). A large number of SUMO conjugation substrates have been reported in vertebrates (Seeler and Dejean, 2003). For different substrates, SUMO conjugation has been demonstrated to increase stability, to promote subcellular relocalization, or to alter protein–protein interactions.
There are several fundamental questions about the distinctions between different SUMO paralogues. First, it is not generally clear whether conjugation of particular substrates is restricted to single SUMO paralogues or whether different paralogues can be used interchangeably. For a few substrates, strong paralogue preferences have been documented; for instance, RanGAP1 is modified almost exclusively by SUMO-1 in vivo (Saitoh and Hinchey, 2000; see below). Other substrates, such as the promyelocytic leukemia protein (PML), have been reported to be conjugated with both SUMO-1 (Sternsdorf et al., 1999) and SUMO-2/3 (Kamitani et al., 1998b) in vivo. Additionally, the issue of specificity is complicated by the fact that paralogue preference can be compromised when individual SUMO proteins are present at superphysiological concentrations (Kamitani et al., 1998a; Azuma et al., 2003). Because the conjugation of many proteins to SUMO-1 has been demonstrated under conditions where that paralogue is highly overexpressed, categorization of paralogue preference for these substrates cannot be considered definitive until their modification is examined under more physiological conditions. Second, to the extent that there is substrate specificity, it is not known how this specificity arises. This is a particularly interesting question because the same SUMO E1 and E2 enzymes are shared between all paralogues. Finally, it is not known whether the consequences of conjugation to different paralogues are distinct.
To address these questions, we have investigated the dynamic properties of SUMO paralogues in living and proliferating HeLa cells that stably express biofluorescent SUMO chimeras. In live cell analyses, the interphase localization of SUMO-1 differed from SUMO-2 and -3. In particular, nucleoli, nuclear envelopes, and cytoplasmic structures were preferentially occupied by SUMO-1 conjugated proteins. Photobleaching studies revealed that there were substantial differences between the dynamics of SUMO-1 and those of SUMO-2 and –3, as well as significant differences between SUMO-1 dynamics in different subnuclear compartments. Notably, the spatial distribution of SUMO-1 within cells during mitosis again differed markedly from the distributions of SUMO-2 and -3. Together, these findings demonstrate that SUMO-1 shows patterns of utilization that are clearly discrete from the patterns of SUMO-2 and -3 throughout the cell cycle, arguing that it is functionally distinct and specifically regulated in vivo.
MATERIALS AND METHODS
Antibodies and Reagents
Monoclonal mouse antibody (JL-8) against green fluorescent protein (GFP) variants was from BD Biosciences (San Jose, CA). Polyclonal rabbit antibodies against human SUMO-1 were as described previously (Azuma et al., 2003). Rabbit polyclonal antibodies against human SUMO-2/3 were kindly provided by H. Saitoh (Saitoh and Hinchey, 2000). Monoclonal mouse anti-actin antibody was from Sigma-Aldrich (St. Louis, MO). Monoclonal mouse antibody (PG-M3) against PML was from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal rabbit antibodies against RanGAP1 were as described in Joseph et al., 2004. All other reagents were from Sigma-Aldrich unless otherwise stated.
DNA constructs
Full-length (unprocessed) human SUMO-1, SUMO-2, SUMO-3 and nonconjugatable SUMO1G, SUMO2G, SUMO3G (with single C-terminal glycines at amino acid positions 96, 92, and 90, respectively) coding sequences were subcloned into BglII and SalI restriction sites of pEYFP (C1) vector (BD Biosciences Clontech, Palo Alto, CA). The identity of subcloned sequences was confirmed by DNA sequencing. Human Histone 2B coding sequence was subcloned into SalI and BamHI sites of pEYFP (N1) vector (BD Biosciences Clontech). The plasmid construct for expression of the cyan fluorescent protein-PML chimera (CFP-PML) was prepared by excising the PML insert from pEGFP (C1):PML (a gift from Prof. Gerd G. Maul, Wistar Institute, PA) by using BspEI and SalI restriction enzymes and ligation of this fragment into pECFP (C1) (BD Biosciences Clontech).
Cell Culture and Stable Cell Lines
HeLa cells were grown at 37°C, in a humidified atmosphere of 5% CO2 in DMEM with 2 mM glutamine supplemented with 10% fetal bovine serum, 100 U of penicillin/ml, and 100 μg/ml streptomycin. Cells were transfected with plasmids by using Effectene reagent (QIAGEN, Valencia, CA) according to the manufacturer's instructions. For stable cell line selection, 0.5 mg/ml geneticin (Invitrogen, Carlsbad, CA) was added to culture medium 24 h after transfection. Cells were incubated in geneticin-containing culture medium that was refreshed daily for a period of 1 wk after when resistant colonies are reseeded sparsely to culture single cell-derived colonies. Uniformly fluorescent colonies derived from single cells were marked and isolated under an inverted fluorescence microscope. Stably transgenic cells were maintained thereafter in medium containing 0.25 mg/ml geneticin. Frequencies of mitotic cells were recorded 24 h after subculturing 5 × 104 cells/ml into medium without geneticin. At least 500 cells were scored in triplicate experiments involving transgenic and nontransgenic HeLa cells.
Heat Shock and Drug Treatments
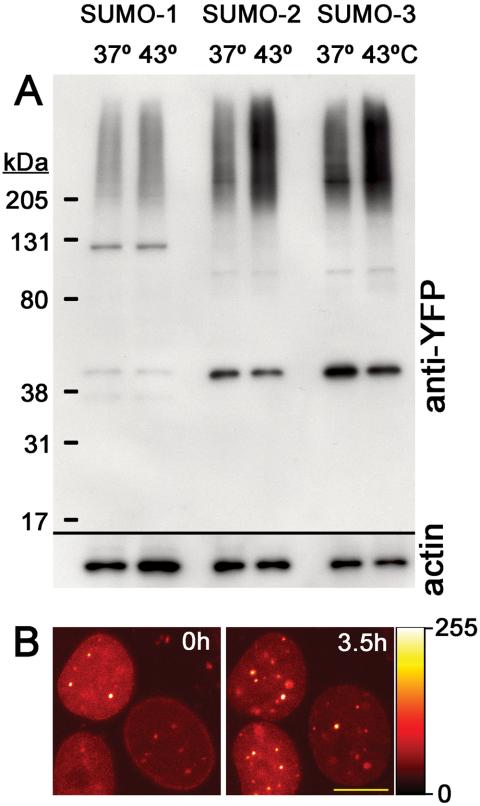
For heat shock treatment (Figure 3A), cells were seeded and grown for 1 d on six-well plates at 37°C. The cell culture medium was removed, and warmed media (43°C) were added. The cells were transferred to a 43°C incubator for 10 min. Control cells were kept 10 min at 37°C after changing culture medium. Total cell lysates from heat-treated and control cells were electrophoresed on SDS-PAGE gels, blotted to polyvinylidene difluoride membrane and subjected to Western blotting analysis using ECL Western detection reagent (Amersham Biosciences, Piscataway, NJ) according to the manufacturer's instructions. For arsenic trioxide (Figure 3B) treatments, transgenic cells grown on LabTekII chambers (Nalge Nunc International, Rochester, NY) were incubated with 1 μM arsenic trioxide. Images of cells were captured before and 3.5 h after addition of the drug.
