Abstract
The matrix metalloproteinase stromelysin-2 is expressed in keratinocytes of the epithelial tongue of skin wounds, suggesting a role in keratinocyte migration. Here, we show that stromelysin-2 enhances migration of cultured keratinocytes. To gain insight into the in vivo activities of stromelysin-2 in epithelial repair, we generated transgenic mice expressing a constitutively active stromelysin-2 mutant in keratinocytes. These animals had no alterations in skin architecture, and the healing rate of skin wounds was normal. Histologically, however, we found abnormalities in the organization of the wound epithelium. Keratinocytes at the migrating epidermal tip were scattered in most sections of mice with high expression level, and there was a reduced deposition of new matrix. In particular, the staining pattern of laminin-5 at the wound site was altered. This may be due to proteolytic processing of laminin-5 by stromelysin-2, because degradation of laminin-5 by this enzyme was observed in vitro. The inappropriate matrix contact of keratinocytes was accompanied by aberrant localization of β1-integrins and phosphorylated focal adhesion kinase, as well as by increased apoptosis of wound keratinocytes. These results suggest that a tightly regulated expression level of stromelysin-2 is required for limited matrix degradation at the wound site, thereby controlling keratinocyte migration.
INTRODUCTION
Proteolytic degradation of matrix proteins occurs in a variety of events that require tissue reorganization, such as embryonic development, wound healing, and cancer progression (Vu and Werb, 2000). Besides proteinases from the serine and cysteine family, matrix metalloproteinases (MMPs) are major players in these processes. Acting on specific protein substrates, these enzymes serve numerous and diverse functions. In addition to removing or remodeling extracellular matrix, secreted MMPs regulate cell–cell and cell–matrix signaling. For example, they release, activate or silence growth factors, modify cell surface receptors, and regulate apoptosis and inflammation (McCawley and Matrisian, 2001; Egeblad and Werb, 2002; Parks et al., 2004).
Obviously, the activity of enzymes with such a diversity of functions has to be tightly regulated, and this occurs on the transcriptional and posttranscriptional level. Most MMPs are absent in healthy, resting tissue and are only expressed in response to specific stimuli. The transcription of many MMPs is induced by a variety of growth factors and cytokines and by changes in cell–cell and cell–matrix interactions (Sternlicht and Werb, 2001). Most MMPs are secreted as inactive proenzymes, which have to be activated either by cleavage through other proteinases or by induction of autocatalytic processing (Visse and Nagase, 2003).
Cutaneous wound repair is an excellent model system to study proteinase function and regulation, because it comprises several processes requiring proteinase action: invasion of inflammatory cells as well as migration of fibroblasts and keratinocytes, angiogenesis, wound contraction and finally remodeling of the scar tissue (Martin, 1997; Parks, 1999; Pilcher et al., 1999). However, the role of MMPs in wound healing is ambiguous: on the one hand it has been shown by application of MMP inhibitors to the wound site that at least some MMPs are needed for proper wound closure (Lund et al., 1999). On the other hand, overexpression of proteinases is considered to be a major cause for the inability of chronic wounds to heal (Wysocki et al., 1993; Vaalamo et al., 1996).
Recently, we and others examined the temporal and spatial regulation of several MMPs and tissue inhibitors of metalloproteinases (TIMPs) in mouse and rat wounds (Okada et al., 1997; Madlener et al., 1998). All MMPs examined were up-regulated during the healing process, and each had a distinct time course and localization within the wound, indicating strict temporal and spatial regulation of MMPs. Among the MMPs, stromelysin-2 (St2/MMP-10) is unique due to its exclusive epithelial expression at the tip of the migrating epithelial tongue (Figure 1A) (Madlener et al., 1996). A similar expression pattern for St2 also was reported for normal and chronic human wounds (Saarialho-Kere et al., 1994; Rechardt et al., 2000) and for the injured intestine, where it was analogously located in migrating enterocytes in inflammatory bowel disease (Vaalamo et al., 1998). St2 also has been detected in several carcinomas (Birkedal-Hansen et al., 2000; Bodey et al., 2001; Kerkela et al., 2001; Mathew et al., 2002). In spite of this interesting expression pattern, little is as yet known about the in vivo function of this enzyme. In vitro, it can degrade elastin, nonfibrillar collagens, proteoglycans, gelatin, and casein (Nicholson et al., 1989; Murphy et al., 1991), but on a functional level it is much less well characterized than stromelysin-1 (MMP-3) or other MMPs.
Figure 1.
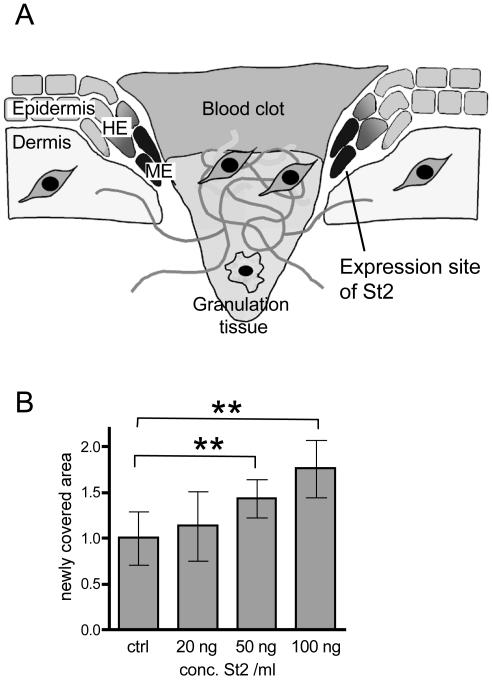
St2 enhances keratinocyte migration. (A) The localization of St2 mRNA in migrating keratinocytes of 5-d wounds is shown schematically. The expression site of St2 is marked in black. HE, hyperproliferative epithelium; ME, migrating epithelium. (B) Primary human keratinocytes were grown to confluence and treated with mitomycin C to block proliferation, and the monolayer was subsequently wounded with a pipette tip. Different concentrations of active recombinant St2 were added to the cells in serum-free medium. The wounded cell layer was photographed immediately after injury and 16 h later. The clear area at the beginning and after 16 h was measured using the Openlab software. The area, which was newly covered with cells, was calculated and set as 1 for the control (ctrl) dishes. Eight pictures per value were analyzed (n = 8). Statistical analysis was performed using the GraphPad Prism4 software. p values are p = 0.003 for comparison of ctrl with 50 ng/ml St2 and p = 0.0019 for comparison of ctrl with 100 ng/ml St2, according to Mann-Whitney U test for non-Gaussian distributions.
