Abstract
The actin cytoskeleton is essential for osteoclasts main function, bone resorption. Two different organizations of actin have been described in osteoclasts, the podosomes belt corresponding to numerous F-actin columns arranged at the cell periphery, and the sealing zone defined as a unique large band of actin. To compare the role of these two different actin organizations, we imaged osteoclasts on various substrata: glass, dentin, and apatite. Using primary osteoclasts expressing GFP-actin, we found that podosome belts and sealing zones, both very dynamic actin structures, were present in mature osteoclasts; podosome belts were observed only in spread osteoclasts adhering onto glass, whereas sealing zone were seen in apico-basal polarized osteoclasts adherent on mineralized matrix. Dynamic observations of several resorption cycles of osteoclasts seeded on apatite revealed that 1) podosomes do not fuse together to form the sealing zone; 2) osteoclasts alternate successive stationary polarized resorption phases with a sealing zone and migration, nonresorption phases without any specific actin structure; and 3) apatite itself promotes sealing zone formation though c-src and Rho signaling. Finally, our work suggests that apatite-mediated sealing zone formation is dependent on both c-src and Rho whereas apico-basal polarization requires only Rho.
INTRODUCTION
Bone is a mineralized tissue that confers multiple mechanical and metabolic functions to the skeleton. It is formed by an organic matrix, called osteoïd, constituted mainly of collagen I but also of numerous noncollagenic proteins, which is ultimately mineralized by calcium apatite crystals (He and George, 2004). Bone homeostasis is maintained through two distinct cell types: osteoblasts synthesizing the osteoïd and the bone resorbing multinucleated osteoclasts. Osteoclast function in bone resorption requires both the dissolution of crystalline apatite and the enzymatic degradation of the organic bone matrix. During bone resorption, osteoclasts are polarized, similarly to epithelial cells, with an apical membrane at the contact with bone and a basolateral membrane at its opposite (Mulari et al., 2003a). Transcytosis occurs by endocytosis of degraded materials at membranes at the matrix level to exocytosis at top of the cells. In bone, protons and enzyme secretions are restricted to the resorption lacuna which is, in turn, limited by a characteristic F-actin organization of the osteoclast, called the sealing zone. In actively resorbing osteoclasts, the sealing zone is formed by a large circular band of actin surrounded by a double ring of vinculin (Vaananen et al., 2000). In osteoclasts seeded on glass or plastic, F-actin is organized in dynamic structures called podosomes, composed of a small F-actin column surrounded by proteins that are also found in focal adhesions such as vinculin and paxillin (Pfaff and Jurdic, 2001). Podosomes were first seen in cells transformed by the v-src oncogene (Marchisio et al., 1987), and since then it has been shown that c-src plays an essential role in sealing zone formation and bone resorption, both in vitro and in vivo. Podosomes have been implicated in extracellular matrix (ECM) degradation, cell invasion, and cell migration in vitro (Linder and Aepfelbacher, 2003). We have previously shown that individual podosomes form part of the subcellular structures that self-organize during the osteoclast differentiation process. Podosome clusters in early osteoclasts evolve into dynamic rings at intermediate stages and end up forming peripheral podosome belts in mature cells (Destaing et al., 2003). Shape and molecular composition analogies between podosome belts and sealing zones of bone resorbing osteoclasts have led to the hypothesis that the sealing zone derives from the podosome belt by fusion of podosomes (Lakkakorpi and Vaananen, 1991, 1996). It is generally believed that on bone, spread osteoclast–exhibiting podosomes belts will get activated for resorption by unknown mechanisms. During this process they become apico-basal polarized with specific submembrane domains and form the sealing zone (Vaananen et al., 2000). Resorption then is associated with polarization and sealing zone formation.
Osteoclasts are able to adhere to various substrates including glass, plastic, bone, dentin, or crystals of various chemical compositions (calcium apatite, calcium carbonate; Jones et al., 1984; Razzouk et al., 1999). Nevertheless, they can only resorb mineralized matrices because it has been shown that demineralized bone cannot be resorbed (Chambers et al., 1984; Nakamura et al., 1996; Yovich et al., 1998). Moreover, during the resorption process osteoclasts are also able to migrate leading to typical resorption trails. Interactions between osteoclasts and ECM are thought to be mediated by specific glycoproteins of the matrix such as fibronectin or vitronectin associating with integrins. αvβ3 integrin is generally regarded as the major receptor in osteoclasts mediating, upon ligation with extracellular proteins, signaling cascades of tyrosine phosphorylation through c-src, paxillin, and c-cbl to cytoskeleton (Sanjay et al., 2001; Nakamura et al., 2003; Miyazaki et al., 2004). Nevertheless, role of integrins does not explain either specific osteoclast activation to resorb bone nor migration at the bone surface.
Rho GTPases are critical actors in actin organization (Etienne-Manneville and Hall, 2002). In the GTP-bound form, they interact and activate various effectors such as Ser/Thr kinases or adaptor proteins, which have been found to be essential for actin remodeling (Ren et al., 1999). Rho and Rac GTPase activities are necessary to maintain podosomes in osteoclast-like cells (Razzouk et al., 1999; Ory et al., 2000). In osteoclasts, RhoA inhibition disrupts sealing zone structure and blocks bone resorption, whereas expression of a constitutively active mutant form of Rho stimulates resorption (Chellaiah et al., 2000). Various organics components of bone matrix are known to be able to induce signaling in osteoclast, but the role of its mineral part has not been taken into account.
To further analyze actin cytoskeletal organization during the osteoclast resorption/migration process, we imaged actin-GFP dynamics in osteoclasts adhering to different substrates, containing apatite or not. We have never observed any resorbing osteoclast exhibiting podosomes. We show here, that on mineralized matrix, resorbing osteoclasts alternate between resorption itself, with an apico-basal polarization together with a sealing zone and migration, with a flat morphology without any specific actin structure. In addition, our results indicate that apatite crystal itself induces sealing zone formation and osteoclast polarization, whereas these two processes could be differently controlled by Rho GTPase and c-Src pathways.
MATERIALS AND METHODS
Reagents
pEGFP-Actin vector was from Clontech (Palo Alto, CA). Recombinant human RANK-L and human M-CSF were produced as previously described (Destaing et al., 2003; Bourette et al., 1993, respectively). Culture media were from Life Technologies (Rockville, MD). The GolgiPlus reagent was from BD Pharmigen (San Diego, CA). The c-src inhibitor PP2 (Calbiochem, San Diego, CA) was used at 10 μM. Global tyrosine phosphorylation was evaluated by immunofluorescence with two antiphosphotyrosine antibodies (4G10) and (PY20; Santa Cruz Biotechnology, Santa Cruz, CA). Anti c-src (clone B-12) was from Santa Cruz Biotechnology; antiphospho-src (p Tyr418) were from Ab-Cam (ab4816; Cambridge, United Kingdom) and Sigma Chemical (St. Louis, MO); antivinculin (clone Vin11–5) was from Sigma and anti-β-tubulin (clone N357) from Amersham Life Science (Piscataway, NJ); mAb 26C4 anti RhoA was a generous gift from Dr. J. Bertoglio (France). F-actin distribution was revealed with Alexa Fluor-546-phalloidin (Molecular Probes, Eugene, OR). Exoenzyme C3, used at 400 nM, was a generous gift from Dr. P. Boquet (INSERM U452, France).