Figure 3.
YFP-SUMO response to stress. (A) YFP-SUMO-2 and YFP-SUMO-3 show increased conjugation with heat stress, but YFP-SUMO-1 does not. Western analysis of heat stressed (43°C; 10 min) or control (37°C; 10 min) YFP-SUMO–expressing (full-length) cell lines, indicating preferential up-regulation of YFP-SUMO-2 and YFP-SUMO-3 conjugation. Blot was probed with GFP antibodies (top) and actin antibodies as a loading/blotting control (bottom). (B) As2O3 treatment and increase of YFP-SUMO-1 signal at the PML bodies of live cells. YFP-SUMO-1 was imaged in cells before (0 h) and after 3.5 h of As2O3 treatment. Note the number and intensity increase of PML nuclear bodies inside the nuclei after treatment. Images were presented as glow-scale intensity coding palette shown on the right. Bar, 10 μm.
Immunofluorescence
Cells were grown on poly-l-lysine–coated coverslips, washed in phosphate-buffered saline (PBS), and fixed 12 min at ambient temperature with 4% paraformaldehyde in PME buffer (PBS supplemented with 5 mM each of MgCl2 and EGTA). Cells were then permeabilized with 0.5% Triton-X-100 for 10 min. After washing with PME, cells were blocked for 10 min in 5% fish gelatin in PME and incubated 1 h with primary antibodies diluted 1:200 in blocking solution. Coverslips were washed and incubated 45 min with Alexa Fluor 594- or Alexa Fluor 647-conjugated secondary antibodies (Molecular Probes, Eugene, OR) diluted 1:400 in blocking solution. Unbound antibodies were washed and cells were briefly incubated in 100 ng/ml 4′,6-diamidino-2 phenylindole HCl (DAPI) to stain DNA and mounted in Fluoromount-G mounting solution (Southern Biotechnology Associates, Birmingham, AL).
Microscopy
Fluorescence microscopy was performed on an LSM510 META confocal microscope (Carl Zeiss MicroImaging, Thornwood, NY), equipped with 40× Plan Neofluar (numerical aperture [NA] 1.3, Oil, differential interference contrast [DIC]) and 100× Plan Apochromat (NA 1.4, Oil, DIC) objectives. The 40× objective was used for bleaching studies; otherwise, the 100× objective was used for image acquisition. We used a 543 nm HeNe laser (5-mW output, detection LP560 nm) for immunolocalization of Alexa Fluor 594-labeled proteins. For detection of microtubules (Supplemental Figure S2), Alexa Fluor 647-conjugated antibodies were visualized by using 633 nm HeNe laser (15-mW output, detection LP650 nm) The 458-nm and 514-nm lines of an Argon laser (25-mW nominal output, detection BP 475–525 nm and BP 530–600 nm) were used for analyses of CFP-conjugated and yellow fluorescent protein (YFP)-conjugated proteins, respectively. DAPI images were captured using 364-nm line of Enterprise II (ML UV) ion laser from Coherent (Santa Clara, CA) (800mW nominal output, detection BP 385–470 nm).
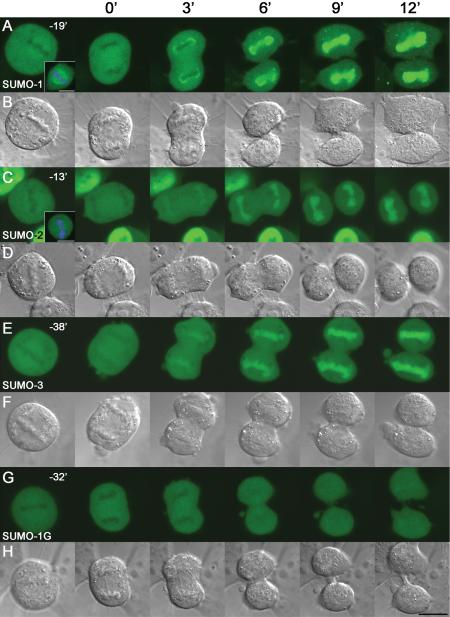
All live analyses were done using CO2-independent medium (Invitrogen) and LabTekII coverslip-bottom chambers at 37°C. For live cell imaging of mitotic cells (Figure 8), 1-d-old cultures were used. Metaphase cells were selected by morphology and followed until cleavage furrow formation became apparent in late anaphase, which was chosen as the reference time point (time 0). Images were then captured every 3 min until the end of mitosis. Zeiss confocal microscopy software (version 3.2) was used for capturing images, which were then analyzed by Adobe Photoshop 7.0 (Adobe Systems, Mountain View, CA).
Figure 8.
Time-lapse imaging of YFP-SUMO chimeras during mitosis. Mitotic divisions of transgenic cells were recorded every 3 min after beginning of cell membrane constriction at late anaphase (designated as 0 min). Single metaphase pictures were taken not to induce artifacts or bleaching of signal while laser scanning. Upper right corner of metaphase pictures shows the time of capture before the start of cytokinesis. Insets at A and C show fixed metaphase cells, also stained with the DNA dye DAPI. YFP-SUMO-1 (A) differed from YFP-SUMO-2 (C) and SUMO-3 (E) with enhanced spindle signal and a distinct perinuclear enrichment. Nonconjugatable YFP-tagged SUMO1 (G) showed only a diffused cytoplasmic signal excluded from the chromosomal area at metaphase and anaphase. B, D, F, and H show corresponding DIC pictures of mitotic cells. Bars, 10 μm.