To gain insight into the in vivo activities of St2 and to determine the consequences of interfering with the tightly balanced proteinase system in wounded skin, we generated St2-transgenic animals. Here, we show that overexpression of a constitutively active St2 mutant in the epidermis results in a disorganized migrating epithelium; degradation of newly formed matrix, including laminin-5; partial loss of cell–cell contacts of the migrating keratinocytes; and an increased rate of apoptosis of wound edge keratinocytes.
MATERIALS AND METHODS
In Vitro Migration Assays
Primary human keratinocytes were prepared as described previously (Rheinwald and Green, 1975) and cultured in keratinocyte serum-free medium (Invitrogen, Basel, Switzerland), supplemented with 0.1 ng/ml epidermal growth factor and 25 μg/ml bovine pituitary extract. Cells were used for experiments from passages 3 to 5.
Cells were grown to confluence and treated with 10 μg/ml mitomycin C (Sigma Chemie, Munich, Germany) for 2 h. A scratch was made within the cell layer with a sterile pipette tip. Cells were washed with phosphate-buffered saline (PBS) and kept in K-SFM containing various concentrations of recombinant St2 protein (R&D Systems, Abingdon, United Kingdom). The same area was photographed under phase contrast directly after scratching and 16 h later. Areas, which were not covered by cells, were measured using the Openlab software.
Plasmid Construction
The isolation of the murine stromelysin-2 cDNA from a murine wound cDNA library was described previously (Madlener and Werner, 1997). For generation of a constitutively active stromelysin-2 mutant (referred to as “St2*”), a point mutation leading to a change from valine at position 94 to glycine was inserted using the site-directed mutagenesis kit (Stratagene, Heidelberg, Germany). The mutated cDNA as well as the wild-type sequence were subcloned into the eukaryotic expression vector pcDNA3 (Invitrogen).
For expression in the epidermis of transgenic animals the full-length murine St2* cDNA was inserted into an expression cassette that includes the pBluescript KSII+ cloning vector (Stratagene, La Jolla, CA), a 2-kb human keratin 14 promoter (Munz et al., 1999), followed by a 0.65-kb rabbit β-globin intron and a transcription termination/polyadenylation fragment [poly(A), 0.65 kb] of the human growth hormone gene. The St2* cDNA was inserted between the intron and the poly(A) fragment (Figure 1B).
Generation and Identification of Transgenic Mice
Standard procedures were followed to generate transgenic mice. Fertilized eggs were obtained after superovulation and mating of B6D2F1 or FVB/N females with males of the same strain. The 4.9-kb insert was separated from vector sequences, purified, and injected into the pronuclei of fertilized oocytes. Microinjected zygotes were transferred into the oviducts of pseudopregnant surrogate mothers. Mouse tail DNA was analyzed for integration of the transgene by Southern blotting by using the rabbit β-globin intron fragment as a probe (founder analysis). The progeny was genotyped by polymerase chain reaction by using 5′ β-globin 5′-GGA TCC TGA GAA CTT CAG GGT GAG-3′ as a 5′ primer and 3′ β-globin 5′-CAG CAC AAT AAC CAG CAC GTT GCC-3′ as a 3′ primer at an annealing temperature of 53°C. The primers hybridize to the β-globin intron sequence of the transgene construct and give a product of the size of 620 base pairs.
Wounding and Preparation of Wound Tissue
Male and female mice (2–3 mo old) were anesthetized by i.p. injection of ketamine (75 mg/kg)/xylazine (5 mg/kg). Two full-thickness excisional wounds, 5 mm in diameter, were made on either side of the dorsal midline by excising skin and panniculus carnosus as described previously (Werner et al., 1994). Wounds were left uncovered and harvested at different time points after injury. For expression analysis, the complete wounds, including 2 mm of the epithelial margins were excised and immediately frozen in liquid nitrogen. Nonwounded back skin served as a control. For histological analysis, the complete wounds were isolated, bisected, and either directly embedded in tissue-freezing medium without prior fixation or fixed overnight in 95% ethanol/1% acetic acid or 4% paraformaldehyde (PFA) and embedded in paraffin. Sections (8 μm) from the middle of the wound were stained with hematoxylin/eosin. Only animals of the same age and sex were used for direct histological comparison, and histological assessment was performed independently by two experienced investigators. All animal experiments were performed with permission from the local veterinary authorities.
Bursting Strength Assay
Mice were anesthetized by i.p. injection of ketamine/xylazine (see above). On the day of wounding the hair on the animals' back was shaved and treated with a depilatory agent (Pilca Perfect; Stafford-Miller Continental, Oevel, Belgium). Two full-thickness incisions (1 cm) were made at each site of the dorsal midline. The skin margins were closed with stripes of Fixomull stretch plaster (Beiersdorf AG, Hamburg, Germany). Mice were sacrificed on day 5 postwounding, and bursting strength of the wounds was determined using a biomechanical tissue characterization device (BTC 2000; Surgical Research Laboratory Incorporation, Nashville, TN) according to the manufacturer's protocol for the nonhuman disruptive linear incision analysis.
RNA Isolation, RNase Protection Assay, and In Situ Hybridization
Isolation of total cellular RNA and RNase protection assays were performed as described previously (Werner et al., 1993). All protection assays were performed at least in duplicate with different sets of RNAs from independent experiments. The probe for mouse St2 is complementary to nucleotide (nt) 1162–1472 (accession no Y13185). As a loading control, the RNA was hybridized with a probe for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (nt 566–685 of the murine cDNA, accession no. NM_008084). Radioactive in situ hybridization was performed as described previously (Wilkinson et al., 1987), by using the St2 cDNA template described above.
Transient Transfection of Human Embryonic Kidney (HEK)-293 Cells
HEK-293 cells (American Type Culture Collection, Manassas, VA) were transiently transfected with the pcDNA3-St2/St2* expression constructs by using the calcium phosphate transfection method (Chen and Okayama, 1987). Briefly, 1 × 106 cells in 6-cm-dishes were transfected with 4 μg of the plasmid in a calcium phosphate precipitate. After a 5-h incubation cells were washed with HEPES buffer (142 mM NaCl, 10 mM HEPES pH 7.3, and 6.7 mM KCl), kept in DMEM/10% fetal calf serum overnight, and then changed to serum-free DMEM. Conditioned media were collected after 48 h and concentrated with AMICON filter devices (Millipore, Bedford, MA). Aliquots of the supernatants were treated with 1.5 mM aminophenylmercuric acetate (APMA) for activation of MMPs, and samples were used for Western blot analysis.
