Abstract
Current imaging diagnostic techniques are often insensitive to the underlying pathological changes following mild traumatic brain injury (TBI) or concussion so much so that the explicit definition of these uncomplicated mild brain injuries includes the absence of radiological findings. In the US military, this is complicated by the natural tendency of service members to down play symptoms for fear of removal from their unit particularly in combat making it challenging for clinicians to definitively diagnose and determine course of treatment. Questions remain regarding the long-term impact of these war-time brain injuries. The objective of the current study was to evaluate the long-term imaging sequelae of blast concussion in active-duty US military and leverage previous longitudinal data collected in these same patients to identify predictors of sustained DTI signal change indicative of chronic neurodegeneration. In total, 50 blast TBI and 44 combat-deployed controls were evaluated at this 5-year follow up by advanced neuroimaging techniques including diffusion tensor imaging and quantitative volumetry. While cross-sectional analysis of regions of white matter on DTI images did not reveal significant differences across groups after statistical correction, an approach flexible to the heterogeneity of brain injury at the single-subject level identified 74% of the concussive blast TBI cohort to have reductions in fractional anisotropy indicative of chronic brain injury. Logistic regression leveraging clinical and demographic data collected in the acute/sub-acute and 1-year follow up to determine predictors of these long-term imaging changes determined that brain injury diagnosis, older age, verbal memory and verbal fluency best predicted the presence of DTI abnormalities 5 years post injury with an AUC of 0.78 indicating good prediction strength. These results provide supporting evidence for the evolution not resolution of this brain injury pathology, adding to the growing body of literature describing imaging signatures of chronic neurodegeneration even after mild TBI and concussion.
Abbreviations: A-P, anterior–posterior; DR-BUDDI, Diffeomorphic Registration for Blip-Up blip-Down Diffusion Imaging; DTI, Diffusion Tensor Imaging; EPI, Echo Planar Imaging; EPV, events-per-variable; FA, Fractional Anisotropy; FLAIR, Fluid attenuation inversion recovery; MPRAGE, Magnetization prepared rapid gradient-echo; TBI, Traumatic Brain Injury; TORTOISE, Tolerably Obsessive Registration and Tensor Optimization Indolent Software Ensemble; US, United States
Keywords: Diffusion tensor imaging, Concussion, Neurodegeneration, Traumatic brain injury
Highlights
-
•
Design: prospective, observational, longitudinal research study
-
•
Patients: concussive blast (n = 50), combat-deployed control (n = 44)
-
•
Diffusion tensor imaging analyzed 5 yr post-injury, highly predicted by 1 yr outcomes.
-
•
Imaging abnormalities appear to evolve from sub-acute, to 1-year, to 5-year scan.
-
•
Findings indicate chronic neurodegeneration in majority of blast concussion patients.
1. Introduction
In the US military, it is estimated that roughly 350,000 service members suffered a head injury. Of these, 82.3% had a mild, uncomplicated TBI or concussion (DVBIC, 2016), the long-term impact of which is just beginning to be appreciated. Many of these service members are young males, 20–30 years old, who have decades of life to live with the complex and often debilitating impact of war-time brain injury. A challenge with this mild/concussive brain injury population is that our current diagnostic techniques are often insensitive to the underlying pathological changes. As such, the explicit definition of these uncomplicated mild brain injuries includes the absence of radiological findings on CT and/or conventional MRI (Casscells, 2007). This leaves ambiguity over the true nature of the patient's exposure and reliance of the clinician on the self-endorsement from the patient regarding the specific details of the event.
In populations, such as the military and those in athletics, there exists an inherent motivation to ‘down-play’ or omit details of the event for fear of being removed from combat or play. Interest has grown in new imaging techniques that may be sensitive to the subtler underlying pathophysiological changes hypothesized to occur during a concussion, offering a more unbiased approach to screening and evaluation. Diffusion tensor imaging (DTI) (Pierpaoli et al., 1996) is one such MRI application thought to provide greater sensitivity to microstructural white matter changes sustained at the time of injury. It has been shown in preclinical models of TBI (Mac Donald et al., 2007a, Mac Donald et al., 2007b) and concussion (Bennett et al., 2012, Brody et al., 2015) to be highly correlated with brain injury pathology.
While the application of DTI has been fairly well explored in civilian mild TBI research for more than a decade (Arfanakis et al., 2002, Bazarian et al., 2007, Inglese et al., 2005, Niogi and Mukherjee, 2010, Niogi et al., 2008a, Niogi et al., 2008b), the approach has more recently gained interest in military service members with mild or concussive brain injury (Bazarian et al., 2013, Hayes et al., 2015, Jorge et al., 2012, Mac Donald et al., 2013, Mac Donald et al., 2011, Miller et al., 2016) in an attempt to elucidate microstructural changes following in particular blast exposures. The current literature has primarily utilized cross-sectional studies of veterans already separated from the service with reliance on retrospective endorsement of exposures and self report (Bazarian et al., 2013, Hayes et al., 2015, Jorge et al., 2012, Levin et al., 2010, Matthews et al., 2012, Miller et al., 2016, Morey et al., 2013, Sorg et al., 2014, Taber et al., 2015, Trotter et al., 2015). While few studies have been provided the opportunity to examine active-duty US military (Ware et al., 2016, Yeh et al., 2014), to our knowledge we maintain one of the very few prospective, observational, longitudinal studies that incorporate imaging and clinical evaluation following combat-deployed service members from the time of injury (Adam et al., 2015, Mac Donald et al., 2011) to multiple points of long-term outcome including 1-year (Han et al., 2014, Mac Donald et al., 2011, Mac Donald et al., 2016, Mac Donald et al., 2014, Macdonald et al., 2014) and now 5-year evaluation. The objective of the current study was to evaluate the long-term imaging sequelae of blast concussion in active-duty US military and leverage the previous longitudinal data collected in these patients to identify predictors of sustained DTI signal change indicative of chronic neurodegeneration.