For fluorescence recovery after photobleaching (FRAP) experiments, five single scans (region of interest frame size, 215 × 215 pixels; pixel time, 1.6 μs; zoom, 3×) were acquired, followed by a bleach pulse of 700 ms (14 iterations) by using a spot radius of 2 μm. Single section images were then collected at 0.5-s intervals, whereas laser power attenuated to 0.05% of the bleach intensity. For fluorescence loss in photobleaching (FLIP) experiments, five single scans (frame size, 512 × 512 pixels; pixel time, 1.6μs; zoom, 3×) were initially acquired, and then a spot radius of 2 μm was repeatedly bleached and imaged at intervals of 1.5 s. Each bleaching lasted 500 ms (10 iterations). For imaging, laser power was attenuated to 0.05% of the bleach intensity. A nucleoplasmic or nucleolar region of interest (2-μm radius circle) that was 10 μm away from the bleach foci was analyzed for depletion in FLIP calculations. An average of five measurements on different cells and half recovery/depletion values were plotted for each cell line.
FRAP recovery and FLIP depletion curves were generated from background subtracted images. The fluorescence signal measured in the region of interest was normalized to the change in total fluorescence according to the following calculation (Phair and Misteli, 2000): Irel = (T0It)/(TtI0), where T0 is total cellular intensity during prebleach, Tt the total cellular intensity at time point t, I0 the average intensity in the region of interest during prebleach, and It the average intensity in the region of interest in time point t.
RESULTS
Expression of YFP-conjugated SUMO Chimeras
To examine the in vivo dynamics of SUMO conjugation, we expressed each of the SUMO paralogues as a full-length, unprocessed proteins, fused at their N termini to yellow fluorescent protein (YFP-SUMO-1, YFP-SUMO-2, YFP-SUMO-3) in HeLa cells. In addition to these full-length forms, we expressed control YFP fusions of each paralogue (YFP-SUMO-1G, YFP-SUMO-2G, YFP-SUMO-3G), which were truncated one amino acid before the normal SUMO processing sites. The control fusion proteins thus possessed a single glycine reside at their C termini, rendering them unconjugatable (Johnson et al., 1997). Notably, similar attempts to select stable cell lines expressing YFP fusions with the mature, processed SUMO proteins were unsuccessful, possibly suggesting that HeLa cells are unable to tolerate elevated levels of the conjugatable forms of SUMO proteins.
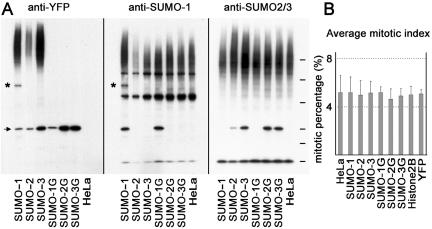
We examined the expression level of each of the proteins by Western blot analysis by using antibodies against GFP that recognize YFP (Figure 1A, left), as well as antibodies against SUMO-1 (middle) and SUMO-2/3 (right). In the transgenic cells, unconjugated YFP-SUMO fusion proteins were detected as single bands of the expected size (arrow at left), but no corresponding band was found in the untransfected HeLa cells (far right lane in each panel). All three transgenic lines expressing full-length YFP-SUMOs showed high-molecular-weight smears of conjugation products, indicating that the YFP-SUMO fusion proteins are effectively processed by hydrolases and successfully conjugated. As expected, the nonconjugatable chimeras were not incorporated into conjugated species. Western blotting of cells expressing YFP-SUMO-2, YFP-SUMO-3, YFP-SUMO-2G, and YFP-SUMO-3G showed levels of unconjugated fusion proteins that were closely comparable with the levels of unconjugated endogenous SUMO-2/3 (Figure 1A, right). Western blotting of cells expressing YFP-SUMO-1 and YFP-SUMO-1G showed that free forms of both SUMO-1 fusion proteins were more abundant than the levels of the unconjugated endogenous SUMO-1 (Figure 1A, middle). None of the fusion proteins significantly altered the level of conjugated species recognized by either anti-SUMO-1 or anti-SUMO-2/3 antibodies, suggesting that their expression did not grossly distort SUMO conjugation patterns overall.
Figure 1.
Expression of YFP-SUMO fusion proteins in HeLa cells. (A) Expression levels of YFP-SUMOs. Western analysis of cells expressing YFP conjugates of both full length and unconjugatable (single glycine) forms of SUMO-1, SUMO-2, and SUMO-3. Same blot was probed with GFP antibodies (left), antibodies against human SUMO-1 (middle), or SUMO-2/3 (right). Asterisks denote YFP-SUMO-1–conjugated Ran-GAP1; arrow indicates the positions of full-length and unconjugatable forms of YFP-SUMO-1, YFP-SUMO-2, and YFP-SUMO-3 (left). Lanes are as indicated at the bottom of the panels. The letter “G” stands for single glycine (unconjugatable) versions of respective YFP-conjugated SUMO cell lines. Last lane is the untransfected HeLa parent line. Molecular weight markers (top to bottom) are 210, 134, 82, 41, 32, and 18 kDa. (B) YFP-SUMO expression does not alter mitotic index. Mitotic index frequencies of control HeLa cells and cells expressing YFP-SUMO conjugates. More than 500 cells were scored for each cell line in triplicate experiments by using phase contrast microscopy analyses of live cells.
Notably, only YFP-SUMO-1 displayed a prominent conjugation product that migrated with a mobility of roughly 120 kDa (Figure 1A, asterisk, left and middle). A novel band of the same molecular weight was recognized in the YFP-SUMO-1 cell line by anti-RanGAP1 antibodies (F.A., unpublished observations), showing that this band arises from YFP-SUMO-1 conjugation to RanGAP1. The absence of a corresponding band in the YFP-SUMO-2– and YFP-SUMO-3–expressing cell lines demonstrates that neither SUMO-2 nor SUMO-3 is abundantly conjugated to RanGAP1, indicating the specificity of conjugation for this substrate. These data are consistent with the findings of Saitoh and Hinchey (2000) that >95% of modified RanGAP1 was conjugated to SUMO-1 in vivo.