Osteoclast Differentiation
Spleen cells from 6-wk-old OF1 male mice, from actin-GFP mice (precious gift from Dr. Matus, FMI Basel) or RAW actin-GFP (Destaing et al., 2003) were cultured for 8 d on glass coverslips in differentiation medium: α-MEM medium containing 10% fetal calf serum (FCS; Hyclone, Perbio Science, France), M-CSF, and RANK-L (Destaing et al., 2003).
Bone and In Vitro Matrix Support for Resorption Tests
After the differentiation process on plastic dishes, mature osteoclasts were removed after four washes with PBS by using PBS-EDTA 0.25 mM during 30 min, and after centrifugation osteoclasts were seeded on various substratum. Osteoclasts were seeded on dentin slices (a generous gift from Dr Takahashi, Japan) and incubated in differentiation medium for 2 d.
The ACCs (apatite collagen complexes) were prepared using the method described previously by Shibutani et al. (2000). Briefly, glass slides (14-mm diameter, Menzel-Glaser, Braunschweig, Germany) coated with calf skin type I collagen, type III collagen or BSA (Sigma Chemical) were incubated for 7 d at 37°C in 10 ml of 200 mM Tris-buffered saline (TBS) at pH 8.5, containing alkaline phosphatase (0.13 mg/ml), egg yolk phosvitin (0.13 mg/ml), and the cross-linking reagent dimethyl suberimidate hydrochloride (1 mg/ml). The cross-linked, collagen-coated glass slides were then washed several times with TBS to remove unreacted chemicals and byproducts. These glass slides were then incubated for 3 h at 37°C in 10 ml of 200 mM TBS containing alkaline phosphatase (0.13 mg/ml) and egg yolk phosvitin (0.13 mg/ml). Then they were incubated for 20 h at 37°C in 10 ml of 6 mM calcium β-glycerophosphate solution buffered at pH 8.5 with 200 mM TBS. The cycles of 3- and 20-h incubation periods were repeated 7–14 times, depending on the amount of precipitated calcium phosphate. After washing with PBS, the glass slides were dried.
GTP-GTPase Affinity Precipitation Assay
The GST-RBD (Rho-binding domain) construct was kindly provided by Dr. M. Schwartz (Scripps Research Institute, La Jolla, CA) and produced as described (Ren et al., 1999). Analysis for bound GTP-Rho by Western blotting were carried out using mAb 26C4 against RhoA, as described (Ory et al., 2002) and quantified using Image Quant software (Buckinghamshire, United Kingdom).
Scanning Electron Microscopy
Mouse spleen and RAW-derived osteoclast cultures were seeded either on glass coverslips, ACC, or dentin slices for 24 or 48 h before being fixed in glutaraldehyde 1% in Na/diK buffer 0.15 M for 30 min (Sigma Chemical). Samples were dehydrated through a graded ethanol series (50, 70, 95, and 100%), critical point-dried in CO2 (HCP-2; Hitachi, Tokyo, Japan), sputtercoated with a thin layer of gold (E-101; Hitachi), and observed by SEM (S-800 FEG; Hitachi), using an accelerating voltage of 15 kV.
Time-Lapse and Confocal Microscopy
Osteoclast were seeded in 35-mm glass-bottom dishes then transferred to observation medium at 37°C as described (Destaing et al., 2003). Dishes were placed on a thermostated stage, and cells were imaged with a Zeiss Laser Scanning Microscope 510 (Axiovert 100M, Jena, Germany) and a 40× (NA 1.0) Zeiss Plan-Apochromat objective. Meta Imaging Series 4.5 (Universal Imaging, West Chester, PA) was used to make AVI movies (see Supplementary Videos). Quantitation was done using Zeiss LSM 510 software. For immunofluorescence, cells were imaged with a Zeiss LSM 510, using a 63× (NA 1.4) Plan Neo Fluor objective.
FRAP Quantification
For actin-GFP, the life-span of the sealing zone being not much larger than the FRAP fluorescence recovery after photobleaching characteristic time, the fluorescence recovery had to be measured specifically within sealing zone that existed during the whole recovery time. The region corresponding to the sealing zone is located by using the ROI function of LSM 510 software. The interpolation of the experimental data by an exponential law was performed with Igor Pro4.0 (WaveMetrics, Lake Oswego, OR).
X-Ray Diffraction Analysis
The long range orders of crystals, which were synthesized using the method presented above and then dried, were determined by powder x-ray diffraction using a CPS 120 INEL goniometer diffractometer (curved counter) at 30 mA and 50 kV. The Cobalt Kalpha1 radiation was used (1.78892 Å). The individual diagrams were cumulated for 3 h to improve the signal/noise ratio. Hydroxyapatite powder and human bone powder were also analyzed for comparison.
RESULTS
Mature Osteoclasts Form Either Podosome Belt or Sealing Zone According to Their Substratum
Osteoclasts can adhere onto several substrates and then exhibit different actin structures. Classically, when nonresorbing bone, they are supposed to exhibit podosomes, small cylindrical actin structures surrounded by many proteins such as vinculin (Pfaff and Jurdic, 2001). We have shown that along osteoclastogenesis on glass substrate, the pattern of podosomes evolves from clusters in early stages to dynamic rings at intermediate stages to belts organized at the cell periphery in mature osteoclasts (Figure 1A; Destaing et al., 2003). In contrast, when resorbing bone, osteoclasts polarize like epithelial cells with baso-lateral and apical membranes at the bone surface level (Mulari et al., 2003a). Then they exhibit a sealing zone, which is a large band of actin with vinculin on each side delineating the resorption pit where the ruffled membrane is formed and resorption takes place (Figure 1A; Lakkakorpi et al., 1991). It has been proposed that sealing zone results from the fusion of podosomes when osteoclasts resorption is activated (Lakkakorpi and Vaananen, 1991, 1996).
Figure 1.