2. Materials and methods
Participants in this study were originally enrolled into one of four previous cohorts (Adam et al., 2015, Mac Donald et al., 2015, Mac Donald et al., 2011, Mac Donald et al., 2016, Mac Donald et al., 2014, Macdonald et al., 2014). This is the 5-year evaluation in a continued prospective, observational, longitudinal research study. In this publication, we report the 5-year imaging outcomes in comparison to our 1-year clinical outcomes (Mac Donald et al., 2016, Mac Donald et al., 2014) in our two main subject groups; concussive blast TBI and combat-deployed control. Inclusion criteria have been reported elsewhere (Mac Donald et al., 2015, Mac Donald et al., 2011, Mac Donald et al., 2016). Briefly, participants were service members, deployed to the combat theater, between 2008 and 2013, in which original enrollment was completed either directly in Afghanistan (Adam et al., 2015, Mac Donald et al., 2015) or following medical evacuation to Landstuhl Regional Medical Center in Germany (Mac Donald et al., 2011, Mac Donald et al., 2016, Mac Donald et al., 2014, Macdonald et al., 2014). Diagnosis of head injury was determined by trained medical personnel working in the TBI clinics in Afghanistan or Germany. For the concussive blast TBI group, all available clinical histories indicated blast exposure plus another mechanism of head injury such as a fall, motor vehicle crash, or being struck by a blunt object. None suffered an isolated blast injury. All concussive blast TBI subjects met the Department of Defense definition for mild, uncomplicated traumatic brain injury. All combat-deployed controls were clinically evaluated to be free of signs and symptoms of head injury with no history of TBI diagnosis, psychiatric diagnosis and no history of blast exposure.
This study was approved by the University of Washington Institutional Review Board with additional approval from the US Army Medical Research and Materiel Command Institutional Review Board, and carried out in accordance with the approved protocol. Reconsent for this 5-year evaluation was provided by all participants; no surrogate consent was allowed. Active-duty military subjects were not paid for participation per government guidelines, though travel expenses to the University of Washington in Seattle were covered. Service members who had since separated from the service by the time of this 5-year follow-up were paid $250 plus travel expenses for participation.
2.1. Imaging acquisition
MRI scans were completed on a 3T Philips Achieva with a 32-channel head coil. Each imaging session lasted ~ 31 min and included a 1 mm isotropic MPRAGE (5:13), 1 mm isotropic 3D T2-weighted image (5:22), 1 mm isotropic 3D T2-Star (3:41), 2D FLAIR collected at an in-plane resolution of 1 × 1 mm with a slice thickness of 4 mm, no gaps (2:56), and a 32 direction 2 mm isotropic diffusion sequence acquired with reverse polarity (A-P, P-A), b = 1000 s/mm2, and 6 non-diffusion weighted images for diffusion tensor imaging (DTI) analysis (each 6:39).
2.2. Diffusion tensor imaging (DTI) post-processing and analysis
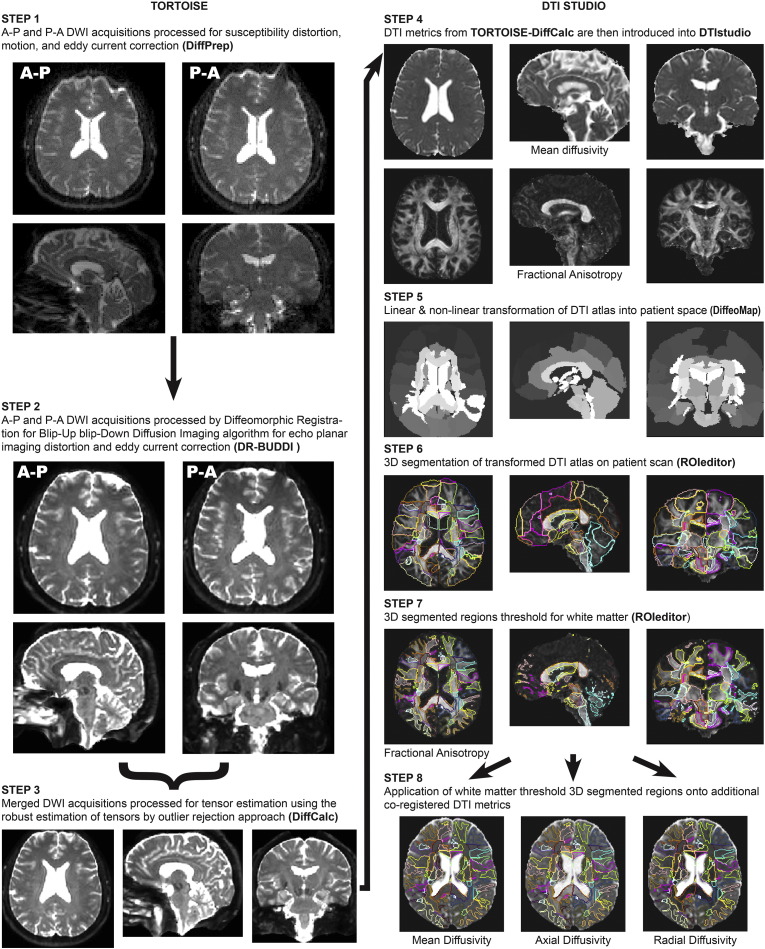
Fig. 1 graphically depicts the DTI post-processing pipeline utilized for this study using imaging data from a participant. The first portion of the pipeline uses the analytical methods constructed by Dr. Carlo Pierpaoli and colleagues at the NIH called TORTOISE - Tolerably Obsessive Registration and Tensor Optimization Indolent Software Ensemble (Pierpaoli et al., 2010a, Pierpaoli et al., 2010b). For reverse polarity data, each DWI acquisition both A-P and P-A is initially run through DiffPrep (Rohde et al., 2004, Wu et al., 2008) in TORTOISE for susceptibility distortion correction, motion correction, eddy current correction, and registration to a 3D high resolution structural image (Fig. 1, Step 1). For EPI distortion correction, the diffusion images were registered to the 1 mm isotropic T2 image using non-linear b-splines. Eddy current and motion distortion were corrected using standard affine transformations, followed by re-orientation of the b-matrix for the rotational aspect of the rigid body motion. Following DiffPrep, the output images from both the A-P and P-A DWI acquisitions were then sent through Diffeomorphic Registration for Blip-Up blip-Down Diffusion Imaging (Irfanoglu et al., 2015) (DR-BUDDI) in TORTOISE for further EPI distortion and eddy current correction that can be completed with diffusion data that has been collected with reverse polarity (Fig. 1, Step 2). This step combines the reverse polarity imaging data to create a single, cleaned, DWI data set that is then sent through DiffCalc (Basser et al., 1994, Chang et al., 2005, Chang et al., 2012, Koay et al., 2006, Koay et al., 2009, Mangin et al., 2002, Pierpaoli et al., 2010a, Pierpaoli et al., 2010b, Rohde et al., 2005) in TORTOISE (Fig. 1, Step 3). This step completes the tensor estimation (Hoy et al., 2014) using the robust estimation of tensors by outlier rejection (RESTORE) (Chang et al., 2005) approach. Following tensor estimation, a variety of DTI metrics can be derived (examples are shown in Fig. 1, Step 4). For this study, we specifically focused on fractional anisotropy (FA) as our main metric for analysis, but also derived the mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) images for exploratory evaluation.