To confirm that expression of YFP-SUMO chimeras did not disrupt the growth or division of stable cell lines, we determined the mitotic index of each of the stable cell lines (Figure 1B). Parental HeLa cells and transgenic cell lines all displayed comparable mitotic index values at 24 h after subculturing. Additionally, when plated at a uniform density, all cell lines reached confluence essentially simultaneously (F.A., unpublished observations). These data showed that the transgenic cell lines were proliferating with efficiency comparable to nontransgenic HeLa cells and that there is no significant block at a particular cell cycle stage.
Distribution of YFP-conjugated SUMO Chimeras within Transgenic Cells
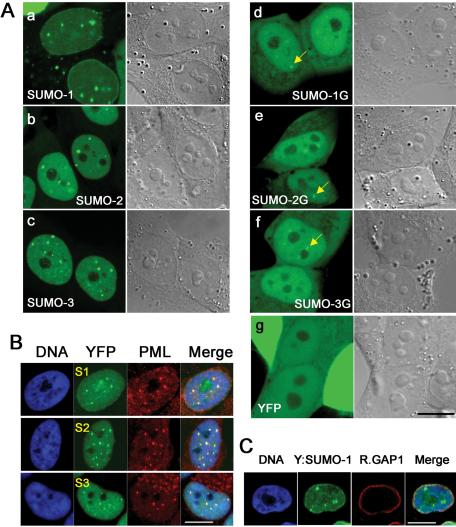
We next determined the interphase localization of YFP-SUMO proteins in each stable cell line (Figure 2A). YFP-SUMO-1, YFP-SUMO-2, and YFP-SUMO-3 all showed accumulation within the nucleoplasm and in punctate nuclear structures. Fixed cells showed similar features that costained brightly with antibodies against the PML protein (Figure 2B), suggesting that these structures corresponded to PML bodies (Takahashi et al., 2004). Notably, there were several differences between the distribution of YFP-SUMO-1 in live cells and the distributions of either YFP-SUMO-2 or YFP-SUMO-3 (Figure 2A, a–c): YFP-SUMO-1 accumulated at the nuclear envelope and within nucleoli, but YFP-SUMO-2 and YFP-SUMO-3 did not. The exclusive accumulation of YFP-SUMO-1 on the nuclear rim (Figure 2, A and C) is consistent with the observation that only SUMO-1 becomes conjugated with RanGAP1. Many cells also showed YFP-SUMO-1 in cytoplasmic dots, which were highly reminiscent of small cytoplasmic structures that contain the RanBP2 and Ran-GAP1 proteins (Saitoh et al., 1996). YFP-SUMO-2 and YFP-SUMO-3 did not accumulate in similar cytoplasmic sites. Nonconjugatable YFP-SUMO-1G, YFP-SUMO-2G, and YFP-SUMO-3G were broadly distributed throughout the nucleus and cytosol (Figure 2A, d–f). Notably, each of the nonconjugatable forms were more concentrated in the nucleus than YFP alone (g). Interestingly, each of these nonconjugatable chimeras occasionally showed a punctate nuclear accumulation that were similar to PML bodies (d–f; arrows) Transient transfection of SUMO-1G, -2G, and -3G stable cell lines with a plasmid expressing a cyan fluorescent protein-PML fusion protein (CFP:PML) confirmed that these foci correspond to PML bodies (Supplemental Figure S1). This observation strongly indicates that protein-protein interactions may contribute toward the accumulation of all three SUMO paralogues within PML nuclear bodies in a manner that does not rely upon their conjugation to cellular proteins.
Figure 2.
Distribution of YFP-SUMO chimeras in interphase cells. (A) Distribution of YFP-SUMOs in live cells. Stable transgenic cell lines were cultured on coverslip-bottom chambers and observed live for interphase localization patterns: (a) YFP-SUMO-1, (b) YFP-SUMO-2, (c) YFP-SUMO-3, (d) YFP-SUMO-1G, (e) YFP-SUMO-2G, (f) YFP-SUMO-3G, and (g) YFP. Arrows in d–f indicate PML body-like signal at the nucleoplasm of nonconjugatable SUMO transgenic cells. Fluorescence pictures (left) were paired with corresponding DIC images (right), indicating position of nuclei and nucleoli. (B) All SUMO paralogues localize to PML bodies. Colocalization of formaldehyde fixed YFP fusion proteins (green) with immunolocalized endogenous PML protein (red). S1, S2, and S3 stands for YFP-conjugated SUMO-1, SUMO-2, and SUMO-3, respectively. DNA is stained with DAPI (blue) and merged images are shown at the last column. (C) Distribution of YFP-SUMO-1 and RanGAP1 in fixed cells. Colocalization of formaldehyde-fixed YFP-SUMO-1 (green) with immunolocalized endogenous RanGAP1 (red). DNA is stained with DAPI (blue) and merged image is shown at the last column. Bars, 10 μm.
It is difficult to compare these distributions to the localization of endogenous SUMO proteins, because the immunofluorescence pattern obtained in our hands with antibodies against either SUMO-1 or SUMO-2/3 is highly dependent upon the particular antibody used and upon fixation conditions. However, we were able to test that the fusion proteins responded to physiological stimuli in a manner similar to the endogenous proteins in several contexts. First, Saitoh and Hinchey (2000) demonstrated that SUMO-2 and -3 conjugation are greatly increased in response to heat shock, whereas SUMO-1 conjugation does not respond similarly. We tested whether the differences between SUMO paralogues also were reflected in the fusion proteins by analyzing the stable transgenic cell lines before or after a brief heat shock treatment (43°C, 10 min) through Western blotting with anti-GFP antibodies. YFP-SUMO-2 and YFP-SUMO-3 conjugation were elevated (Figure 3A), but YFP-SUMO-1 conjugation was not substantially altered by heat treatment of this duration. This finding indicates that the behavior of the fusion proteins recapitulates the behavior of the endogenous SUMO-1, -2, and -3 proteins.