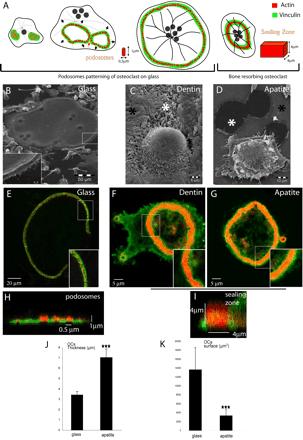
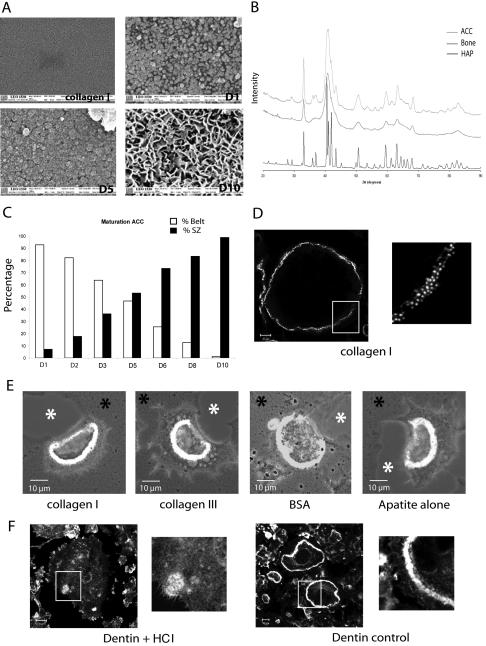
Formation of podosome belt in spread osteoclasts or sealing zone in apico-basal polarized osteoclasts is dependent on extracellular matrix. (A) Scheme of the different actin structures observed in osteoclasts. Osteoclasts seeded on glass form podosomes, small cylinders of actin surrounded by vinculin. Podosomes organize into three different structures along differentiation namely clusters, rings, and belts into mature osteoclasts (Destaing et al., 2003). On bone when resorbing, they form a sealing zone, a large circular band of actin surrounded by vinculin. Differentiated osteoclasts were plated on either glass (B, E, and H), dentin (C–F), or apatite (D, G, and I); scanning electron microscopy images of osteoclasts adherent on their respective substratum are shown (B–D). Mature osteoclasts adherent on glass are large flat cells with a swollen area at site of podosome belt (B, inset). In contrast, on dentin or apatite substrate-resorbing osteoclasts are contracted. Black asterisks indicate nonresorbed matrix and white asterisks resorption pits (C and D). (E) A single osteoclast on a glass coverslip is shown with a podosome belt; the inset is a zoom of the area outlined to show podosomes (bar, 20 μm). (F and G) Osteoclast on dentin and apatite exhibiting a sealing zone; inset is a zoom of the area outlined showing the absence of podosomes and the presence of a large sealing zone (bar, 5 μm). (H and I) Z-cuts of podosome belt and sealing zone. Actin is shown in red and vinculin in green. (J and K) Graphical representation of surface area and thickness of osteoclasts adherent on either glass coverslips or apatite-coated glass coverslips, measured using Zeiss LSM 510 software. Stars indicate statistically significant results using a Student' t test (p < 0.0001; n = 30).
We first asked whether these two actin structures, podosome belt and sealing zone, represent different stages of osteoclast maturation or whether their formation depends on the substratum. After 8 d of differentiation on plastic dishes, mature multinucleated osteoclasts were removed with PBS-EDTA 0.25 mM during 20 min. They were then seeded either on glass coverslips, or ACC glass coverslips, or dentin slices and observed 24 h later. ACC substratum mimics bone matrix (see Figure 3), allowing to directly visualize resorbing cells. As previously described, osteoclasts on glass were spread out (Figure 1B) and exhibited typical podosome belts with polymerized actin dots ∼0.5 μm wide and 1 μm high, surrounded by vinculin (Figure 1, E and H). In contrast, when cells were adherent on mineralized matrix, either dentin or apatite, they rounded up, acquiring an apico-basal polarized phenotype and were able to resorb matrix (Figure 1, C and D). In addition, they exhibited a sealing zone, represented by a large band of actin ∼4 μm wide and 4 μm high surrounded by vinculin (Figure 1, F, G, and I). On glass, the area covered by osteoclasts was about four times greater than osteoclasts on apatite (Figure 1K), whereas they were twice as thin (Figure 1J). So, mature osteoclasts were able to acquire a fully differentiated phenotype, including a sealing zone associated with an apico-basal polarization only in the presence of mineralized matrix.
Figure 3.
Apatite mineral triggers sealing zone formation. (A) Scanning electron microscopy showing the nature of the coating formed along the mineralization process of ACC slides. Apatite particles appeared on day 1, grew, and covered the entire surface by day 5. On day 10, three-dimensional bone-like apatite structures were formed. (B) X-ray diffraction patterns for the substrate ACC in the range of 20–90° 2 theta is presented. As can be seen, ACC substrate showed the characteristic peaks of apatite (002, 310) with some unresolved large broad peaks typical of a poorly crystalline apatitic calcium phosphate. By comparison of the ACC diagram with those of bone samples and hydroxyapatite, we could see that, if the same apatitic phase is common to all the samples, bone as ACC showed a poorly crystalline diagram, compared with hydroxyapatite, which logically presents a highly crystalline phase. (C) Mature osteoclasts were seeded on coverslips at different time points during the mineralization process and stained for actin 24 h later. The percentage of osteoclasts exhibiting podosome belts vs. sealing zones along the mineralization process is shown (n = 200 at each day). (D and E) Whereas osteoclasts seeded on collagen I exhibited podosome belts, they formed sealing zone as long as organic coating (collagen I, collagen III or BSA) was mineralized. (F) In contrast to dentin, osteoclasts adherent on HCl demineralized dentin slices did not form clearly recognizable actin structures.
Sealing Zones Do Not Evolve from Podosome Belts by Podosome Fusion
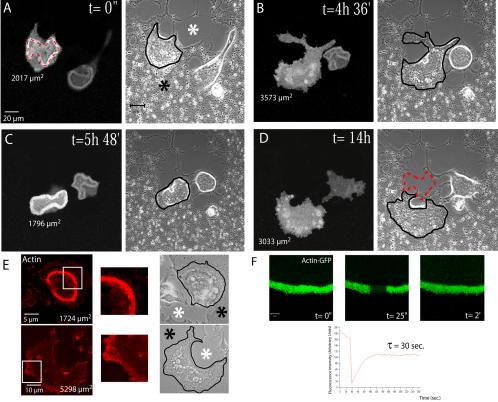
Our results with fixed cells suggest that the formation of a podosome belt is not a prerequisite for the formation of a sealing zone. On the other hand, previous studies had suggested that sealing zones were formed by the fusion of podosomes (Lakkakorpi and Vaananen, 1996). To test these opposite hypotheses we carried out live cell imaging on osteoclasts expressing a recombinant GFP-actin. Mature osteoclasts, differentiated on glass, were plated on ACC glass coverslips and observed by time-lapse confocal microscopy. In the representative example presented in Figure 2 and Supplementary Video 1, we observed two successive cycles of sealing zone formation associated with resorption (Figure 2, A and C) interrupted by two intermediate periods without clearly recognizable actin structures (Figure 2, B and D; t = 4 h 36 min and 14 h). In this experiment, the life span of the sealing zone during a resorption cycle was on average 2 h 15 min. The sealing zones we observed were bona fide, as indicated by their association with resorption activity. They preceded and delineated precisely the resorption pits as exemplified in Figure 2, A and D (sealing zone position is marked with red dotted lines). We imaged several de novo formation of sealing zones, which appear to expand centrifugally from small induction centers. By contrast, we did not observe any podosome belts formation and, obviously, no transition from podosome belts to sealing zone. To rule out the possibility that in these experiments GFP fluorescence was too weak and did not allow actin detection, we performed actin-phalloidin staining on fixed osteoclasts on ACC matrix. We could confirm that they either exhibited a sealing zone or no specific actin structures (Figure 2E). Hence, our observations do not support the hypothesis that podosome fusion leads to the formation of sealing zone and that podosome belts are precursors of the sealing zones.