Fig. 1.
Diffusion Tensor Imaging Post-Processing Pipeline.
Following this post-processing in TORTOISE, 3D image stacks for MD and FA were introduced into DTIstudio (Oishi et al., 2009, Zhang et al., 2010) for segmentation of the DTI atlas (Oishi et al., 2011) onto each participants DTI data set in ‘patient space’ through the Diffeomap program in DTIstudio using both linear and non-linear transformations (Fig. 1, Step 5). This is a semi-automated process that allows for the extraction of DTI metrics within each 3D–atlas-based region of interest providing a comprehensive sampling throughout the entire brain into 189 regions including ventricular space. An example of the overlaid transformed DTI atlas on a patient's FA image completed in the ROIeditor program in DTIstudio is shown in Fig. 1, Step 6. For this study, selection of regions was limited to regions of white matter, as the main hypothesis regarding DTI was that there would be reductions in white matter integrity observed with FA related to brain injury. This reduced the number of regions used for further analysis to 78. To select only white matter, FA images were then thresholded at 0.2 or greater (Fig. 1, Step 7), and this final 3D segmentation was then applied to all other co-registered DTI metrics and the data within each DTI metric for the 78 regions of interest was extracted in ROIeditor for further analysis (Fig. 1, Step 8).
2.3. Volumetric segmentation
Additional post-processing of the 3D T1-weighted high resolution structural MPRAGE image was performed using Freesurfer (Fischl et al., 2002) for volumetric segmentation. Freesurfer is an automatic segmentation program for observing volumetric data using a structural T1-weighted image. This process is divided into two primary parts. The first part consists of sub-cortical/white matter surface creation and segmentation of the individual structures. The second part provides reconstruction of the cortical surface, created from the underlying white matter surface followed by parcellation of the cortical areas. The current study focused on primarily whole brain volume (MacKenzie et al., 2002) and subcortical region comparisons given previous literature on brain injury (Bigler et al., 1997) and PTSD (Morey et al., 2012) reporting reductions in total brain volume (MacKenzie et al., 2002) and volumetric reductions in regions such as the hippocampus (Bigler et al., 1997) and amygdala (Morey et al., 2012).
2.4. Statistical analysis
Group differences in patient characteristics were assessed statistically using two-sided Fisher's exact and Mann-Whitney tests as appropriate. Group differences in 5-year volumetric and imaging measures were evaluated using standard linear and logistic regression with adjustment for imbalances in patient characteristics. Significance values were corrected for multiple comparisons using the Holm-Bonferroni method for controlling the familywise Type-I error rate (Holm, 1979).
Prediction of multiple-abnormalities was carried out with multivariate logistic regression based on TBI diagnosis, age, and 1-year clinical outcome data (Mac Donald et al., 2016, Mac Donald et al., 2014, Macdonald et al., 2014) which included measures of neurobehavior, neuropsychological function, and psychiatric symptomatology along with demographic information about the participants (e.g. education, race, gender, etc.). A “best-subset” approach was used to identify the specific combination of these predictors that minimized Akaike's Information Criterion (AIC) (Akaike, 1973) a likelihood-based metric that favors overall model parsimony over individual covariate significance. The events-per-variable (EPV) ratio for the best AIC model was 7.5, which while lower than the minimum recommended EPV of 10–15 is likely still valid given the conditions of this particular analysis (Vittinghoff and McCulloch, 2007). The area under the receiver operating characteristic (ROC) curve was calculated to assess the overall predictive accuracy of the model.
3. Results
In total 94 participants, 50 concussive blast TBI and 44 combat-deployed controls, completed a scan for this 5-year study evaluation. Due to image quality and/or scanner hardware issues that arose on some of the scanning days, 86 of the 94 scans were viable for further analysis; 46 concussive blast TBI and 40 combat-deployed controls (Table 1). All scans were evaluated by a board-certified neuroradiologist and determined to be unremarkable for signs of brain injury pathology consistent with the radiological interpretations previously identified in these subjects (Mac Donald et al., 2013, Mac Donald et al., 2011). Groups did not differ in gender, race, or number of officer vs. enlisted but did significantly differ on age, education, and branch of service. Due to these findings, all results were adjusted by age, education, gender, officer/enlisted, branch of service, and race. All cross-sectional associations between measures were adjusted for these factors by fitting linear (continuous) and logistic (binary) regression models, and adjusted p-values are reported.
Table 1.