Second, treatment of HeLa cells with As2O3 promotes the SUMO-1 modification of the PML protein and the accumulation of PML and SUMO-1 into enlarged PML bodies (Muller et al., 1998). We tested whether YFP-SUMO-1 was similarly sequestered into PML bodies after As2O3 treatment: we imaged five different clusters of YFP-SUMO-1 before and after a 3.5-h treatment with 1 μM As2O3. In all cells, we found that As2O3 promoted clear accumulation of YFP-SUMO-1 within PML bodies. Typical results for the accumulation of YFP-SUMO-1 in PML bodies after As2O3 treatment are shown in Figure 3B. Interestingly, both YFP-SUMO-2 and YFP-SUMO-3 also frequently accumulated to higher levels in PML bodies after As2O3 treatment, although this accumulation was not universally seen in all cells (F.A., unpublished observations). We do not know what factors distinguished those cells that accumulated YFP-SUMO-2 and YFP-SUMO-3 in PML bodies after As2O3 treatment from those that did not. Overall, we observed that the YFP-fused SUMO proteins behaved similarly to the endogenous proteins, to the extent that they mimicked previously described physiological responses to heat shock or As2O3 treatment.
FRAP and FLIP Analysis of YFP-SUMO Chimeras
Having established that YFP-SUMO-1, YFP-SUMO-2, and YFP-SUMO-3 mimic substrate specificity and responses to physiological stress when expressed in our stable cell lines, we wished to use these cells to compare the dynamics of individual SUMO paralogues. Toward this end, we performed FRAP and FLIP experiments.
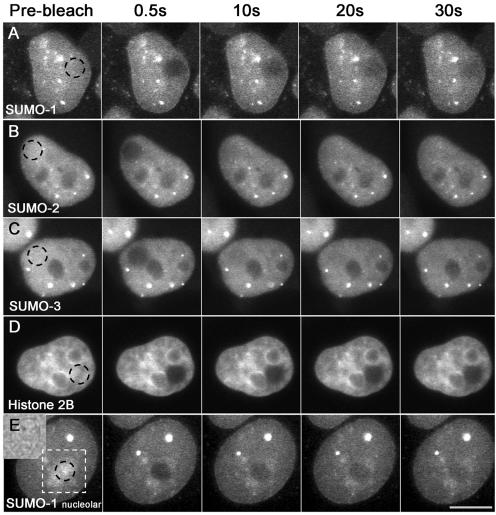
FRAP analyses of YFP-SUMO transgenic cell lines are shown in Figure 4. Recovery after bleaching in the nucleoplasm of each cell line revealed that YFP-SUMO-1 was less mobile (Figure 4A) than YFP-SUMO-2 (Figure 4B) or YFP-SUMO-3 (Figure 4C), which had nearly identical recovery rates. In each case, YFP-SUMO recovery rates were substantially faster than the recovery of an immobile chromatin bound fusion protein, Histone 2B-YFP (Figure 4D; Kanda et al., 1998), but slower than YFP alone. Notably, FRAP analysis of the nucleolar YFP-SUMO-1 population showed it was less dynamic than that of nucleoplasmic population (Figure 4E). We also observed that recovery rates were apparently slower for all SUMO paralogues when PML-body associated populations were bleached, although it was difficult to quantitate this difference because of the limited area, irregular size, and motion of PML bodies. Our data thus indicated that recovery rates vary both between paralogues and between subnuclear compartments.
Figure 4.
FRAP analysis of YFP-SUMO chimeras. A spot of 2-μm-radius circle (dotted) was bleached within the nuclei or nucleoli of transgenic cell line, as indicated, and recovery of fluorescence was recorded in the bleached area (see Materials and Methods). Recovery of YFP-SUMO-1 (A) was slower than that of YFP-SUMO-2 (B) and YFP-SUMO-3 (C). Nucleolar FRAP analysis of YFP-SUMO-1 is shown in E. Bright field picture of the area where the nucleolus is located (dotted square) is shown as an inset of E. D shows Histone2B-YFP FRAP as a control experiment. Bar, 10 μm.
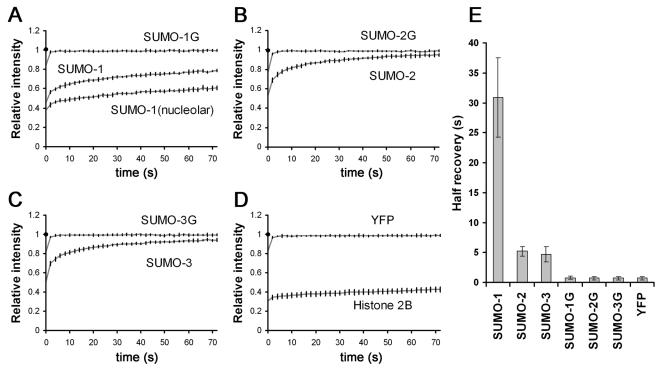
To quantitate the recovery rates, we measured FRAP recoveries of full-length and nonconjugatable YFP-SUMO proteins in five separate cells, and the averages from each series of experiments is presented in Figure 5. In each case, the recovery rates of YFP-SUMO-1, YFP-SUMO-2, and YFP-SUMO-3 were dependent upon their capacity to undergo activation and conjugation to cellular proteins: nonconjugatable YFP-SUMO-1G, YFP-SUMO-2G, and YFP-SUMO-3G proteins were all highly dynamic, with total recovery times of <3 s. Due to extremely fast recovery rates, FRAP techniques did not allow us to differentiate the recovery differences between nonconjugatable SUMO-G forms and YFP alone.
Figure 5.
FRAP analysis of YFP-SUMO chimeras. Average recovery curves of five individual cells from each cell line and standard deviations are shown in A to D. Half recovery durations and standard deviations are plotted in E.