Figure 2.
Resorbing osteoclasts on apatite alternate resorption with apico-basal polarization and sealing zone together with spread phases to migrate. (A–D) Osteoclasts derived from the spleen of actin-GFP mice were plated on mineralized substratum (ACC). Images were extracted from Supplementary Video 1 and time of imaging is indicated. Mineralized matrix is indicated by the black asterisk and resorbed area by the white asterisk. Cells were successively imaged using a fluorescein-type filter set and by phase contrast. Contours of osteoclasts are underlined in black. Delineation of the sealing zone by the red dotted line (A) indicates that it matches perfectly with resorbed areas (see the same red line on the final light transmission, image D). Osteoclast surface area at each time point is indicated below the cell. A large surface area, >3000 μm2, corresponded to spreading of nonresorbing osteoclasts without sealing zone. In contrast, cell surfaces between 1700 and 2000 μm2 corresponded to actively resorbing osteoclasts exhibiting a sealing zone (bar, 20 μm). (E) Actin of osteoclasts seeded on apatite was stained with phalloidin-RITC to confirming that on apatite, osteoclasts exhibit either sealing zone when polarized or no recognizable actin structures when spread during migration. (F) FRAP analysis of the sealing zone. Images are extracted from one of several experiments. Experimental data fit an exponential law with characteristic time of 30 s.
Apico-basal Polarization Correlates with the Presence of a Sealing Zone
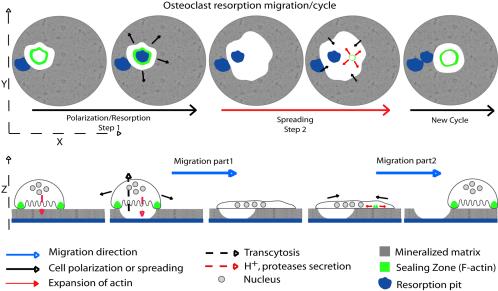
Our observations also pointed to a correlation between osteoclast apico-basal polarization, sealing zone formation and resorption. Indeed, during resorbing phases, cells exhibited a sealing zone and a smaller surface area compared with cells in transition phases lacking the sealing zone (∼1850 vs. 3000 μm2; Figure 2, A–D, and Supplementary Video 1). Hence, when seeded on apatite, osteoclasts display a cycle composed of two distinct steps. First, they initiate a sealing zone, reduce their adhesive surface, and round up. After resorption, a second step starts with the disassembly of the sealing zone and a spreading of the cell body without any characteristic actin structures. This spreading step corresponds to the first part of the migration process; after spreading, the osteoclast reduce again their surface by the formation of a new sealing zone. This contraction corresponds to the second part of the migration process (see Supplementary Video 1 and Figure 7).
Figure 7.
Representation of osteoclast and its actin dynamic during resorption and migration. Schematic representation of osteoclast and its actin cytoskeleton during resorption on apatite mineralized substrate. They alternate between polarized morphology with sealing zone during resorption and spread morphology without any specific actin structures during migration.
Sealing Zones Are Dynamic Actin Structures
We have previously shown that within short lived podosomes, polymerized actin is the site of permanent exchange of monomers, with a characteristic dynamical time of 20–40 s (Destaing et al., 2003). We wondered whether the dynamic sealing zone was also the site of active polymerization/depolymerization for actin. To this end, we performed FRAP analysis on sealing zones from resorbing osteoclasts derived from RAW actin-GFP. Similarly to podosomes, actin recoveries in sealing zone fitted with an exponential law (Figure 2F) with a characteristic dynamical time of 30 s (Figure 2F). Hence, the sealing zone is a site of active actin-remodeling like podosome, which can explain its ability to expand inside the cell. At higher magnification, we imaged small apatite crystals being removed in the immediate proximity of the sealing zone (Supplementary Video 2). This presumably occurred by a partial dissolution of apatite as a result of acidification by the osteoclast.
Crystalline Apatite Triggers Sealing Zone Formation
The nature of the inducing signal of sealing zone formation and bone resorption is still a matter of debate. It has been shown that osteoclasts can resorb any substrates including various minerals (Jones et al., 1984), whereas others have shown that they cannot resorb demineralized bone (Chambers et al., 1984; Nakamura et al., 1996; Yovich et al., 1998). From our experiments reported above, it is clear that sealing zone is observed only when osteoclasts are adherent to a mineralized matrix and in the process of bone resorption. We decided to revisit the role of the bone matrix components in sealing zone formation and apico-basal polarization. We took advantage of the ACC system in which glass coverslips are first coated with collagen I before initiation of a slow mineralization process, which takes place in the presence of β-glycerophosphate, resulting in the formation of apatite mineralized matrices in ∼10 d (Shibutani et al., 2000). We first better characterized the mineralization process by scanning electron microscopy (Figure 3A). Collagen I coating presented an amorphous surface, whereas on day 1 of the mineralization process, the first crystalline apatite nanosize particles were formed (Figure 3A). Later, crystals grew on the surface and were interconnected to cover the entire surface, resulting, on day 10, in the formation of three-dimensional crystals that covered the slide (Figure 3A). X-ray diffraction analysis confirmed that, as published by Shibutani et al. (2000), these crystals corresponded to crystalline apatite similar to bone mineral (Figure 3B).
Mature osteoclasts were seeded on coverslips taken at various stages of the progressive mineralizing process. On collagen I, 100% of osteoclasts exhibited podosome belts as seen on glass or plastic (Figure 3, C and D). On day 1 and 2, when coverslips were still mostly covered by collagen, 93–83% of osteoclasts exhibited a podosome belt, respectively (Figure 3C). As early as day 3, with mineralization progressing, more osteoclasts appeared polarized with few resorption pits and by day 5, half (53%) of the osteoclasts had a sealing zone and the other half still exhibited podosome belts (Figure 3C). The proportion of osteoclasts exhibiting sealing zones then regularly increased up to 98% when seeded on coverslips fully mineralized at day 10 of the mineralization process. These experiments suggested that apatite mineral itself rather than collagen I triggers sealing zone formation.
To emphasize the role played by apatite crystals rather than organic components of the matrix, glass coverslips were either uncoated or coated with various proteins and then mineralized. Osteoclasts seeded on these coverslips were then stained for actin. Figure 3E shows that sealing zones were formed in presence of mineral whether it was coated on collagen I, collagen III, BSA, or glass. This conclusion was reinforced by the following experiments in which osteoclasts were seeded on top of HCl-demineralized dentin. In such conditions, there was no more sealing zone observed when compared with control dentin (Figure 3F).