Culture of Primary Mouse Keratinocytes
Murine epidermal keratinocytes were isolated from pools of St2* transgenic mice and control littermates as described previously (Caldelari et al., 2000), with the exception that 2- to 4-d-old mice were used instead of embryos and that cells were seeded at a density of 2 × 105 cells/cm2. Cells were grown to 90% density in defined keratinocyte serum-free medium (Invitrogen), and conditioned media were collected. For Western blot analysis, supernatants were precipitated with 4 volumes of acetone.
Preparation of Protein Lysates and Western Blot Analysis
Cultured cells were lysed in 20 mM Tris/HCl (pH 8.0), 1% (vol/vol) Triton-X-100, 137 mM NaCl, 2 mM EDTA, 10% (vol/vol) glycerol, 0.5 mM 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF), and 0.15 U/ml aprotinin. Proteins were separated by SDS-PAGE under reducing conditions and transferred to nitrocellulose filters. Antibody incubations were performed in 5% nonfat dry milk in PBS/0.1% Tween 20. An anti-St2-antibody was raised in rabbits against the synthetic peptide TRHVMDKGFPRQITDDFP, corresponding to amino acids 413–429 of the mouse St2 protein. This antibody does not cross-react with stromelysin-1 (St1/MMP3), as determined by Western blot analysis of COS-7 cells transfected with St1 and St2 expression vectors (Madlener and Werner, 1997; our unpublished data). Antibodies against different regions of laminin-5 (anti-α3IIIa, anti-α3LG4-5, anti-γ2LE4-6, and anti-γ2L4m) have been described previously (Sasaki et al., 2001).
In Vitro Cleavage of Laminin-5
Human laminin-5 was purified from primary human keratinocytes as described previously (Rousselle and Aumailley, 1994; Tasanen et al., 2004). Laminin-5 (6 ng/μl) was incubated with recombinant human stromelysin-2 (R&D Systems) at enzyme: substrate ratios between 1:5 and 1:50 in the presence of 1 mM APMA at 37°C for different time periods. Controls without enzyme were incubated in assay buffer alone (50 mM Tris/HCl, pH 7.5, 150 mM NaCl, 10 mM CaCl2, 0.05% Brij35, 100 μg/ml bovine serum albumin (BSA), 0.5 mM AEBSF, 1 μg/ml pepstatin, and 1 μg/ml leupeptin). Reactions were stopped by addition of Laemmli sample buffer, and samples were analyzed by Western blotting.
Immunofluorescence
Methanol-fixed frozen sections (8 μm) or dewaxed ethanol/acetic acid fixed paraffin sections from the middle of the wound or from tail skin were incubated overnight at 4°C with the primary antibodies diluted in PBS containing 3% BSA and 0.025% NP-40. After three 10-min washes with PBS/0.1% Tween 20, sections were incubated for 1 h with the secondary antibodies, washed again, mounted with Mowiol (Hoechst, Frankfurt, Germany), and photographed with a Zeiss Axioplan fluorescence microscope (Carl Zeiss, Oberkochen, Germany). The following antibodies were used: a rabbit anticollagen IV antibody (Timpl et al., 1978), rabbit anti-laminin-5 antibodies (anti-α3IIIa, anti-α3LG4-5, anti-γ2LE4–6, and anti-γ2L4m; Sasaki et al., 2001); a rabbit anti-phospho-focal adhesion kinase (pY397) antibody (diluted 1:100; BioSource, Nivelles, Belgium); a rabbit anti-phospho-protein kinase B (Akt) antibody (diluted 1:50; Cell Signaling Technology, Beverly, MA); a rabbit anti β1-integrin antibody (kindly provided by Dr. S. Johansson, The Biomedical Center, Uppsala University, Uppsala, Sweden); a rat anti-α6-integrin antibody (BD Biosciences PharMingen, San Diego, CA); a rabbit anti-cleaved caspase-3 antibody (diluted 1:100; Cell Signaling Technology); and a goat anti-type II keratin antibody (diluted 1:500; Abcam, Cambridge, United Kingdom). As secondary antibody, a goat anti-rabbit-Cy3 antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) was used for single stainings; for double immunofluorescence, a donkey anti goat-Alexa-488 antibody (Molecular Probes, Leiden, The Netherlands), a donkey anti-rat-Cy3 antibody, and a donkey anti-rabbit-Cy3 antibody (Jackson ImmunoResearch Laboratories) were used.
Detection of Proliferating Cells by Labeling with 5′-Bromodeoxyuridine (BrdU)
Mice were injected intraperitoneally with BrdU (250 mg/kg in 0.9% NaCl; Sigma Chemie) and sacrificed 2 h after injection. Bisected wounds were fixed in ethanol/acetic acid as described above. Sections were incubated with a peroxidase-conjugated monoclonal antibody directed against BrdU (Roche Diagnostics, Rotkreuz, Switzerland) and stained using the 3,3′-diaminobenzidine peroxidase substrate (Sigma Chemie). Counterstaining was performed with hematoxylin.
Electron Microscopy
Mice were lethally anesthetized with pentobarbital (700 mg/kg) and perfused with 4% PFA in PBS. Wounds were kept overnight in fixation solution, rinsed in PBS, and treated with 2% OsO4 for 2 h. After washing, they were stained in 1% uranyl acetate, dehydrated through a series of graded ethanols, and embedded in araldite resin. Semithin sections (1 μm) were cut with a glass knife by using an ultramicrotome (Reichert, Bensheim, Germany) and stained with methylene blue. Ultrathin sections (50–80 nm) for electron microscopic observation were processed on the same microtome with a diamond knife and placed on Formvar-coated copper grids. Sections were stained with uranyl acetate and lead nitrate. The transmission electron microscopy was performed with a Zeiss 902A electron microscope (Carl Zeiss).
RESULTS
Recombinant St2 Enhances Migration of Cultured Keratinocytes
St2 is expressed exclusively at the tip of the migrating epithelium in murine and human wounds (Saarialho-Kere et al., 1994; Madlener et al., 1996; Rechardt et al., 2000; Figure 1A, expression site of St2 marked in black). This expression site suggests a role of St2 in keratinocyte migration during wound healing. To address this hypothesis, we first performed in vitro migration assays with cultured primary human keratinocytes in the presence of various concentrations of recombinant human St2. As shown in Figure 1B, St2 significantly enhanced the migration of keratinocytes after scratch wounding in a dose-dependent manner.