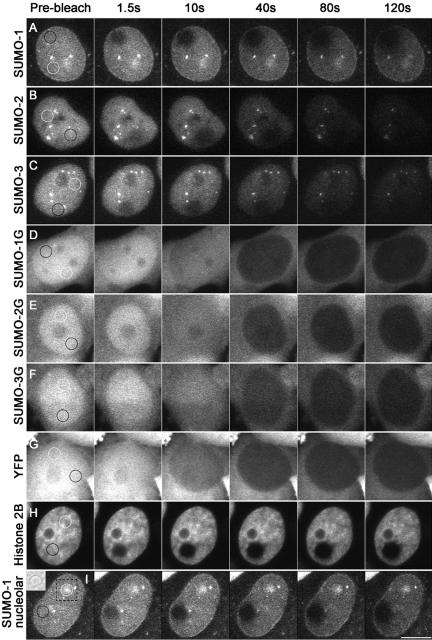
FLIP analyses of YFP-SUMO transgenic cell lines are shown in Figure 6. Nucleoplasmic spots of 2-μm radius (black circles) were repetitively bleached and loss of fluorescence has been measured 10 μm away from the bleaching foci (white circles). Nucleoplasmic YFP-SUMO-1 (Figure 6A) was more resistant to bleaching than either YFP-SUMO-2 or YFP-SUMO-3 (Figure 6, B and C), whereas YFP-SUMO-2 and YFP-SUMO-3 were indistinguishable from each other. The nucleolar population of YFP-SUMO-1 was more resistant to photobleaching than the nucleoplasmic population (Figure 6). The populations of all three conjugatable YFP-SUMO proteins associated to PML bodies also seemed to be more resistant than the nucleoplasmic populations (Figure 6, A–C), although it was again difficult to precisely quantitate the rate of fluorescence loss in PML bodies because of their irregular size and motion. On the other hand, YFP-SUMO-1G, YFP-SUMO-2G, YFP-SUMO-3G proteins associated to PML bodies did not seem to be resistant to photobleaching, suggesting that the interactions through which they accumulated at these sites are highly dynamic (Figure 6, D, F). Together, these experiments confirm that there are differences in between the dynamics of SUMO paralogues with the nucleoplasm, and between populations of YFP-SUMO-1 within various subnuclear compartments.
Figure 6.
FLIP analysis of YFP-SUMO chimeras. A spot of 2-μm-radius circle (black line) was bleached within the nuclei or nucleoli of transgenic cells expressing YFP-SUMO-1 (A), YFP-SUMO-2 (B), or YFP-SUMO-3 (C) and loss of fluorescence at 10 μm away from the bleaching foci (white circle) was recorded and plotted (see Materials and Methods). Similar experiments were performed with nonconjugatable YFP-SUMO-1G– (D), YFP-SUMO-2G– (E), or YFP-SUMO-3G (F)–expressing cells, as well as with cells expressing YFP alone (G) or Histone2B-YFP (H). Nucleolar FLIP for YFP-SUMO-1 is shown in I. Bright field picture of the area where the nucleolus is located (dotted square) is shown as an inset in I. Bar, 10 μm.
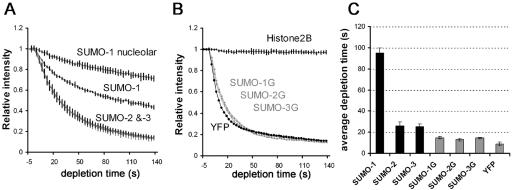
To quantitate these differences, we measured FLIP rates of full-length and nonconjugatable YFP-SUMO proteins in five separate cells. Figure 7 shows the averages from each series of experiments. In addition to confirming the conclusions discussed above, this quantitation revealed that nonconjugatable SUMO proteins were less mobile than YFP alone. It is possible that the measurably slower FLIP rates of the nonconjugatable YFP-SUMO chimeras in comparison with YFP alone may partially reflect their interactions with less mobile nuclear proteins.
Figure 7.
FLIP analysis of YFP-SUMO chimeras. Average depletion curves of five individual cells from each line and their standard deviations are shown as indicated in A and B. Half depletion durations are plotted in C, with standard deviations.
Time Lapse Imaging of YFP-SUMO Chimeras during Mitosis
To assess the comparative behavior of SUMO paralogues in mitosis, the transgenic YFP-SUMO-1–, YFP-SUMO-2–, and YFP-SUMO-3–expressing cells were grown on coverslip bottom chambers. Prometaphase and metaphase cells were selected from these asynchronous cultures and imaged live as they proceeded through mitosis and into the subsequent G1 phase (Figure 8). To reduce artifacts, chromosome dynamics and the timing of mitosis in these cells were followed by DIC microscopy rather than DNA intercalating fluorescent dyes. To minimize laser-induced cell damage that could disrupt progression of the cells through mitosis, one single scan of metaphase stage was taken until cleavage furrow invagination. In all cases, timing is represented with respect to the first evident invagination of the cleavage furrow during late anaphase.
During metaphase and anaphase, YFP-SUMO-1 was broadly distributed throughout the cells (Figure 8A), excluding the chromosomal region. It was not excluded from the region containing spindle microtubules, but rather slightly enhanced in this area of the cell. The distribution of YFP-SUMO-1 to the spindle was even more evident on fixed and extracted cells (Figure 8A, first panel inset), which could be further demonstrated by comparison of the YFP-SUMO-1 signal to immunofluorescent staining with antibodies against β-tubulin (Supplemental Figure S2). Nonconjugatable YFP-SUMO-1G (Figure 8G) showed neither exclusion nor concentration in the region of mitotic spindles, and the spindle-associated YFP-SUMO-1G fraction was not resistant to detergent extraction after fixation. These data indicate that the concentration of YFP-SUMO-1 on spindles resulted from its conjugation to spindle-associated proteins. It is likely that YFP-SUMO-1 conjugation to RanGAP1 contributes toward its localization on spindles (Joseph et al., 2002), although it also is possible that the enhanced localization of YFP-SUMO-1 to spindles may reflect its conjugation to other proteins as well. YFP-SUMO-2 and YFP-SUMO-3 were also generally distributed throughout cells in metaphase and anaphase, excluding the chromosomal region (Figure 8, C and E). Unlike YFP-SUMO-1, however, they did not show concentration on or near the spindle microtubules. The faint YFP signal associated to spindles in the YFP-SUMO-2 and YFP-SUMO-3 cell lines was not resistant to detergent extraction after fixation (Figure 8C, first panel inset, and Supplemental Figures S2B and S2C), further suggesting that SUMO-2 and SUMO-3 do not become as abundantly conjugated to spindle proteins as SUMO-1 does.