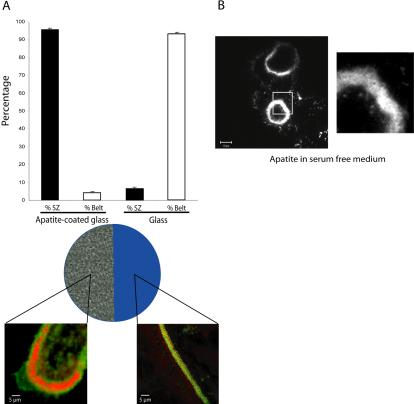
Our results strongly suggest that apatite mineral itself triggers osteoclasts polarization and sealing formation. However, although very unlikely, it was still possible that these two events were induced by microenvironmental factors such as calcium concentrations, serum components or even factors directly secreted by osteoclasts because it has been shown that they can secrete osteopontin, for example (Chellaiah et al., 2003b). To test this possibility we prepared hemislides with half the surface coated with apatite and the other half was glass. For that purpose one half of apatitecoated coverslips was soaked in HCl (6 N) to remove apatite. Then, mature osteoclasts were seeded on these hemislides and stained for actin and vinculin 12 h later. Ninety-three percent of osteoclasts adherent on the glass part of the coverslip showed classical podosome belts, whereas 96% of the osteoclasts adherent on the apatite showed a typical sealing zone (Figure 4A), thus ruling out the potential role of serum or medium components. In addition, when seeded on dentin in serum free medium, osteoclasts still exhibited a sealing zone (Figure 4B). Finally, osteoclasts adherent on apatite were treated 6 h in presence of 1 μg/ml GolgiPlug to block Golgi apparatus and then protein secretion. In such conditions osteoclasts still exhibited sealing zone (unpublished data). Therefore, we can conclude that formation of the sealing zone requires adhesion to apatite alone and is not mediated by other factors.
Figure 4.
Sealing zone formation is not triggered by microenvironmental modifications. (A) To further confirm the role of extracellular matrix on osteoclast actin organization, we used apatite-coated slides. Half the slide was first immersed in HCl (6 N) to remove apatite crystals. Mature osteoclasts were then seeded on these slides for 24 h. Osteoclasts exhibit sealing zones when adherent on the mineral part and podosome belts on glass. The graph represents the percentage of osteoclasts exhibiting podosome belts or sealing zones according to the substratum (n = 150). (B) Mature adherent osteoclasts still exhibit a sealing zone on apatite in serum-free medium ruling out the putative role of medium components.
C-src Is Required for Apatite-dependent Sealing Zone Formation
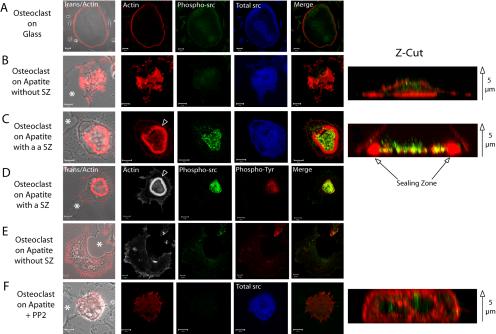
The fact that apatite crystals induced sealing zone formation in osteoclasts was reminiscent of neutrophil activation by monosodium urate monohydrate crystals in acute gouty arthritis (Gilbert et al., 2003). This prompted us to check whether apatite was triggering signal transduction pathways at the adhesion site. According to the well described role played by c-src phosphorylation in osteoclast adhesion and resorption (Violette et al., 2001; Lakkakorpi et al., 2003), we checked whether c-src could be involved in sealing zone formation. We compared, by immunofluorescence, both the level and subcellular localization of total as well as activated c-src, using two different antibodies. In unpolarized osteoclasts exhibiting, either a podosome belt on glass (Figure 5A) or spread on apatite (Figure 5B), levels of total or phosphorylated c-src were comparable and diffusely expressed within cells. In sharp contrast, in polarized apatite resorbing osteoclasts (Figure 5C), phosphorylated c-src showed increased expression and relocalization within the actin ring of the sealing zone, at the level of the ruffled membrane, as shown on the Z-cut section. We verified that c-src was the main tyrosine phosphorylated target by repeating these experiments with both antiactivated c-src and two antiphosphotyrosine antibodies. Figure 5D clearly indicated that, in apatite resorbing osteoclasts exhibiting a sealing zone, phosphorylated c-src colocalized with proteins phosphorylated on tyrosine within the sealing zone, suggesting that it represents an essential tyrosine kinase. To further confirm the major role played by c-src, we made use of PP2 a c-src family inhibitor. Osteoclasts seeded on apatite were treated for 1 h in the presence of PP2, and actin organization, as well as total and activated c-src, were observed by confocal microscopy. As expected, c-src inhibition suppressed the sealing zone, whereas the total c-src level remained unaltered (Figure 5F). However, PP2-mediated c-src inhibition did not modify osteoclast polarization, as shown by Z-cut reconstitution (compare Z-cuts in Figure 5F with unpolarized osteoclasts in Figure 5B and polarized osteoclasts in Figure 5C; Supplementary Video 3). These data confirmed previous results obtained on isolated osteoclasts from c-src–/– mice, showing a defect in bone resorption linked with the absence of sealing zone (Soriano et al., 1991; Horne et al., 1992). Altogether, these results indicate that in osteoclasts, apatite triggers c-src activation and relocalization, both of which are necessary for sealing zone formation but not for polarization.
Figure 5.
c-src is required for apatite-dependent sealing zone formation but not for polarization. Mature osteoclasts seeded for 24 h on glass or on ACC and associated with resorption pits (asterisk) were observed by confocal microscopy after actin staining by phalloidin or immunolabelling with antic-src or antiphosphotyrosine antibodies. Z-cut sections are provided to confirm polarization (actin in red) and phosphorylated c-src localization (green). Arrow: from bottom to top of the cells. (A) On glass, osteoclasts exhibited typical peripheral podosome belts with diffuse total and activated c-src (bar, 20 μm). (B) On apatite, in the absence of sealing zone, actin and c-src were distributed throughout the cells, whereas (C) in the presence of a sealing zone (arrowhead), activated c-src labeling was more intense and localized within the area delineated by the sealing zone. Merged images of actin and phosphorylated c-src are presented (A and F; bar, 10 μm). (D) Phosphorylated c-src is the main phosphotyrosine target in bone resorbing osteoclasts as shown by their precise colocalization within the sealing zone area, whereas (E) they are diffusely distributed in its absence (bar, 10 μm). (F) After 1 h of PP2 treatment of osteoclasts adherent on apatite, osteoclasts still associated with resorption pits had lost their sealing zone, and activated c-src and phosphotyrosine labeling was diffuse (bar, 10 μm). Nevertheless, cells were still polarized as shown by the Z-cut section. Merged images of phosphorylated c-src and phosphotyrosine proteins are presented (D and E).