Generation of a Constitutively Active St2 Mutant
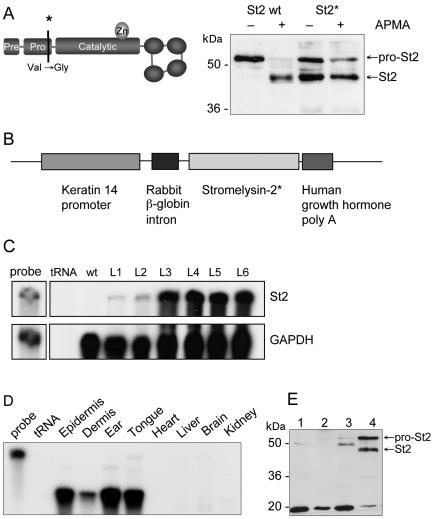
To determine the activities of St2 in vivo, a cDNA encoding a constitutively active form of this enzyme was generated. As demonstrated for St1 (MMP-3; Sanchez-Lopez et al., 1988; Park et al., 1991; Sympson et al., 1994) and matrilysin (MMP-7; Witty et al., 1994), specific point mutations in the proregion of these enzymes lead to autocatalytic activation of the enzyme. Taking advantage of the high homology between St1 and St2, especially in the proregion, we introduced an equivalent point mutation into the St2 cDNA, changing Val94 to Gly94 (Figure 2A, left). To verify the effect of the mutation, the mutated (St2*) and wild-type St2 cDNAs were cloned into the eukaryotic expression vector pcDNA3 and used for transient transfections of HEK-293 cells. Cell lysates and concentrated supernatants were analyzed by Western blotting. Aliquots of the supernatants were treated with APMA to activate proSt2. As shown in Figure 2A, right, wild-type St2 was secreted as inactive proenzyme, whereas the mutation led to autocatalytic processing of ∼50–60% of the total St2* protein. Surprisingly, no further activation of the mutated protein occurred after incubation with APMA, whereas the wild-type protein was fully activated in the presence of APMA. Comparison of cell lysates and supernatants showed that the autocatalytic processing occurs extracellularly, because only the proform of St2* was found in the cell lysates (our unpublished data). Therefore, effects seen would not be due to intracellular processing of St2*.
Figure 2.
Generation of transgenic mice expressing a constitutively active St2 mutant in the epidermis. (A) Introduction of a point mutation into the proregion of St2, changing Val94 to Gly94. Expression constructs of wild type (St2 wt) or mutated St2 (St2*) were used for transient transfection of HEK-293 cells. Equal volumes of the concentrated supernatants were left untreated or were treated with APMA and analyzed by Western blotting using an antibody against the carboxy terminus of St2 (right panel). (B) The construct for transgenic expression of St2* includes the human K14 promoter, the rabbit β-globin intron, the murine St2* cDNA, and the human growth hormone poly(A). (C) Total RNA (20 μg) was isolated from the back skin of transgenic animals (F1 generation) from different founder lines and from a wild-type animal and analyzed for the presence of St2 mRNA by RNase protection assay. As a loading control, the RNAs also were hybridized to the housekeeping gene gapdh. tRNA (20 μg) was used as a negative control; 1000 cpm of the hybridization probe was loaded in the lane labeled “probe” and used as a size marker. (D) RNA from different tissues or organs was analyzed for the presence of St2* mRNA by RNase protection assay as described in C. (E) Detection of the St2*-protein in cultured primary keratinocytes derived from wild-type (lanes 1 and 2) and St2* transgenic animals (lanes 3 and 4). Total cell lysates (40 μg; lanes 1 and 3) or acetoneprecipitated conditioned media (lanes 2 and 4) were analyzed by Western blotting using the St2-antibody described above.
Generation of Transgenic Mice Expressing High Levels of St2* in the Skin
To express St2* in the epidermis of transgenic mice, the mutated St2* cDNA was placed under the control of the human keratin 14 promoter (Figure 2B). This promoter targets the expression of transgenes to the basal epidermal keratinocytes and the outer root sheath keratinocytes of the hair follicles (Vassar et al., 1989; Munz et al., 1999; Wankell et al., 2001). Several transgenic lines were generated with FVB/N inbred background, named FVB-Tg(K14St2)Zbz, and with B6D2F2 hybrid oozytes, named Tg(K14St2). Expression of the transgene was determined in the F1 generation by RNase protection assay. Two lines on FVB/N background (L3 and L4) and two on B6D2 mixed background (L5 and L6) expressed high levels of the transgene, one B6D2-line (L2) had intermediate levels, and another (L1) expressed weak levels of St2* mRNA (Figure 2C). Endogenous St2-mRNA was not detectable in unwounded skin. The transgene had integrated at different sites of the genome in the different founder mice, as confirmed by Southern blotting (our unpublished data). All founders gave rise to transgenic offspring. As expected from the activity of the promoter, expression of the transgene was only found in the skin (shown in Figure 2D for epidermis from tail skin) as well as in the tongue, but not in other organs such as heart, liver, brain, or kidney, as determined by RNase protection assay. The weak signal found in the dermis (Figure 2D) resulted from expression in hair follicles (our unpublished data). Radioactive in situ hybridization confirmed that expression of the transgene was restricted to the basal layer of the epidermis and to the hair follicles (our unpublished data). St2* protein was detected in the supernatant of cultured primary keratinocytes derived from transgenic animals (Figure 2E, lane 4), but not in the cell lysate (lane 3), indicating efficient secretion of the overexpressed protein. Consistent with the results obtained by transient transfection of HEK-293 cells, ∼50% of the secreted protein had the size of the processed form.
St2*-overexpressing Mice Are Grossly Normal and Have an Unaltered Skin Architecture
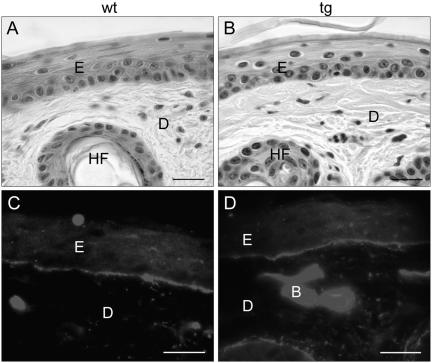
ST2* transgenic mice showed no visible differences compared with control littermates and reached the same average life span. The fur looked normal, and histological analysis of tail skin revealed no differences in skin architecture (Figure 3, A and B). These findings were confirmed by immunofluorescence staining of tail skin for the basement membrane proteins type IV collagen (Figure 3, C and D) and laminin-1 (our unpublished data) and for the keratinocyte differentiation markers keratin 14, keratin 10, and loricrin in back skin (our unpublished data). No difference in the rate of keratinocyte proliferation was observed as determined by BrdU incorporation (our unpublished data).