We observed other differences as the cells progressed through telophase. At early telophase, soon after formation of the cleavage furrow, YFP-SUMO-1 abruptly localized to the periphery of the newly forming nuclei (Figure 8A, 3 min after the onset of cleavage furrow contraction). The appearance of YFP-SUMO-1-specific cytoplasmic foci was closely coincident with this transition (Figure 8A). The accumulation of YFP-SUMO-1 within the reforming nuclei occurred slightly later (6–9 min after furrow contraction). Both of these patterns required the conjugation of YFP-SUMO-1, because YFP-SUMO-1G neither localized to reforming nuclear envelopes nor accumulated within the nuclei during this interval (Figure 8G). YFP-SUMO-2 and YFP-SUMO-3 accumulation on chromosomes began somewhat earlier than YFP-SUMO-1 accumulation, within 3–6 min of furrow ingression (Figure 8, C and E), but neither protein showed even transient accumulation at the forming nuclear envelopes. The sequestration of YFP-SUMO-2 and YFP-SUMO-3 within the nuclei continued to increase steadily as cells progressed through telophase into G1 phase, when both proteins resumed their interphase distributions. Similar to YFP-SUMO-1G, nonconjugatable YFP-SUMO-2G and YFP-SUMO-3G were not enriched on spindles during mitosis, and they showed accumulation within nuclei only after enclosure of nuclear envelope in G1 phase (Supplemental Figure S3), indicating that the capacity to become conjugated is important for redistribution of SUMO-2 and -3 at the end of mitosis. Together, these results show that SUMO-2 and SUMO-3 behave very similarly during mitosis, but their behavior can be distinguished from SUMO-1, which shows different timing of accumulation and localization patterns throughout the cell cycle.
DISCUSSION
To ascertain the functional differences between the three SUMO paralogues in mammalian cells, we have examined the localization and dynamic behavior of YFP-SUMO fusion proteins. A major finding of these experiments was that YFP-SUMO-1 behaves in a manner that is highly distinct from either YFP-SUMO-2 or YFP-SUMO-3. First, although the pattern of YFP-SUMO-1 localization was overlapping with YFP-SUMO-2 and YFP-SUMO-3 in the nucleoplasm and in PML bodies, YFP-SUMO-1 also uniquely localized to nucleoli, the nuclear envelope, and cytoplasmic foci (Figure 2A). Second, YFP-SUMO-1 showed remarkably different dynamics from YFP-SUMO-2 or YFP-SUMO-3, with slower rates of both recovery in FRAP experiments and depletion in FLIP experiments (Figures 5 and 7). This was true even when apparently indistinguishable regions of the nucleoplasm were bleached, suggesting that the distinct dynamics of YFP-SUMO-1 are an intrinsic property of this protein, rather than a secondary effect of its sequestration with different subnuclear regions. Third, as previously shown for endogenous SUMO proteins (Saitoh and Hinchey, 2000), YFP-SUMO-1 shows substantially different responses to physiological stimuli, such as heat stress (Figure 3A).
It has previously been shown that RanGAP1 becomes modified exclusively by SUMO-1 (Saitoh and Hinchey, 2000). This preference could underlie the finding that only the YFP-SUMO-1 paralogue was found at the nuclear envelope and within cytosolic dots resembling annulate lamelli. In addition, our data suggest that SUMO-1 also has unique roles within the nucleolus. In budding yeast, Smt3p plays an important role in nucleoli. Mutants in the Smt3p protease Smt4p show deficient localization of condensin subunits to ribosomal DNA (Strunnikov et al., 2001), suggesting that they have some role in Condensin targeting or in rDNA chromatin structure. Only YFP-SUMO-1 localizes to nucleoli in human cells, indicating that these functions may be performed uniquely by SUMO-1 in interphase vertebrate cells. Topoisomerase-I has been demonstrated to be a nucleolar substrate for SUMO conjugation, which is redistributed toward the nucleoplasm in response to its chemical inhibition by camptothecin in a manner that can be inhibited by a dominant negative Ubc9 mutant (Mo et al., 2002; Rallabhandi et al., 2002). We found the nucleolar concentration of YFP-SUMO-1 also was dramatically reduced in response to camptothecin, such that it becomes largely excluded from nucleoli within 20–30 min of drug treatment, consistent with the notion that topoisomerase I may be a major nucleolar substrate of SUMO-1 (F.A., unpublished observation).
The overall dynamics of each YFP-SUMO fusion protein should be strongly influenced by several different factors. First, the rates of conjugation and deconjugation determine the fraction of time that each paralogue spends in conjugated forms. It is clear that all of the fusion proteins spent a significant fraction of their time in conjugated complexes, because their dynamics was significantly slower than nonconjugatable forms in both FRAP and FLIP assays. Notably, the faster dynamics of the YFP-SUMO-2 and YFP-SUMO-3 proteins would be consistent with the idea that SUMO-2 and -3 conjugates are turned over more rapidly than SUMO-1 conjugates. The relatively larger free pools of YFP-SUMO-2 and YFP-SUMO-3 are also consistent with this notion (Figure 1A, compare middle and right panels; Saitoh and Hinchey, 2000). Second, substrate specificity also should contribute toward the movement of YFP-SUMO proteins within the nucleus. It is self-evident that conjugation to very static protein complexes with low mobility, like core histones, should result in slower dynamics during photobleaching assays than conjugation to highly mobile components of the transcription or RNA processing machineries (Phair and Misteli, 2000). Finally, the fate of conjugated species could differ in a paralogue-specific manner. For instance, if conjugation of a particular substrate to SUMO-1 targets it to a static structure within the nucleus but SUMO-2 or -3 conjugation of the same substrate does not result in this distribution, then this difference would contribute toward the lower mobility of YFP-SUMO-1–conjugated species.
We also have shown that distribution of all three paralogues changes rapidly as cells enter and progress through mitosis, and as they enter G1 phase (Figure 8). Notably, the mitotic behavior of YFP-SUMO-1 was again different from either YFP-SUMO-2 or -3: SUMO-1 was more abundantly present on the mitotic spindle, and it was recruited very early to the reforming nuclear envelope, and later colocalized with chromosomes. SUMO-2 and SUMO-3 were not found on the reassembling nuclear envelope, but accrued on chromosomes at an earlier point in the nuclear reformation process (Figure 8).
In summary, our findings demonstrate that mammalian SUMO paralogues show discrete patterns of behavior and localization throughout the cell cycle, arguing that they are functionally distinct and specifically regulated in vivo. These findings suggest relatively global differences in the in vivo roles of these proteins that may reflect their preferred substrates, the regulation of their conjugation and deconjugation, and the fate of proteins that become covalently attached to particular paralogues.
Supplementary Material
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04–07–0589. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–07–0589.