Rho GTPase-mediated Sealing Zone Formation
In addition to tyrosine phosphorylation and src activation, the activity of Rho GTPase is essential for the polarization of osteoclasts and their resorption activity (Chellaiah et al., 2000). We previously reported that treatment with the specific Rho inhibitor exoenzyme C3 induces an extensive spreading of osteoclast-like cells (Ory et al., 2000). To test whether Rho was also implicated in the formation of the apatite-dependent sealing zone, mature osteoclasts were seeded either on glass or on apatite and treated with C3 toxin at 400 nM for 5 h. Time-lapse or classical confocal microscopy observation after labeling of F-actin revealed that, in osteoclasts adherent on apatite, Rho inhibition induced a transition from a rounded, polarized morphology to one that was flat and spread out (Figure 6, A–C), similar to osteoclasts maintained on glass (Figure 6, B–D and F). Interestingly, in the presence of C3, the sealing zones (Figure 6, E and H) were replaced by podosome belts (Figure 6, G and I, and Supplementary Video 4). Altogether, these results suggested that the Rho GTPase is activated in response to osteoclast adhesion to the apatite matrix. To test this hypothesis, we compared the Rho-GTP content of osteoclasts adherent on plastic vs. osteoclasts adherent on apatite-coated coverslips, by affinity precipitation assays, using GST-RBD. Twelve hours after seeding, the Rho activation levels increased sharply on apatite in comparison with plastic (Figure 6, J and K), indicating that in osteoclasts, Rho activity is required for the formation of the apatite-dependent sealing zone and polarization. We addressed the question of whether c-src was acting upstream or downstream of Rho. Osteoclasts were seeded on plastic or apatite and treated with PP2 and the Rho-GTP level assessed. On plastic seeded osteoclasts, c-src inhibition resulted in a 3.5× increase in GTP bound Rho (Figure 6, J and K), whereas in contrast, on apatite, c-src inhibition did not modify significantly the Rho-GTP/total Rho ratio (Figure 6, J and K), suggesting that under these latter conditions, the Rho and c-src pathways could be separated.
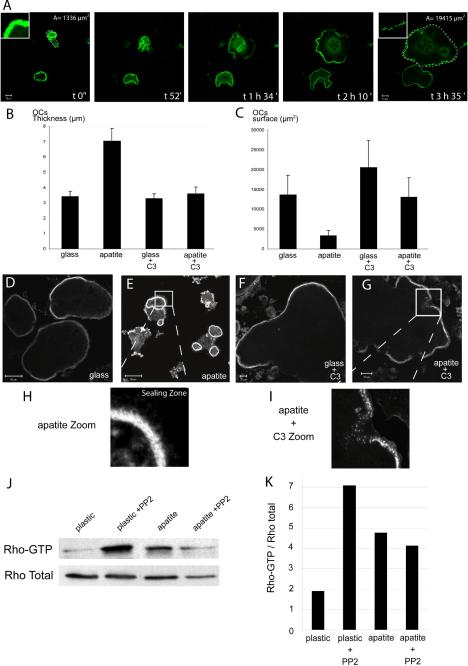
Figure 6.
Rho is required for sealing zone formation and polarization of osteoclast on apatite. Mature osteoclasts adherent on glass or apatite-coated slides were either untreated or treated with exoenzyme C3 (100 nM) for 5 h before being observed by confocal microscopy after actin staining. (A) Images of resorbing osteoclasts on apatite taken at different time point show the transition from polarized (1336 μm2 surface area) to largely spread cells (1945 μm2 at time 3 h 35 min) as well as the transition from sealing zone to podosome belt (insets). (B and C) Graphical representation of the thickness and surface area of osteoclasts, seeded either on glass or apatite and treated, or not, with C3. Mineral substratum increased osteoclast average thickness (B) and decreased surface area (C and E) compared with glass, (C and D) indicating that they were polarized (n = 30), as measured using Zeiss LSM 510 software. Exoenzyme C3–mediated Rho inhibition completely abrogated apatite-dependent sealing zone formation. In the presence of exoenzyme C3, osteoclasts were flat and spread (B, C, F, and G). Osteoclasts seeded on apatite, in the presence of C3, exhibited podosome belts (G and I) instead of the sealing zones seen in untreated cells (E and H). (J) Differentiated osteoclasts were seeded on plastic or apatite for 12 h, and Rho activity was assessed by pull-down binding assays. After 12 h on apatite, the level of Rho-GTP was clearly increased compared with plastic. In presence of PP2 after 12 h on plastic, the level of Rho-GTP increased, but was maintained on the apatite substratum. This experiment has been repeated three times with similar results, and one of them is shown here. (K) Rho GTP vs. total Rho ratio was evaluated by Western blotting and quantitated using Image Quant software.
DISCUSSION
The actin network organization in multinucleated osteoclast is rather different than in other cell types because two different structures have been described so far, podosomes and sealing zone, but the relationship between these two structures is not yet clear. Our study provides the first dynamic study of the actin based sealing zone in resorbing osteoclasts and then provide new information on its formation, its regulation, and its function. We have characterized three different stages of osteoclasts with different actin structures. On uncoated or protein-coated glass coverslips they are large flat cells with podosomes, whereas on mineralized substrates they exhibit two different morphologies to allow resorption and migration. During resorption per se, they are apico-basally polarized, as epithelial cells, with a sealing zone at their basal level but then spread, without any recognizable actin structures. It has to be noticed that an osteoclast associated with a resorption pit can be either polarized when in the process of resorption or spread when in the migrating phase. These successive events between stationary resorption phases of polarized osteoclasts exhibiting sealing zone and spread nonresorption phases are responsible for migration as an inchworm like progression (Figure 7). We can speculate that these cycles of cell rounding and cell spreading in absence of typical actin structures usually observed in other migrating cells could be driven entirely by regulating myosin II contraction in the cell cortex. Indeed, myosin II has been shown to be localized in resting and activated osteoclasts, giving some basis for this hypothesis (Krits et al., 2002).
By using actin-GFP–expressing osteoclasts, we have previously identified podosome patterning in differentiating osteoclast adherent onto glass (Destaing et al., 2003) and here we characterized sealing zone. Both structures are clearly different in size since podosomes are small cylinders of actin of ∼0.5-μm diameter and 1-μm height. They are associated together as clusters in early osteoclasts, which evolve along osteoclastogenesis into dynamic unstable rings to end up in mature osteoclast into belts at the cell periphery (Destaing et al., 2003). In contrast, sealing zone is a continuous band of actin of 4 μm large for 4 μm height. FRAP experiments have revealed that both structures are sites of constant flux of actin with similar characteristic dynamical recovery time of about 30 s. Taking into account the large difference of size between individual podosome and sealing zone, we can conclude that actin dynamic in the sealing zone is higher than in the podosome. Using immunofluorescence on fixed osteoclasts, it has been proposed that during activation of resorption, podosome belts fuse together to form the sealing zone (Lakkakorpi and Vaananen, 1996). In our experimental set up, using apatite-coated coverslips mimicking bone mineral as assessed by x-ray diffraction, we have been able to follow live osteoclasts expressing actin-GFP. From all our observations we can first conclude that podosomes are not formed when osteoclasts are adherent onto mineralized substrates and second, that podosome belts are not precursor of the sealing zone. This raises the intriguing question of the role, if any, played by podosomes in osteoclasts. Moreover, our results confirm earlier results that sealing zone is the specific actin structure for bone resorption (Lakkakorpi et al., 1993) and suggest that all components necessary for mineral dissolution, such as proton pump or vacuolar ATPase, could be closely associated to the sealing zone as recently observed (Mulari et al., 2003b).