Figure 3.
Unaltered skin architecture in St2* transgenic mice. (A and B) Hematoxylin and eosin staining of paraffin sections from tail skin of wild-type (A) and transgenic (B) mice. (C and D) Immunofluorescence staining for collagen IV on ethanol/acetic acid fixed paraffin sections from wild-type (C) and transgenic (D) mice. E, epidermis; D, dermis; HF, hair follicle; B, blood vessel. Bars, 20 μm.
Normal Wound Closure in St2* Transgenic Mice
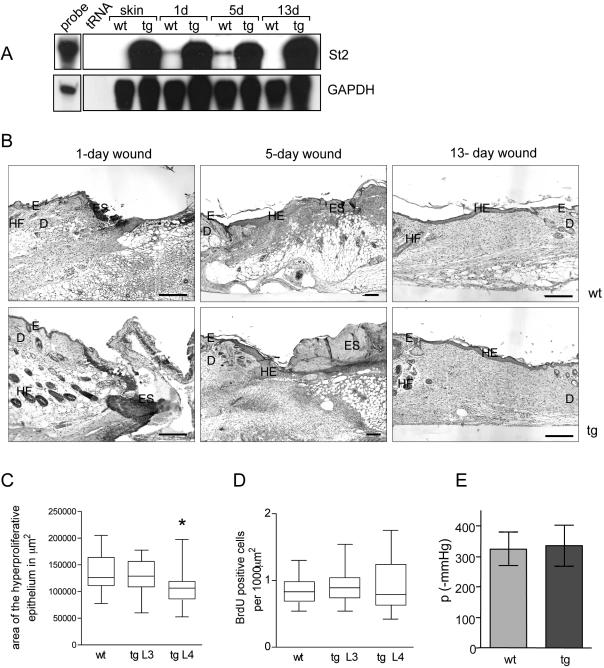
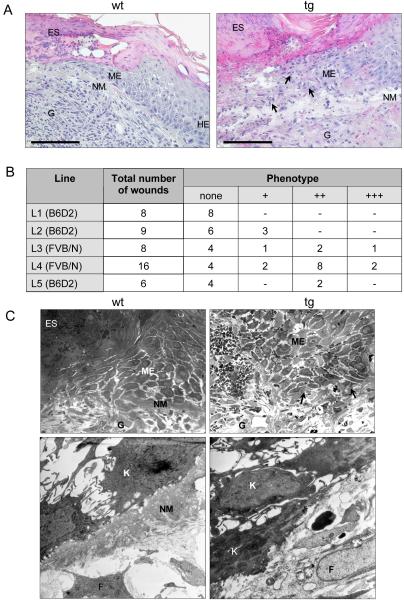
To examine the influence of St2* overexpression on wound healing, full-thickness excisional wounds were generated on the back of 9- to 12-wk-old male and female transgenic mice and control littermates. The initial wound healing analysis (Figures 4B and 5, A and B) was performed with all six transgenic lines. For a more detailed analysis, we focused on the two high-expressing lines on FVB/N background (L3 and L4). All experiments were performed with hemizygous transgenic mice. Only littermates of the same sex were used for a direct comparison. RNase protection assays confirmed a strong overexpression of transgene-derived St2* mRNA compared with the injury-induced endogenous St2 mRNA in wild-type animals at all time points after wounding (Figure 4A). At the histological level, no abnormalities in inflammation and granulation tissue formation and no obvious differences in the rate of reepithelialization were observed in 1- and 5-d wounds (Figure 4B). After 13 d, wounds in control and transgenic mice were closed and showed a similar area of granulation tissue (Figure 4B). Measurement of the area of the hyperproliferative epithelium in 5-d wounds revealed a slightly reduced reepithelialization rate in the transgenic animals of one line, but not in the others (Figure 4C). The difference in size of the wound epithelium was not caused by altered proliferation, as determined by measurement of BrdU incorporation in 3(our unpublished data) and 5-d wounds (Figure 4D).
Figure 4.
Normal wound closure, keratinocyte proliferation, and bursting strength in St2* transgenic mice. (A) Relative expression levels of endogenous and transgene-derived St2 in intact skin and after wounding. Samples of total cellular RNA (20 μg) from normal and wounded skin, derived from wild-type and St2*-transgenic animals, were analyzed by RNase protection assay for the expression of St2 as described in the legend to Fig. 2C. (B) Full-thickness excisional wounds were generated on the back of wild-type (wt) and St2*-transgenic (tg) mice. Sections from the middle of 1-, 5-, and 13-d wounds were stained with hematoxylin and eosin. D, dermis; E, epidermis; ES, eschar; HE, hyperproliferative epithelium; G, granulation tissue; HF, hair follicle. Bar, 200 μm. (C) Measurement of the size of the hyperproliferative epithelium in 5-d wounds by using Openlab software. The hyperthickened epithelium on each side of the wound was measured separately, and only animals of the same age and sex from a single wounding experiment were used for direct comparison. In total, 19 wounds from control mice, 14 wounds from transgenic mice of line 3, and 17 wounds from line 4 were analyzed; wounds were from two independent wounding experiments. Statistical analysis was performed with GraphPad Prism4 software. The box extends from 25th to 75th percentile, with the horizontal line indicating the median (the 50th percentile) and the whiskers marking the highest and lowest values. p values according to Student's t test are p = 0.33 for comparison of wild-type mice with transgenic mice of line 3 and p = 0.045 for comparison of wild-type mice with transgenic mice of line 4. (D) Analysis of the proliferation rate of keratinocytes in 5-d wounds. Mice were injected with BrdU and sacrificed 2 h later. Ethanol/acetic acid-fixed sections were immunostained with an antibody against BrdU. BrdU-positive cells in the hyperproliferative epithelium were counted using the Openlab software and are depicted as the number of BrdU-positive cells per 1000 μm2 of epithelium. Statistical analysis was performed as described in C. Number of wounds analyzed: n = 13 for the wild-type mice (wt) and n = 10 for both transgenic (tg) lines. (E) Biomechanical tissue characterization displays no significant difference in bursting strength of 5-d full-thickness incisional wounds from wild-type (wt) or transgenic (tg) mice. Wound strength was measured as maximum amount of negative pressure (mmHg) required for bursting. 21 wounds from seven wild-type mice and 24 wounds from seven transgenic mice were analyzed in two independent experiments.
Figure 5.