Imaging Participant Characteristics.
| Characteristic | Combat CTL (n = 40) | Concussive blast TBI (n = 46) | p-Value |
|---|---|---|---|
| Age in years | |||
| Mean (stdev) | 35 ± 8 | 31 ± 7 | 0.01 |
| Education in years | |||
| Mean (stdev) | 16 ± 2.6 | 13 ± 1.5 | 0.0001 |
| Gender - no (%) | |||
| Male | 36 (90%) | 45 (98%) | 0.17 |
| Female | 4 (10%) | 1 (2%) | |
| Race/ethnicitya - no (%) | |||
| White | 28 (70%) | 34 (74%) | 0.81a |
| African American | 8 (20%) | 5 (11%) | |
| Hispanic/Latino | 4 (10%) | 5 (11%) | |
| Asian | 0 | 2 (4%) | |
| Branch of service - no (%) | |||
| US Army | 26 (65%) | 41 (89%) | 0.009b |
| US Air Force | 8 (20%) | 0 | |
| US Marine Corps | 3 (7.5%) | 5 (10%) | |
| US Navy | 3 (7.5%) | 0 | |
| Military rank - no (%) | |||
| Enlisted | 39 (88%) | 44 (96%) | 0.26 |
| Officer | 5 (12%) | 2 (4%) | |
Age, education: Mann-Whitney U Test, all other characteristics: Fisher's exact test
White vs. Other
Army vs. Other
3.1. Diffusion tensor imaging findings
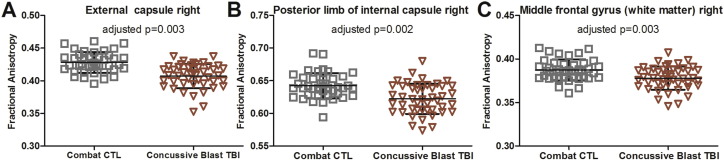
Cross-sectional analysis of each DTI region of interest identified a multitude of regions in which concussive blast TBI subjects were observed to have reduced fractional anisotropy in comparison to combat-deployed controls. The three most significant regions were the right external capsule, right posterior limb of the internal capsule, and right middle frontal gyrus white matter (Fig. 2). However, no region remained significant after strict correction for multiple comparisons (i.e. for 78 regions, p < 0.0006). Exploratory evaluation of mean diffusivity, axial diffusivity, and radial diffusivity in these regions identified significant increase in signal across all diffusivity measures in concussive blast TBI patients compared to controls (range of p = 0.02–0.00001). In contrast, no significant volumetric differences were observed suggesting these DTI changes were not from frank atrophy but from more subtle degradation of the white matter (range of p = 0.1–0.6).
Fig. 2.
Reduced fractional anisotropy in concussive blast TBI at 5-year outcome. At the group level, reductions in fractional anisotropy were observed across a variety of regions the most significantly impacted being the right external capsule (A), the right posterior limb of the internal capsule (B), and the right middle frontal gyrus white matter (C). No region of interest cross sectional comparison remained significant after strict correction for multiple comparisons (i.e. p < 0.0006).
Given the heterogeneity of brain injury, the lack of significant group level differences after proper statistical correction was no surprise and is in line with previously published work (Levin et al., 2010). To appreciate the heterogeneity of brain injury, we next asked the question: How many concussive blast TBI patients would have 2 or more regions of abnormally low FA? As we have done previously (Mac Donald et al., 2011), abnormality was defined as 2 standard deviations from the mean of the control for each region since it is well known that FA naturally varies throughout the brain and one must not assume a universal value across all regions. In order to compute this for each control participant, a ‘leave-one-out’ approach was employed. For each control, the scan data from that subject was omitted and the mean FA and standard deviation for the ‘group control – 1’ were then recomputed for each of the 78 white matter regions of interest. The omitted control subject's FA values for each region were then compared to the new mean and standard deviation and the number of regions falling greater than two standard deviations from the mean were summed. This was then iteratively completed for all 40 combat-deployed control subjects. Using this method which is more flexible to the heterogeneity of brain injury, revealed a significant number of concussive blast TBI patients to have 2 or more regions of abnormality (Fig. 3). Specifically, 74% (34/46) of concussive blast TBI patients were found to have 2 or more regions of abnormal FA and this was highly significant by chi-square compared to combat-deployed controls (p = 0.0003).
Fig. 3.
Number of abnormal regions of fractional anisotropy in concussive blast TBI and combat-deployed controls at 5-year outcome. To account for the potential heterogeneity of brain injury, the total number of abnormal regions of FA were summed for each participant. Abnormality was defined as a value that was > 2 standard deviations below the mean of the control value for that region. To compute this summation for control participants, a “leave one out” approach was employed and the mean and standard deviation by region were rederived for each control omitting their scan data. The graph summarizes the percent of participants by group with abnormal regions.
3.2. Quantitative volumetric findings
Quantitative volumetric analysis revealed that the DTI findings were not likely influenced by significant reductions in the brain anatomy but rather from microstructural injury to the regions themselves. As shown in Table 2, there were no significant differences in the total volume for any of the regions sampled by Freesurfer volumetric segmentation analysis. Evaluation at the single subject level again investigating the number of regional volumes that were > 2 standard deviations below the mean of the control only identified 5 concussive blast TBI patients and 5 combat-deployed controls meeting this criterion, which is well within the range of what would be expected by chance for these group sizes (p = 0.91, chi-square).
Table 2.
Volumetric analysis.