Abbreviations used: GFP, green fluorescent protein; YFP, yellow fluorescent protein.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Azuma, Y., Arnaoutov, A., and Dasso, M. (2003). SUMO-2/3 regulates topoisomerase II in mitosis. J. Cell Biol. 163, 477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachant, J., Alcasabas, A., Blat, Y., Kleckner, N., and Elledge, S.J. (2002). The SUMO-1 isopeptidase Smt4 is linked to centromeric cohesion through SUMO-1 modification of DNA topoisomerase II. Mol. Cell 9, 1169–1182. [DOI] [PubMed] [Google Scholar]
- Dieckhoff, P., Bolte, M., Sancak, Y., Braus, G.H., and Irniger, S. (2004). Smt3/SUMO and Ubc9 are required for efficient APC/C-mediated proteolysis in budding yeast. Mol. Microbiol. 51, 1375–1387. [DOI] [PubMed] [Google Scholar]
- Johnson, E.S., Schwienhorst, I., Dohmen, R.J., and Blobel, G. (1997). The ubiquitin-like protein Smt3p is activated for conjugation to other proteins by an Aos1p/Uba2p heterodimer. EMBO J. 16, 5509–5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph, J., Liu, S. T., Jablonski, S. A., Yen, T. J., Dosso, M. (2004). The RanGAP1-RanBP2 complex is essential for microtubule-kinetochore interactions in vivo. Curr. Biol. 14, 611–617. [DOI] [PubMed] [Google Scholar]
- Joseph, J., Tan, S.H., Karpova, T.S., McNally, J.G., and Dasso, M. (2002). SUMO-1 targets RanGAP1 to kinetochores and mitotic spindles. J. Cell Biol. 156, 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamitani, T., Kito, K., Nguyen, H.P., Fukuda-Kamitani, T., and Yeh, E.T. (1998a). Characterization of a second member of the sentrin family of ubiquitin-like proteins. J. Biol. Chem. 273, 11349–11353. [DOI] [PubMed] [Google Scholar]
- Kamitani, T., Nguyen, H.P., Kito, K., Fukuda-Kamitani, T., and Yeh, E.T. (1998b). Covalent modification of PML by the sentrin family of ubiquitin-like proteins. J. Biol. Chem. 273, 3117–3120. [DOI] [PubMed] [Google Scholar]
- Kanda, T., Sullivan, K.F., and Wahl, G.M. (1998). Histone-GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr. Biol. 8, 377–385. [DOI] [PubMed] [Google Scholar]
- Melchior, F., Schergaut, M., and Pichler, A. (2003). SUMO: ligases, isopeptidases and nuclear pores. Trends Biochem. Sci. 28, 612–618. [DOI] [PubMed] [Google Scholar]
- Mo, Y.Y., Yu, Y., Shen, Z., and Beck, W.T. (2002). Nucleolar delocalization of human topoisomerase I in response to topotecan correlates with sumoylation of the protein. J. Biol. Chem. 277, 2958–2964. [DOI] [PubMed] [Google Scholar]
- Muller, S., Matunis, M.J., and Dejean, A. (1998). Conjugation with the ubiquitin-related modifier SUMO-1 regulates the partitioning of PML within the nucleus. EMBO J. 17, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phair, R.D., and Misteli, T. (2000). High mobility of proteins in the mammalian cell nucleus. Nature 404, 604–609. [DOI] [PubMed] [Google Scholar]
- Rallabhandi, P., Hashimoto, K., Mo, Y.Y., Beck, W.T., Moitra, P.K., and D'Arpa, P. (2002). Sumoylation of topoisomerase I is involved in its partitioning between nucleoli and nucleoplasm and its clearing from nucleoli in response to camptothecin. J. Biol. Chem. 277, 40020–40026. [DOI] [PubMed] [Google Scholar]
- Saitoh, H., Cooke, C.A., Burgess, W.H., Earnshaw, W.C., and Dasso, M. (1996). Direct and indirect association of the small GTPase ran with nuclear pore proteins and soluble transport factors: studies in Xenopus laevis egg extracts. Mol. Biol. Cell 7, 1319–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh, H., and Hinchey, J. (2000). Functional heterogeneity of small ubiquitin-related protein modifiers SUMO-1 versus SUMO-2/3. J. Biol. Chem. 275, 6252–6258. [DOI] [PubMed] [Google Scholar]
- Seeler, J.S., and Dejean, A. (2003). Nuclear and unclear functions of SUMO. Nat. Rev. Mol. Cell. Biol. 4, 690–699. [DOI] [PubMed] [Google Scholar]
- Stade, K., Vogel, F., Schwienhorst, I., Meusser, B., Volkwein, C., Nentwig, B., Dohmen, R.J., and Sommer, T. (2002). A lack of SUMO conjugation affects cNLS-dependent nuclear protein import in yeast. J. Biol. Chem. 277, 49554–49561. [DOI] [PubMed] [Google Scholar]
- Stead, K., Aguilar, C., Hartman, T., Drexel, M., Meluh, P., and Guacci, V. (2003). Pds5p regulates the maintenance of sister chromatid cohesion and is sumoylated to promote the dissolution of cohesion. J. Cell Biol. 163, 729–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternsdorf, T., Puccetti, E., Jensen, K., Hoelzer, D., Will, H., Ottmann, O.G., and Ruthardt, M. (1999). PIC-1/SUMO-1-modified PML-retinoic acid receptor alpha mediates arsenic trioxide-induced apoptosis in acute promyelocytic leukemia. Mol. Cell. Biol. 19, 5170–5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunnikov, A.V., Aravind, L., and Koonin, E.V. (2001). Saccharomyces cerevisiae SMT4 encodes an evolutionarily conserved protease with a role in chromosome condensation regulation. Genetics 158, 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, Y., Lallemand-Breitenbach, V., Zhu, J., and de The, H. (2004). PML nuclear bodies and apoptosis. Oncogene 23, 2819–2824. [DOI] [PubMed] [Google Scholar]
- Tanaka, K., Nishide, J., Okazaki, K., Kato, H., Niwa, O., Nakagawa, T., Matsuda, H., Kawamukai, M., and Murakami, Y. (1999). Characterization of a fission yeast SUMO-1 homologue, pmt3p, required for multiple nuclear events, including the control of telomere length and chromosome segregation. Mol. Cell. Biol. 19, 8660–8672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.