Bone resorption by osteoclasts is thought to be mediated by integrins, and specially vitronectin receptor heterodimer αVβ3 through interactions with mainly glycoproteins of the ECM (Faccio et al., 2002). Nevertheless, in β3 knock-out mice, characterized by an osteosclerosis, the bone phenotype is not due to a complete inhibition of osteoclast activity as for c-src knock-out. This observation suggests that this integrin could be only partially implicated in osteoclast-mediated bone resorption (McHugh et al., 2000; Faccio et al., 2003). To resorb bone in physiological conditions, osteoclast activity can be divided into three main steps, namely, adhesion onto bone, resorption per se, and migration. Our results, presented here indicate that apatite mineral is able to activate resorption that means apico-basal polarization and sealing zone formation. Differentiated osteoclasts must express an unknown receptor that senses mineralized substrate surfaces and can activate c-src and Rho GTPase simultaneously. Rho is known to be necessary for sealing zone formation and plays a crucial role in the resorption process (Chellaiah et al., 2000, 2003a). We have shown here that Rho activation is mediated by apatite. Moreover, Rho inhibition in apatite resorbing osteoclasts disrupt apico-basal polarization and sealing zones, which reorganize into podosome belts as observed in osteoclasts seeded on glass. Nevertheless, if Rho activation is necessary for sealing zone formation, it is not sufficient because expression of the constitutively activated form of Rho (Rho V14) in glass-adherent osteoclasts does not allow sealing zone formation (unpublished data). We can propose that apatite-mediated Rho activation is a part of the sealing zone formation and of the apico-basal polarization of resorbing osteoclasts. Similarly, in response to apatite-mediated signals, c-src tyrosine kinase is activated, but overall relocalized inside the sealing zone. c-src has been known for a long time as necessary for the resorbing activity of osteoclasts because its inactivation in mice leads to severe osteopetrosis due to the presence of numerous inactive osteoclasts at the bone surface (Soriano et al., 1991; Horne et al., 1992). We have shown that src is implicated in the sealing zone formation but not in the apico-basal polarization. We propose that this difference is due to c-src relocalization, which, in resorbing osteoclasts, may be in separate compartments than activated Rho. This also indicates that the signaling pathways of osteoclasts are different when seeded on bone or nonphysiological matrices, implying different membrane receptors.
At present, we can only speculate on the nature of this apatite receptor. It is known that apatite can bind molecules such as proteins, because it is used as a chromatographic support for protein purification (Putnam and Takahashi, 1988). Moreover, it is known that immune cells are responsive to signals induced by urate crystals in gouty arthritis (Gilbert et al., 2003). Therefore, the existence of a specific receptor for mineral crystals, at the membrane surface of osteoclasts is not unlikely. In addition, it has been reported that osteoclasts can as well resorb avian egg shell or mollusk shell containing calcite and aragonite crystals, respectively (Jones et al., 1984). However, our results raise the possibility that it is not apatite per se that is recognized but its three-dimensional structure. Indeed, during the slow mineralization process of ACC coverslips, mature osteoclasts exhibit mostly sealing zones, instead of podosome belts, after only 5 or 6 d of mineralization (Figure 3), whereas apatite already covers the collagen coat as soon as day 1 or 2. This would fit with the possibility that apatite has to mature and organize to trigger signaling in osteoclasts. Identification of such a receptor will be the next challenge, because it could be an interesting therapeutic target for bone diseases in which osteoclasts are implicated.
Supplementary Material
Acknowledgments
We thank M. Mazzorana for help in electron microscopy and S. Ory, I. Machuca, E. Bonnelye, and M. Pfaff for critical reading of the manuscript. This work was supported by Ligue contre le cancer (Rhône) and ARC and CNRS (dynamique et réactivité des assemblages biologiques). O.D. and F.S. are recipients of MENRT grants.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04–06–0522. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–06–0522.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Bourette, R.P., Mouchiroud, G., Ouazana, R., Morle, F., Godet, J., and Blanchet, J.P. (1993). Expression of human colony-stimulating factor-1 (CSF-1) receptor in murine pluripotent hematopoietic NFS-60 cells induces long-term proliferation in response to CSF-1 without loss of erythroid differentiation potential. Blood 81, 2511–2520. [PubMed] [Google Scholar]
- Chambers, T.J., Thomson, B.M., and Fuller, K. (1984). Effect of substrate composition on bone resorption by rabbit osteoclasts. J. Cell Sci. 70, 61–71. [DOI] [PubMed] [Google Scholar]
- Chellaiah, M.A., Biswas, R.S., Rittling, S.R., Denhardt, D.T., and Hruska, K.A. (2003a). Rho-dependent Rho Kinase activation increases CD44 surface expression and bone resorption in osteoclasts. J. Biol. Chem. 278, 29086–29097. [DOI] [PubMed] [Google Scholar]
- Chellaiah, M.A., Kizer, N., Biswas, R., Alvarez, U., Strauss-Schoenberger, J., Rifas, L., Rittling, S.R., Denhardt, D.T., and Hruska, K.A. (2003b). Osteopontin deficiency produces osteoclast dysfunction due to reduced CD44 surface expression. Mol. Biol. Cell 14, 173–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellaiah, M.A., Soga, N., Swanson, S., McAllister, S., Alvarez, U., Wang, D., Dowdy, S.F., and Hruska, K.A. (2000). Rho-A is critical for osteoclast podosome organization, motility, and bone resorption. J. Biol. Chem. 275, 11993–12002. [DOI] [PubMed] [Google Scholar]
- Destaing, O., Saltel, F., Geminard, J.C., Jurdic, P., and Bard, F. (2003). Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol. Biol. Cell 14, 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville, S., and Hall, A. (2002). Rho GTPases in cell biology. Nature 420, 629–635. [DOI] [PubMed] [Google Scholar]
- Faccio, R., Grano, M., Colucci, S., Villa, A., Giannelli, G., Quaranta, V., and Zallone, A. (2002). Localization and possible role of two different alpha v beta 3 integrin conformations in resting and resorbing osteoclasts. J. Cell Sci. 115, 2919–2929. [DOI] [PubMed] [Google Scholar]