| Region (volume in mm3) | Combat CTL | Concussive blast TBI | Adjusted p-value |
|---|---|---|---|
| Total intracranial volume | 1,551,448 ± 137,596 | 1,552,199 ± 121,377 | 0.78 |
| Total cortex volume | 486,964 ± 46,368 | 497,723 ± 40,154 | 0.87 |
| Total cortical white matter volume | 503,029 ± 50,826 | 486,445 ± 50,752 | 0.17 |
| Left thalamus | 8617 ± 785 | 8847 ± 886 | 0.46 |
| Right thalamus | 7526 ± 677 | 7518 ± 556 | 0.64 |
| Left caudate | 3866 ± 514 | 3822 ± 441 | 0.91 |
| Right caudate | 3939 ± 504 | 3880 ± 462 | 0.96 |
| Left putamen | 5826 ± 633 | 5863 ± 560 | 0.95 |
| Right putamen | 5461 ± 558 | 5595 ± 485 | 0.86 |
| Left pallidum | 1594 ± 196 | 1572 ± 197 | 0.59 |
| Right pallidum | 1741 ± 167 | 1724 ± 183 | 0.80 |
| Left hippocampus | 4355 ± 376 | 4351 ± 438 | 0.75 |
| Right hippocampus | 4515 ± 476 | 4442 ± 423 | 0.58 |
| Left amygdala | 1609 ± 181 | 1613 ± 193 | 0.95 |
| Right amygdala | 1783 ± 227 | 1783 ± 195 | 0.82 |
3.3. Predictors of long-term imaging sequelae of concussive blast TBI
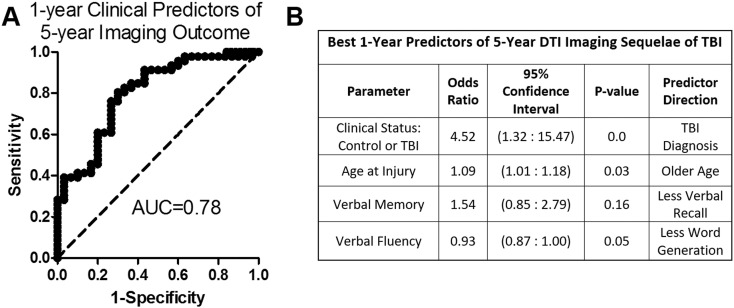
Leveraging an extensive battery of neurological, neuropsychological, and psychiatric clinical outcomes data previously collected at 1-year evaluation (Mac Donald et al., 2015, Mac Donald et al., 2016, Mac Donald et al., 2014, Macdonald et al., 2014) in these same participants allowed us to explore what early clinical and demographic factors best predicted the likelihood of sustained imaging abnormalities at 5-year evaluation. Of the 86 participants, whose 5-years scans were viable for analysis, 76 had complete clinical assessment data from 1-year testing. Logistic regression using the dichotomized number of DTI abnormalities of ‘0–1’ or ‘2 or more’ identified the best fit model by Akaike information criterion to include concussive brain injury diagnosis, older age at injury with each increase of one year corresponding to a 9% increase of the odds, performance on the California Verbal Learning Test long delayed free recall (a measure of verbal memory), and performance on the controlled oral word association (a measure of verbal fluency) (Fig. 4, Area Under the Curve = 0.78). Given the somewhat arbitrary nature of the ‘2 or more’ cutoff for imaging abnormalities, we also ran the model using the dichotomized number of DTI abnormalities with a ‘3 or more’, and ‘4 or more’ cutoff. Running the logistic regression on dichotomized DTI abnormalities of ‘0–2’ and ‘3 or more’ identified the same top model as shown in Fig. 4 for ‘0–1’ vs. ‘2 or more’ (Area Under the Curve = 0.74). Running the logistic regression using the dichotomization of ‘0–3’ and ‘4 or more’ DTI abnormalities, the same model was the third top model with only minimal difference in factor strengths (Area Under the Curve = 0.79).
Fig. 4.
Predictors of 5-year DTI Imaging Sequelae of Concussive blast injury. Receiver-operator curve (A) and parameter table (B) for best fit logistic regression model of 1-year clinical predictors of 5-year imaging abnormalities. The best model by Akaike information criterion contained the diagnoses of TBI, age at time of injury, performance on the California Verbal Learning Test long delay free recall (a measure of verbal memory), and performance on the controlled auditory word association (a measure of verbal fluency).
4. Discussion
Overall, a substantial number (74%) of concussive blast TBI service members were found to have sustained imaging changes, evidenced by significant reductions in FA on DTI, 5 years after exposure even though conventional MRI collected at the very same time was unremarkable for brain injury pathology. While cross-sectional analysis at the group level did not differentiate these patients from combat-deployed controls, an analysis approach flexible to the heterogeneity of brain injury at the single subject level identified a large portion of concussive blast TBI patients with multiple regions of abnormality at this chronic time point. Reductions in FA in some of these same participants have been previously observed at the acute (Adam et al., 2015), sub-acute (Mac Donald et al., 2011), and 1-year (Mac Donald et al., 2011) time point following injury. It was surprising to identify significant numbers of these uncomplicated mild TBI cases with sustained imaging sequelae now at 5 years post injury. We believe this provides supporting evidence for the evolution not resolution of this brain injury pathology, adding to the growing body of literature describing imaging signatures of chronic neurodegeneration (Farbota et al., 2012, Ramlackhansingh et al., 2011).
By logistic regression, risk factors for these sustained imaging changes included TBI diagnosis, older age at the time of injury, verbal memory, and verbal fluency at 1-year post injury with good predictive strength (AUC = 0.78). While the predictive strength is good not excellent, these findings do provide important clinical and demographic factors for clinicians to consider early on in triage decisions about intensive rehabilitation. The goal with this approach was to understand, even for those without access to advanced neuroimaging capabilities, what factors should be strongly considered to assist in determination of focused treatment. These findings are in line with prior studies in veterans also reporting reductions in fractional anisotropy by DTI using different analytical methods (Hayes et al., 2015, Jorge et al., 2012, Miller et al., 2016, Morey et al., 2013, Trotter et al., 2015). In addition, previous work in civilian TBI (Lingsma et al., 2015) studies have observed older age as an important predictor of poor outcome in addition to recent work in veterans (Trotter et al., 2015) suggesting that this may be a universal risk factor irrespective of injury mechanism or exposure.
Strengths of this study include the prospective, observational, longitudinal study design, ability to leverage previously collected clinical and imaging data for comparison to 5-year data, a rigorous image processing approach, radiological interpretation blinded to the clinical status of the participant, and an analytical approach sensitive to the heterogeneity of brain injury at the single subject level that removes the confounds of tractography (Thomas et al., 2014). Limitations include modest group size, lack of multiple advanced neuroimaging techniques, demographically imbalanced groups that had to be adjusted for in the statistical analysis, heterogeneous treatment across centers in theater and in the US after injury, possible unmeasured covariates including unmeasured heterogeneity of injury severity across cohorts that may influence long-term imaging outcome and lack of direct comparison of imaging data from the acute (Adam et al., 2015)/sub-acute (Mac Donald et al., 2011), 1-year (Mac Donald et al., 2011), and 5-year time point given the disparities in MRI hardware and scan acquisition available at each evaluation. While the current findings highlight a greater number with DTI abnormalities than previous findings reported at 1-year (Mac Donald et al., 2011), it is unclear whether this discrepancy is due to scanner hardware and post-processing differences or directly related to injury severity or related to some ongoing neurodegenerative process. Large efforts are underway however to reprocess the earlier imaging data so that it can be used for such an analysis in the years to come.