Disorganized keratinocytes at the tip of the migrating epithelium in wounds of St2* transgenic mice. (A) Hematoxylin and eosin staining of sections from 5-d wounds from wild-type (wt) and St2* transgenic (tg) mice at high magnification. HE, hyperproliferative epithelium; ME, migrating tip of the epithelium; G, granulation tissue; ES, eschar. Bar, 50 μm. (B) Histological grading of the extent of keratinocyte scattering and abnormal matrix deposition at the wound edge for each transgenic line. Definition of the grades: +, slight loss of matrix at few distinct spots along the wound epidermis; ++, matrix loss and keratinocyte scattering at several spots at the epithelial tip; +++, severe matrix loss and keratinocyte scattering affecting all migrating keratinocytes. Mouse lines are arranged according to increasing expression levels of the transgene. The genetic background of the lines is indicated in parentheses. (C) Ultrastructural analysis of 5-d wounds from wild-type (wt) and St2*-transgenic (tg) mice. Semithin sections (1 μm) from paraformaldehyde-fixed, araldite-embedded wounds were stained with methylene blue (top). G, granulation tissue; ME, migrating keratinocytes. Areas lacking matrix deposition in transgenic animals are marked with arrowheads. Bottom, electron microscopy from the tip of the migrating epithelium. The picture shows the border between epidermis and granulation tissue. K, keratinocyte; F, fibroblast.
Because St2* is a secreted enzyme, we assessed whether the transgene protein affected the formation of the new dermal tissue. For that purpose, we generated full-thickness incisional wounds and measured the bursting strength after 5 d, when incisional wounds are fully closed. However, no differences between control and transgenic animals were detected (Figure 4E).
To assess whether the overexpression of St2* was compensated by counterregulation of other MMPs or inhibitors, we performed RNase protection assays to determine the expression of St1 (MMP-3), gelatinase B (MMP-9), and collagenase-3 (MMP-13), which also are expressed in the wounded mouse epidermis (Madlener et al., 1998), and of TIMPs 1, 2, and 3. None of these genes showed an altered expression between wild-type and St2* transgenic mice (our unpublished data). Furthermore, the expression of markers for angiogenesis (vascular endothelial growth factor) and inflammation (interleukin-1β) and of major matrix proteins (collagen I, collagen III, tenascin-C, and fibronectin) was unaltered (our unpublished data).
The Tip of the Migrating Epithelium in Wounds of Transgenic Mice Is Disorganized
Although overexpression of St2* had no obvious effect on the general healing process, striking abnormalities were observed at the histological level. Most noticeably, the tips of the migrating epithelium looked disorganized. Whereas in wild-type mice migrating keratinocytes stayed close together and formed a sharp cell border with a thick layer of new underlying extracellular matrix (indicated with NM in Figure 5A), no clear border of the migrating epithelium was seen in the transgenic mice; keratinocytes were scattered at the tip of the epithelium and lost contact to each other (Figure 5A). The extent of scattering strongly correlated with the loss of new matrix. To quantify this observation, we defined three histological grades of the phenotype (indicated +, ++, and +++ in Figure 5B; see figure legend for specification). The micrograph of the wound of a transgenic mouse in Figure 5A corresponds to a phenotype of grade +++. The severity of the phenotype correlated strongly with the expression level of the transgene (compare Figure 5B with Figure 2C). Furthermore, the phenotype was dependent on the genetic background of the mice. In the inbred strain FVB/N, a more severe phenotype was observed than in mice with B6D2 mixed background (Figure 5B).
Electron microscopy (Figure 5C) showed a layer of newly deposited matrix (NM) underneath the keratinocytes in wild-type mice, which extended almost to the tip of the migrating epithelium and clearly separated the migrating keratinocytes from the granulation tissue. In contrast, in wounds of transgenic mice with a severe phenotype (graded as +++), this matrix layer was missing. The matrix below the migrating keratinocytes is regarded as a precursor of the newly forming basement membrane, because it includes major basement membrane proteins, such as collagen IV, laminin-1 (our unpublished data), and laminin-5 (see below). Electron microscopy analysis, as well as immunostaining for collagen IV and laminin-1, however, showed that the reformation of the basement membrane is not significantly impaired. Furthermore, as soon as the new basement membrane was formed, no more scattering of keratinocytes was observed (our unpublished data).
Altered Deposition of Laminin-5 in Wounds of St2* Transgenic Animals and Processing of Laminin-5 by St2 In Vitro
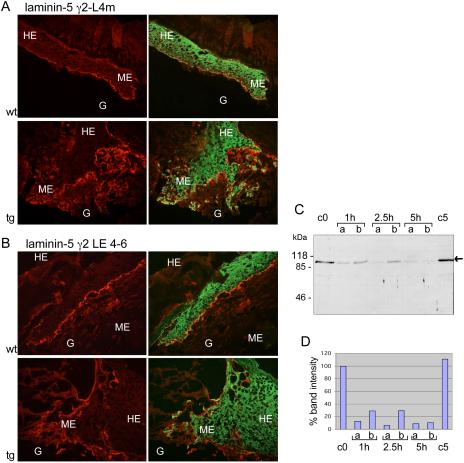
To further characterize the alterations in the deposited matrix below the tip of the migrating epithelium in 5-d wounds of St2* transgenic mice, we analyzed the expression and distribution of various extracellular matrix components, which are known to play an important role in wound healing. Immunofluorescence staining revealed no obvious differences in the expression and localization of fibrin/fibrinogen, fibronectin, collagen IV, laminin-1, and tenascin C (our unpublished data). However, we found striking differences in the expression pattern of laminin-5 by using antibodies against the amino-terminal proregion (γ2-L4m; Figure 6A, top, red) or an internal region (γ2-LE4–6; Figure 6B, top, red) of the γ2 chain in combination with an antibody against type II keratin (green). With both laminin-5 antibodies, we found a sharp band of laminin-5 underneath the keratinocytes in wounds of wild-type animals. By contrast, in wounds of transgenic animals laminin-5 was not correctly deposited in the wound matrix. It was not restricted to the basal site of the keratinocytes, but it looked dispersed. In particular, the antibody against the amino terminus of the γ2 chain stained a more extended area below the migrating keratinocytes. This staining pattern suggests that deposition of laminin-5 in the wound is altered, possibly as a result of aberrant processing/degradation. Similar results also were obtained upon staining for precursor and mature laminin-5 α3 chain (our unpublished data). The observed changes were not caused by marked alterations in the mRNA levels of both laminin-α3 or laminin-γ2, as determined by RNase protection assay (our unpublished data).