- Faccio, R., Novack, D.V., Zallone, A., Ross, F.P., and Teitelbaum, S.L. (2003). Dynamic changes in the osteoclast cytoskeleton in response to growth factors and cell attachment are controlled by {beta}3 integrin. J. Cell Biol. 162, 499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, C., Poubelle, P.E., Borgeat, P., Pouliot, M., and Naccache, P.H. (2003). Crystal-induced neutrophil activation: VIII. Immediate production of prostaglandin E2 mediated by constitutive cyclooxygenase 2 in human neutrophils stimulated by urate crystals. Arthritis Rheum. 48, 1137–1148. [DOI] [PubMed] [Google Scholar]
- He, G., and George, A. (2004). Dentin matrix protein 1 immobilized on type I collagen fibrils facilitates apatite deposition in vitro. J. Biol. Chem. 279, 11649–11656. [DOI] [PubMed] [Google Scholar]
- Horne, W.C., Neff, L., Chatterjee, D., Lomri, A., Levy, J.B., and Baron, R. (1992). Osteoclasts express high levels of pp60c-src in association with intracellular membranes. J. Cell Biol. 119, 1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, S.J., Boyde, A., and Ali, N.N. (1984). The resorption of biological and non-biological substrates by cultured avian and mammalian osteoclasts. Anat. Embryol. (Berl) 170, 247–256. [DOI] [PubMed] [Google Scholar]
- Krits, I., Wysolmerski, R.B., Holliday, L.S., and Lee, B.S. (2002). Differential localization of myosin II isoforms in resting and activated osteoclasts. Calcif. Tissue Int. 71, 530–538. [DOI] [PubMed] [Google Scholar]
- Lakkakorpi, P.T., Bett, A.J., Lipfert, L., Rodan, G.A., and Duong le, T. (2003). PYK2 autophosphorylation, but not kinase activity, is necessary for adhesion-induced association with c-Src, osteoclast spreading, and bone resorption. J. Biol. Chem. 278, 11502–11512. [DOI] [PubMed] [Google Scholar]
- Lakkakorpi, P.T., Helfrich, M.H., Horton, M.A., and Vaananen, H.K. (1993). Spatial organization of microfilaments and vitronectin receptor, alpha v beta 3, in osteoclasts. A study using confocal laser scanning microscopy. J. Cell Sci. 104(Pt 3), 663–670. [DOI] [PubMed] [Google Scholar]
- Lakkakorpi, P.T., Horton, M.A., Helfrich, M.H., Karhukorpi, E.K., and Vaananen, H.K. (1991). Vitronectin receptor has a role in bone resorption but does not mediate tight sealing zone attachment of osteoclasts to the bone surface. J. Cell Biol. 115, 1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkakorpi, P.T., and Vaananen, H.K. (1991). Kinetics of the osteoclast cytoskeleton during the resorption cycle in vitro. J. Bone Miner. Res. 6, 817–826. [DOI] [PubMed] [Google Scholar]
- Lakkakorpi, P.T., and Vaananen, H.K. (1996). Cytoskeletal changes in osteoclasts during the resorption cycle. Microsc. Res. Tech. 33, 171–181. [DOI] [PubMed] [Google Scholar]
- Linder, S., and Aepfelbacher, M. (2003). Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 13, 376–385. [DOI] [PubMed] [Google Scholar]
- Marchisio, P.C., Cirillo, D., Teti, A., Zambonin-Zallone, A., and Tarone, G. (1987). Rous sarcoma virus-transformed fibroblasts and cells of monocytic origin display a peculiar dot-like organization of cytoskeletal proteins involved in microfilament-membrane interactions. Exp. Cell Res. 169, 202–214. [DOI] [PubMed] [Google Scholar]
- McHugh, K.P. et al. (2000). Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J. Clin. Invest. 105, 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki, T., Sanjay, A., Neff, L., Tanaka, S., Horne, W.C., and Baron, R. (2004). Src kinase activity is essential for osteoclast function. J. Biol. Chem. 279, 17660–17666. [DOI] [PubMed] [Google Scholar]
- Mulari, M., Vaaraniemi, J., and Vaananen, H.K. (2003a). Intracellular membrane trafficking in bone resorbing osteoclasts. Microsc. Res. Tech. 61, 496–503. [DOI] [PubMed] [Google Scholar]
- Mulari, M.T., Zhao, H., Lakkakorpi, P.T., and Vaananen, H.K. (2003b). Osteoclast ruffled border has distinct subdomains for secretion and degraded matrix uptake. Traffic 4, 113–125. [DOI] [PubMed] [Google Scholar]
- Nakamura, I., Rodan, G.A., and Duong le, T. (2003). Distinct roles of p130Cas and c-Cbl in adhesion-induced or macrophage colony-stimulating factor-mediated signaling pathways in prefusion osteoclasts. Endocrinology 144, 4739–4741. [DOI] [PubMed] [Google Scholar]
- Nakamura, I., Takahashi, N., Sasaki, T., Jimi, E., Kurokawa, T., and Suda, T. (1996). Chemical and physical properties of the extracellular matrix are required for the actin ring formation in osteoclasts. J. Bone Miner. Res. 11, 1873–1879. [DOI] [PubMed] [Google Scholar]
- Ory, S., Destaing, O., and Jurdic, P. (2002). Microtubule dynamics differentially regulates Rho and Rac activity and triggers Rho-independent stress fiber formation in macrophage polykaryons. Eur. J. Cell Biol. 81, 351–362. [DOI] [PubMed] [Google Scholar]
- Ory, S., Munari-Silem, Y., Fort, P., and Jurdic, P. (2000). Rho and Rac exert antagonistic functions on spreading of macrophage-derived multinucleated cells and are not required for actin fiber formation. J. Cell Sci. 113(Pt 7), 1177–1188. [DOI] [PubMed] [Google Scholar]
- Pfaff, M., and Jurdic, P. (2001). Podosomes in osteoclast-like cells: structural analysis and cooperative roles of paxillin, proline-rich tyrosine kinase 2 (Pyk2) and integrin alphaVbeta3. J. Cell Sci. 114, 2775–2786. [DOI] [PubMed] [Google Scholar]
- Putnam, F.W., and Takahashi, N. (1988). Structural characterization of glycoproteins. J. Chromatogr. 443, 267–284. [DOI] [PubMed] [Google Scholar]
- Razzouk, S., Lieberherr, M., and Cournot, G. (1999). Rac-GTPase, osteoclast cytoskeleton and bone resorption. Eur. J. Cell Biol. 78, 249–255. [DOI] [PubMed] [Google Scholar]
- Ren, X.D., Kiosses, W.B., and Schwartz, M.A. (1999). Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18, 578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjay, A. et al. (2001). Cbl associates with Pyk2 and Src to regulate Src kinase activity, alpha(v)beta(3) integrin-mediated signaling, cell adhesion, and osteoclast motility. J. Cell Biol. 152, 181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibutani, T., Iwanaga, H., Imai, K., Kitago, M., Doi, Y., and Iwayama, Y. (2000). Use of glass slides coated with apatite-collagen complexes for measurement of osteoclastic resorption activity. J. Biomed. Mater. Res. 50, 153–159. [DOI] [PubMed] [Google Scholar]
- Soriano, P., Montgomery, C., Geske, R., and Bradley, A. (1991). Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 64, 693–702. [DOI] [PubMed] [Google Scholar]
- Vaananen, H.K., Zhao, H., Mulari, M., and Halleen, J.M. (2000). The cell biology of osteoclast function. J. Cell Sci. 113(Pt 3), 377–381. [DOI] [PubMed] [Google Scholar]
- Violette, S.M. et al. (2001). Bone-targeted Src SH2 inhibitors block Src cellular activity and osteoclast-mediated resorption. Bone 28, 54–64. [DOI] [PubMed] [Google Scholar]
- Yovich, S., Seydel, U., Papadimitriou, J.M., Nicholson, G.C., Wood, D.J., and Zheng, M.H. (1998). Evidence that failure of osteoid bone matrix resorption is caused by perturbation of osteoclast polarization. Histochem. J. 30, 267–273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.