With considerable cost accumulating from these combat exposures, it seems prudent to identify new techniques that remove self-report bias and provide more definitive evidence of injury. This is of particular importance as many of these soldiers begin to separate from the service and are evaluated by medical review boards for lifelong disability determination. In the absence of strong diagnostic evidence, questions often arise regarding the extent to which events in the service member's record would influence their functional presentation and long-term outcome. Incorporating advanced neuroimaging findings into this consideration could potentially assist in shedding light on what is commonly referred to as the ‘invisible injury of war’, removing ambiguity regarding brain injury exposure. Efforts are currently underway to replicate these findings in additional service members enrolled in the overall prospective, observational, longitudinal study and to extend evaluation to later time points, investigating the impact on aging and long-term outcome.
Funding
Support for this study was provided by a Department of Defense grant through the Chronic Effects of Neurotrauma Consortium (W81XWH-13-2-0095) and by an NIH RO1 grant from NINDS (1R01NS091618-01).
Acknowledgements
We would like to thank the service members, their families, commanding officers, and clinical providers for making this study possible. We are grateful for the assistance of the University of Washington Diagnostic Imaging Sciences team including Serena Bennett, Kris McKown, and Liza Young for their support with the imaging acquisition and logistical planning. Any opinions, findings, conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the U.S. Government, Department of Defense, or the U.S. Department of Veterans Affairs, and no official endorsement should be inferred.
References
- Adam O., Mac Donald C.L., Rivet D., Ritter J., May T., Barefield M., Duckworth J., LaBarge D., Asher D., Drinkwine B., Woods Y., Connor M., Brody D.L. Clinical and imaging assessment of acute combat mild traumatic brain injury in Afghanistan. Neurology. 2015;85:219–227. doi: 10.1212/WNL.0000000000001758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike H. Information theory and an extension of the maximum likelihood principle. In: Petrov B., Csaki F., editors. Second International Symposium on Information Theory. Academiai Kiado; Budapest: 1973. pp. 267–281. [Google Scholar]
- Arfanakis K., Haughton V.M., Carew J.D., Rogers B.P., Dempsey R.J., Meyerand M.E. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am. J. Neuroradiol. 2002;23:794–802. [PMC free article] [PubMed] [Google Scholar]
- Basser P.J., Mattiello J., LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J. Magn. Reson. B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Bazarian J.J., Donnelly K., Peterson D.R., Warner G.C., Zhu T., Zhong J. The relation between posttraumatic stress disorder and mild traumatic brain injury acquired during Operations Enduring Freedom and Iraqi Freedom. J. Head Trauma Rehabil. 2013;28:1–12. doi: 10.1097/HTR.0b013e318256d3d3. [DOI] [PubMed] [Google Scholar]
- Bazarian J.J., Zhong J., Blyth B., Zhu T., Kavcic V., Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J. Neurotrauma. 2007;24:1447–1459. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- Bennett R.E., Mac Donald C.L., Brody D.L. Diffusion tensor imaging detects axonal injury in a mouse model of repetitive closed-skull traumatic brain injury. Neurosci. Lett. 2012;513:160–165. doi: 10.1016/j.neulet.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler E.D., Blatter D.D., Anderson C.V., Johnson S.C., Gale S.D., Hopkins R.O., Burnett B. Hippocampal volume in normal aging and traumatic brain injury. AJNR Am. J. Neuroradiol. 1997;18:11–23. [PMC free article] [PubMed] [Google Scholar]
- Brody D.L., Benetatos J., Bennett R.E., Klemenhagen K.C., Mac Donald C.L. The pathophysiology of repetitive concussive traumatic brain injury in experimental models; new developments and open questions. Mol. Cell. Neurosci. 2015;66:91–98. doi: 10.1016/j.mcn.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casscells S. Defense Aso. Department of Defense; 2007. Traumatic brain injury: definition and reporting. [Google Scholar]
- Chang L.C., Jones D.K., Pierpaoli C. RESTORE: robust estimation of tensors by outlier rejection. Magn. Reson. Med. 2005;53:1088–1095. doi: 10.1002/mrm.20426. [DOI] [PubMed] [Google Scholar]
- Chang L.C., Walker L., Pierpaoli C. Informed RESTORE: a method for robust estimation of diffusion tensor from low redundancy datasets in the presence of physiological noise artifacts. Magn. Reson. Med. 2012;68:1654–1663. doi: 10.1002/mrm.24173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DVBIC . Defense and Veterans Brain Injury Center; 2016. DoD Worldwide TBI Numbers (2000–2016, Q1–Q3) [Google Scholar]
- Farbota K.D., Bendlin B.B., Alexander A.L., Rowley H.A., Dempsey R.J., Johnson S.C. Longitudinal diffusion tensor imaging and neuropsychological correlates in traumatic brain injury patients. Front. Hum. Neurosci. 2012;6:160. doi: 10.3389/fnhum.2012.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., van der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Han K., Mac Donald C.L., Johnson A.M., Barnes Y., Wierzechowski L., Zonies D., Oh J., Flaherty S., Fang R., Raichle M.E., Brody D.L. Disrupted modular organization of resting-state cortical functional connectivity in U.S. military personnel following concussive 'mild' blast-related traumatic brain injury. NeuroImage. 2014;84:76–96. doi: 10.1016/j.neuroimage.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J.P., Miller D.R., Lafleche G., Salat D.H., Verfaellie M. The nature of white matter abnormalities in blast-related mild traumatic brain injury. Neuroimage Clin. 2015;8:148–156. doi: 10.1016/j.nicl.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. A simple sequentially Rejective multiple test procedure. Scand. J. Stat. 1979;6:65–70. [Google Scholar]