Figure 6.
Altered deposition of laminin-5 in wounds of St2* transgenic animals and processing of laminin-5 by St2 in vitro. (A and B) Immunofluorescence staining of frozen sections from 5-d-old wounds of wild-type and transgenic animals for laminin-5 (red) and type II-keratin (green). G, granulation tissue; HE, hyperproliferative epithelium; ME, migrating epithelium. (A) Staining for the N-terminal propeptide of the laminin-5 γ2 chain by using an antibody directed against the L4m region. (B) Staining for the mature laminin-5 γ2 chain by using an antibody against the LE modules 4–6. (C and D) In vitro cleavage of laminin-5 γ2 chain by recombinant St2. (C) Ninety nanograms per lane of purified human laminin-5 was incubated with recombinant active human St2 at enzyme:substrate ratios of 1:5 (lanes a) and 1:50 (lanes b) for the indicated time periods. Samples were analyzed by Western blotting by using the γ2-LE 4-6 antibody. Densitometric quantification is shown in D. The band intensity of the 0 h control was set as 100%.
To determine whether the altered deposition of laminin-5 is the result of direct cleavage by St2*, we incubated laminin-5, purified from the supernatant of human keratinocytes, with recombinant St2. Because cell culture-derived laminin-5 is always partially processed (the α3 chain is present only as the mature 165-kDa form, γ2 chain is processed to 105 kDa), we focused on a potential further processing that had been shown before for some other MMPs (Koshikawa et al., 2000; Pirila et al., 2003; Udayakumar et al., 2003). As shown in Figure 6, C and D, we found a time- and concentration-dependent disappearance of the γ2-chain signal upon treatment with St2, indicating that laminin-5 is indeed a substrate of this enzyme.
Overexpression of St2* Affects β1-Integrin Expression, Focal Adhesion Kinase (FAK) and Akt Phosphorylation, and Apoptosis Signaling in Keratinocytes
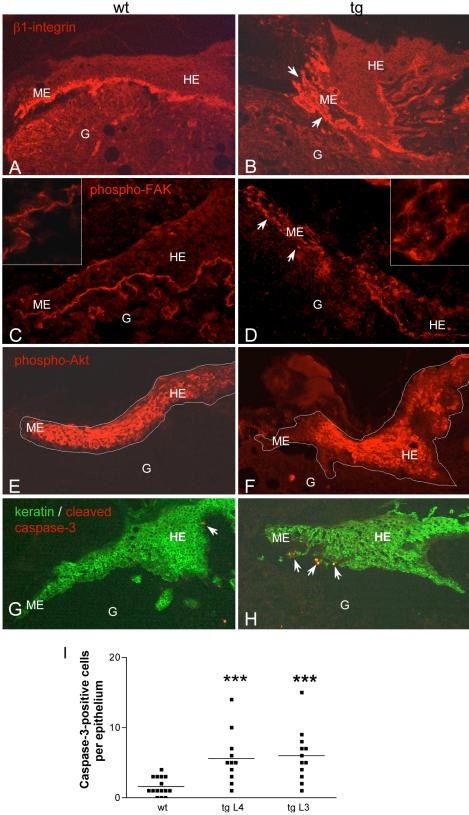
To determine whether the impaired laminin-5 deposition in wounds of transgenic animals also affected cell–matrix interactions, we analyzed the localization of its major binding partners, the integrins α3β1 and α6β4 (Rousselle and Aumailley, 1994). In contrast to wounds from wild-type mice (Figure 7A), the expression of the β1-integrin subunit was not restricted to the basal layer of keratinocytes (Figure 7B). All keratinocytes that were not in close contact to neighboring cells expressed β1-integrins. A similar staining pattern was observed for α6-integrins (our unpublished data). Consistent with the distribution of β1 and α6 integrins, activated FAK was restricted to the basolateral side of basal keratinocytes in wild-type mice (Figure 7C), as determined by staining with a phospho-specific antibody directed against the autophosphorylation site Y397. In contrast, scattered keratinocytes from transgenic animals showed a diffuse signal for phospho-FAK all around the cells (Figure 7D).
Figure 7.
Altered β1-integrin expression and FAK phosphorylation, reduced Akt phosphorylation, and activation of apoptotic signaling in 5-d wounds of transgenic mice. Immunofluorescence staining of frozen (C, D, G, and H) or ethanol/acetic acid-fixed paraffin sections (A, B, E, and F) from 5-d old wounds of wild-type and transgenic animals. G, granulation tissue; HE, hyperproliferative epithelium; ME, migrating epithelium. (A and B) Staining for β1-integrins; scattered keratinocytes are marked with arrowheads. (C and D) staining for phospho-FAK (Y397). A higher magnification of the epithelial tongue (1000×) is shown in the corners of the pictures. (E and F) Staining for phospho-Akt; the borders of the epithelium are marked with a white line. (G and H) Staining for cleaved caspase-3 (red) and type II-keratin (green); apoptotic keratinocytes (yellow) are marked with arrowheads. (I) Number of caspase-3–positive cells per epithelium in wild-type and transgenic animals of two different founder lines. Wound sections were counted regardless of the severity of their phenotype. Statistical analysis was performed using GraphPad Prism4 software. The horizontal line indicates the median (the 50th percentile), and each dot corresponds to one analyzed epithelium. p values are p = 0.0006 for comparison of wt with line 4 and p = 0.0002 for comparison of wt with line 3, according to Student's t test.
In about half of the wounds with a strong phenotype, the changes in the localization of β1 integrins and activated FAK were accompanied by reduced phosphorylation of Akt at the tip of the migrating epithelium (Figure 7F). Because reduced Akt signaling is involved in the induction of apoptosis, we stained 5-d wounds with an antibody against cleaved caspase-3 as a marker for apoptotic cells (Figure 7, G and H, red). Keratinocytes of the hyperproliferative epithelium were counterstained with an antibody against type II keratins (Figure 7, G and H, green). A significantly greater number of caspase-3–positive keratinocytes (yellow in Figure 7, G and H) was found in wounds of transgenic mice (Figure 7, G–I). Although these cells were predominantly found at the tip of the epithelium, occasional positive cells were seen surrounded by neighboring keratinocytes.