- Hoy A.R., Koay C.G., Kecskemeti S.R., Alexander A.L. Optimization of a free water elimination two-compartment model for diffusion tensor imaging. NeuroImage. 2014;103:323–333. doi: 10.1016/j.neuroimage.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglese M., Makani S., Johnson G., Cohen B.A., Silver J.A., Gonen O., Grossman R.I. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J. Neurosurg. 2005;103:298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- Irfanoglu M.O., Modi P., Nayak A., Hutchinson E.B., Sarlls J., Pierpaoli C. DR-BUDDI (Diffeomorphic Registration for Blip-Up blip-Down Diffusion Imaging) method for correcting echo planar imaging distortions. NeuroImage. 2015;106:284–299. doi: 10.1016/j.neuroimage.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge R.E., Acion L., White T., Tordesillas-Gutierrez D., Pierson R., Crespo-Facorro B., Magnotta V.A. White matter abnormalities in veterans with mild traumatic brain injury. Am. J. Psychiatry. 2012;169:1284–1291. doi: 10.1176/appi.ajp.2012.12050600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koay C.G., Chang L.C., Carew J.D., Pierpaoli C., Basser P.J. A unifying theoretical and algorithmic framework for least squares methods of estimation in diffusion tensor imaging. J. Magn. Reson. 2006;182:115–125. doi: 10.1016/j.jmr.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Koay C.G., Ozarslan E., Basser P.J. A signal transformational framework for breaking the noise floor and its applications in MRI. J. Magn. Reson. 2009;197:108–119. doi: 10.1016/j.jmr.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin H.S., Wilde E., Troyanskaya M., Petersen N.J., Scheibel R., Newsome M., Radaideh M., Wu T., Yallampalli R., Chu Z., Li X. Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. J. Neurotrauma. 2010;27:683–694. doi: 10.1089/neu.2009.1073. [DOI] [PubMed] [Google Scholar]
- Lingsma H.F., Yue J.K., Maas A.I., Steyerberg E.W., Manley G.T., Investigators T.-T. Outcome prediction after mild and complicated mild traumatic brain injury: external validation of existing models and identification of new predictors using the TRACK-TBI pilot study. J. Neurotrauma. 2015;32:83–94. doi: 10.1089/neu.2014.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald C., Johnson A., Cooper D., Malone T., Sorrell J., Shimony J., Parsons M., Snyder A., Raichle M., Fang R., Flaherty S., Russell M., Brody D.L. Cerebellar white matter abnormalities following primary blast injury in US military personnel. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald C.L., Johnson A.M., Nelson E.C., Werner N.J., Fang R., Flaherty S.F., Brody D.L. Functional status after blast-plus-impact complex concussive traumatic brain injury in evacuated United States military personnel. J. Neurotrauma. 2014;31:889–898. doi: 10.1089/neu.2013.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald C.L., Adam O.R., Johnson A.M., Nelson E.C., Werner N.J., Rivet D.J., Brody D.L. Acute post-traumatic stress symptoms and age predict outcome in military blast concussion. Brain. 2015;138:1314–1326. doi: 10.1093/brain/awv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald C.L., Dikranian K., Bayly P., Holtzman D., Brody D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J. Neurosci. 2007;27:11869–11876. doi: 10.1523/JNEUROSCI.3647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald C.L., Dikranian K., Song S.K., Bayly P.V., Holtzman D.M., Brody D.L. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp. Neurol. 2007;205:116–131. doi: 10.1016/j.expneurol.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald C.L., Johnson A.M., Cooper D., Nelson E.C., Werner N.J., Shimony J.S., Snyder A.Z., Raichle M.E., Witherow J.R., Fang R., Flaherty S.F., Brody D.L. Detection of blast-related traumatic brain injury in U.S. military personnel. N. Engl. J. Med. 2011;364:2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald C.L., Johnson A.M., Wierzechowski L., Kassner E., Stewart T., Nelson E.C., Werner N.J., Adam O.R., Rivet D.J., Flaherty S.F., Oh J.S., Zonies D., Fang R., Brody D.L. Outcome trends after US military concussive traumatic brain injury. J. Neurotrauma. 2016 doi: 10.1089/neu.2016.4434. Jun 27. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald C.L., Johnson A.M., Wierzechowski L., Kassner E., Stewart T., Nelson E.C., Werner N.J., Zonies D., Oh J., Fang R., Brody D.L. Prospectively assessed clinical outcomes in concussive blast vs nonblast traumatic brain injury among evacuated US military personnel. JAMA Neurol. 2014;71(8):994–1002. doi: 10.1001/jamaneurol.2014.1114. Aug. [DOI] [PubMed] [Google Scholar]
- MacKenzie J.D., Siddiqi F., Babb J.S., Bagley L.J., Mannon L.J., Sinson G.P., Grossman R.I. Brain atrophy in mild or moderate traumatic brain injury: a longitudinal quantitative analysis. AJNR Am. J. Neuroradiol. 2002;23:1509–1515. [PMC free article] [PubMed] [Google Scholar]
- Mangin J.F., Poupon C., Clark C., Le Bihan D., Bloch I. Distortion correction and robust tensor estimation for MR diffusion imaging. Med. Image Anal. 2002;6:191–198. doi: 10.1016/s1361-8415(02)00079-8. [DOI] [PubMed] [Google Scholar]
- Matthews S.C., Spadoni A.D., Lohr J.B., Strigo I.A., Simmons A.N. Diffusion tensor imaging evidence of white matter disruption associated with loss versus alteration of consciousness in warfighters exposed to combat in Operations Enduring and Iraqi Freedom. Psychiatry Res. 2012;204:149–154. doi: 10.1016/j.pscychresns.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D.R., Hayes J.P., Lafleche G., Salat D.H., Verfaellie M. White matter abnormalities are associated with chronic postconcussion symptoms in blast-related mild traumatic brain injury. Hum. Brain Mapp. 2016;37:220–229. doi: 10.1002/hbm.23022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey R.A., Gold A.L., LaBar K.S., Beall S.K., Brown V.M., Haswell C.C., Nasser J.D., Wagner H.R., McCarthy G., Mid-Atlantic M.W. Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Arch. Gen. Psychiatry. 2012;69:1169–1178. doi: 10.1001/archgenpsychiatry.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey R.A., Haswell C.C., Selgrade E.S., Massoglia D., Liu C., Weiner J., Marx C.E., Group M.W., Cernak I., McCarthy G. Effects of chronic mild traumatic brain injury on white matter integrity in Iraq and Afghanistan war veterans. Hum. Brain Mapp. 2013;34:2986–2999. doi: 10.1002/hbm.22117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi S.N., Mukherjee P. Diffusion tensor imaging of mild traumatic brain injury. J. Head Trauma Rehabil. 2010;25:241–255. doi: 10.1097/HTR.0b013e3181e52c2a. [DOI] [PubMed] [Google Scholar]
- Niogi S.N., Mukherjee P., Ghajar J., Johnson C.E., Kolster R., Lee H., Suh M., Zimmerman R.D., Manley G.T., McCandliss B.D. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain. 2008;131:3209–3221. doi: 10.1093/brain/awn247. [DOI] [PubMed] [Google Scholar]
- Niogi S.N., Mukherjee P., Ghajar J., Johnson C., Kolster R.A., Sarkar R., Lee H., Meeker M., Zimmerman R.D., Manley G.T., McCandliss B.D. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am. J. Neuroradiol. 2008;29(5):967–973. doi: 10.3174/ajnr.A0970. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K., Faria A., Jiang H., Li X., Akhter K., Zhang J., Hsu J.T., Miller M.I., van Zijl P.C., Albert M., Lyketsos C.G., Woods R., Toga A.W., Pike G.B., Rosa-Neto P., Evans A., Mazziotta J., Mori S. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer's disease participants. NeuroImage. 2009;46:486–499. doi: 10.1016/j.neuroimage.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K., Faria A., Van Zijl P.C., Mori S. 2 ed. Elsevier; Oxford, UK: 2011. MRI Atlas of Human White Matter. [Google Scholar]
- Pierpaoli C., Jezzard P., Basser P.J., Barnett A., Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C., Walker L., Irfanoglu M.O., Barnett A., Basser P., Chang L.-C., Koay C., Pajevic S., Rohde G., Sarlls J., Wu M. International Society for Magnetic Resonance in Medicince. 18th Annual Meeting. 2010. TORTOISE: an integrated software package for processing of diffusion MRI data. [Google Scholar]
- Pierpaoli C., Walker L., Irfanoglu M.O., Barnett A., Basser P., Chang L.-C., Koay C., Pajevic S., Rohde G., Sarlls J., Wu M. ISMRM 18th annual meeting, Stockholm, Sweden. 2010. TORTOISE: an integrated software package for processing of diffusion MRI data. #1597. [Google Scholar]
- Ramlackhansingh A.F., Brooks D.J., Greenwood R.J., Bose S.K., Turkheimer F.E., Kinnunen K.M., Gentleman S., Heckemann R.A., Gunanayagam K., Gelosa G., Sharp D.J. Inflammation after trauma: microglial activation and traumatic brain injury. Ann. Neurol. 2011;70:374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- Rohde G.K., Barnett A.S., Basser P.J., Marenco S., Pierpaoli C. Comprehensive approach for correction of motion and distortion in diffusion-weighted MRI. Magn. Reson. Med. 2004;51:103–114. doi: 10.1002/mrm.10677. [DOI] [PubMed] [Google Scholar]
- Rohde G.K., Barnett A.S., Basser P.J., Pierpaoli C. Estimating intensity variance due to noise in registered images: applications to diffusion tensor MRI. NeuroImage. 2005;26:673–684. doi: 10.1016/j.neuroimage.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Sorg S.F., Delano-Wood L., Luc N., Schiehser D.M., Hanson K.L., Nation D.A., Lanni E., Jak A.J., Lu K., Meloy M.J., Frank L.R., Lohr J.B., Bondi M.W. White matter integrity in veterans with mild traumatic brain injury: associations with executive function and loss of consciousness. J. Head Trauma Rehabil. 2014;29:21–32. doi: 10.1097/HTR.0b013e31828a1aa4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber K.H., Hurley R.A., Haswell C.C., Rowland J.A., Hurt S.D., Lamar C.D., Morey R.A. White matter compromise in veterans exposed to primary blast forces. J. Head Trauma Rehabil. 2015;30:E15–E25. doi: 10.1097/HTR.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C., Ye F.Q., Irfanoglu M.O., Modi P., Saleem K.S., Leopold D.A., Pierpaoli C. Anatomical accuracy of brain connections derived from diffusion MRI tractography is inherently limited. Proc. Natl. Acad. Sci. U. S. A. 2014;111:16574–16579. doi: 10.1073/pnas.1405672111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter B.B., Robinson M.E., Milberg W.P., McGlinchey R.E., Salat D.H. Military blast exposure, ageing and white matter integrity. Brain. 2015;138:2278–2292. doi: 10.1093/brain/awv139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittinghoff E., McCulloch C.E. Relaxing the rule of ten events per variable in logistic and Cox regression. Am. J. Epidemiol. 2007;165:710–718. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- Ware J.B., Biester R.C., Whipple E., Robinson K.M., Ross R.J., Nucifora P.G. Combat-related mild traumatic brain injury: association between baseline diffusion-tensor imaging findings and long-term outcomes. Radiology. 2016;280:212–219. doi: 10.1148/radiol.2016151013. [DOI] [PubMed] [Google Scholar]
- Wu M., Chang L.C., Walker L., Lemaitre H., Barnett A.S., Marenco S., Pierpaoli C. Comparison of EPI distortion correction methods in diffusion tensor MRI using a novel framework. Med. Image Comput. Comput. Assist. Interv. 2008;11:321–329. doi: 10.1007/978-3-540-85990-1_39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh P.H., Wang B., Oakes T.R., French L.M., Pan H., Graner J., Liu W., Riedy G. Postconcussional disorder and PTSD symptoms of military-related traumatic brain injury associated with compromised neurocircuitry. Hum. Brain Mapp. 2014;35:2652–2673. doi: 10.1002/hbm.22358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhang J., Oishi K., Faria A.V., Jiang H., Li X., Akhter K., Rosa-Neto P., Pike G.B., Evans A., Toga A.W., Woods R., Mazziotta J.C., Miller M.I., van Zijl P.C., Mori S. Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. NeuroImage. 2010;52:1289–1301. doi: 10.1016/j.neuroimage.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]