DISCUSSION
Up-regulation of St2 expression occurs in several normal and pathological processes associated with tissue remodeling, including wound healing (Saarialho-Kere et al., 1994; Madlener et al., 1996; Okada et al., 1997; Madlener et al., 1998; Rechardt et al., 2000), diabetic retinopathy (Saghizadeh et al., 2001), and carcinogenesis (Kerkela et al., 2001). Although this pattern of expression suggests an important role of St2 in repair and disease, functional studies aimed at the elucidation of the in vivo functions of St2 have not yet been performed. In this study, we showed that the expression site of St2 in skin wounds, which is restricted to the tip of the migrating epithelium (Madlener et al., 1998) correlates with a promigratory effect of St2 on keratinocytes in vitro. To determine the activities of St2 in vivo, we generated transgenic mice overexpressing St2 in basal keratinocytes, the cell type that expresses the endogenous protein in wounded skin (Saarialho-Kere et al., 1994; Madlener et al., 1996; Okada et al., 1997; Madlener et al., 1998; Rechardt et al., 2000). To ensure the proteolytic activity of St2 independent of the presence of endogenous activators, we generated a constitutively active St2-mutant, which resulted in autocatalytic processing of ∼50% of the enzyme, similar to the results obtained with equivalent mutations in other MMPs (Sanchez-Lopez et al., 1988; Park et al., 1991; Witty et al., 1994).
The most striking finding of our study were the abnormalities seen in 5-d wounds of St2* transgenic animals. The normal alignment of keratinocytes migrating into the wound was disturbed; keratinocytes were scattered, and the layer of matrix that is normally deposited underneath the migrating keratinocytes was partially lacking. The severity of this phenotype was strongly dependent both on the expression level of the transgene and on the genetic background. In the FVB/N mouse lines with high transgene expression levels, we found these characteristic abnormalities in the sections from most of the wounds. The lack of obvious abnormalities in the remaining sections may be due to individual differences in the expression of the transgene or to differences in the local composition of the wound environment. Because we only analyzed sections from the middle of the wound, it may be possible that we missed phenotypic abnormalities in adjacent areas of the injured tissue. Consistent with this possibility, we often found slight variations in the intensity of the phenotype between serial sections.
Electron microscopy suggested that the scattering of keratinocytes was not due to an impaired capacity to form cell–cell contacts, because the number and subcellular localization of desmosomes looked normal as soon as keratinocytes were neighbored (our unpublished data). These findings indicate that the disorganization of the migrating epithelium is, at least in part, a consequence of the matrix alterations.
A detailed analysis of the matrix composition in 5-d wounds of transgenic animals revealed significant abnormalities in the deposition of laminin-5. Deposits of this matrix protein seemed highly dispersed, resembling the appearance of the matrix structures seen in hematoxylin and eosin stainings. This may be due to fragmentation of laminin-5 by St2*. Consistent with this hypothesis, we showed that laminin-5 is a direct substrate of St2. Most interestingly, much lower enzyme: substrate ratios of St2 were needed for degradation of laminin-5 than reported for other MMPs (Pirila et al., 2003). This is surprising, because St2 has a lower activity than St1 or other MMPs on all other substrates identified so far (Murphy et al., 1991). Collagen IV, an in vitro identified St2 substrate (Murphy et al., 1991), which is highly expressed in wounded skin, seemed not to be affected in St2* transgenic mice, supporting the idea that laminin-5 is the major in vivo substrate of St2*, at least in wounded skin.
Because laminin-5 is a major basement membrane component, it is surprising that neither the basement membrane of nonwounded skin nor the newly formed basement membrane in healing wounds was obviously affected. It seems possible that laminin-5 is immediately bound to α6β4 integrins or to other basement membrane components and thus becomes inaccessible for St2 in nonwounded or regenerated skin. Alternatively, the presence of the MMP inhibitors TIMP-2 and -3 in nonwounded epidermis and dermis (Madlener et al., 1998) might be sufficient to inhibit St2* action.
The altered deposition of laminin-5 in wounded skin is most likely directly responsible for the observed phenotype, especially for the scattering of keratinocytes: Laminin-5 has originally been identified as a “scatter factor” for epithelial cells (Miyazaki et al., 1993), and it is essential both for proper adhesion and for migration of keratinocytes (Goldfinger et al., 1998). It is widely accepted that during migration, keratinocytes initially deposit large amounts of laminin-5 precursor into the matrix. A tightly regulated stepwise processing of laminin-5 modulates the affinity to different integrin receptors and thereby is essential to maintain the balance between adhesion and motility that is required for controlled migration (reviewed by Nguyen et al., 2000b). Whereas processing of the α3-chain of laminin-5 promotes adhesion (Goldfinger et al., 1999), several studies showed that cleavage of the γ2-chain enhances migration of various cell types (Pirila et al., 2003; Kariya and Miyazaki, 2004; Ogawa et al., 2004). Therefore, we propose that in St2 transgenic mice, an excessive cleavage of the laminin-5 γ2 chain disturbs the controlled migration of keratinocytes into the wound, resulting in keratinocyte scattering. Thus, our results strongly suggest that laminin-5 regulates keratinocyte migration in vitro and in vivo through similar mechanisms.
Interestingly, the altered structure of the migrating epithelium was accompanied by abnormal expression of β1-integrins in the hyperproliferative wound epidermis. A major player in integrin signaling is FAK (Giancotti and Ruoslahti, 1999; Miranti and Brugge, 2002). The activation pattern of this intracellular tyrosine kinase in excisional mouse wounds has not been determined yet. Here, we show that activated FAK colocalizes with β1-integrins, forming a sharp band at the epithelial border in normal mice. Similarly, in wounds of transgenic mice, a strong correlation of β1-integrin expression and FAK activation was found. In the scattered keratinocytes, FAK-phosphorylation was not confined to the basal side of the cells but rather was seen as a diffuse signal around the cells. These findings suggest that the abnormal distribution of keratinocyte integrins resulted in alterations of FAK phosphorylation. Another serine/threonine kinase involved in integrin signaling is protein kinase B/Akt (Stupack and Cheresh, 2002). We found that Akt phosphorylation was reduced at the tip of the epithelium in a subset of wounds from transgenic mice. The fact that this reduction was not consistently observed may be explained by the involvement of Akt in several signal transduction pathways, which may not all be affected by St2* overexpression. These pathways may compensate to a variable extent for the abnormal matrix-dependent signaling. Because phosphorylation of Akt is required for its activation, our findings suggest that Akt signaling is reduced in wound keratinocytes of transgenic mice. Consistent with the importance of Akt in the transduction of survival signals from the matrix to the cells (Scheid and Woodgett, 2003), we found a significantly increased activation of apoptotic signaling in keratinocytes in the newly forming epithelium in 5-d-old wounds. This is most likely the consequence of inappropriate matrix contact of the cells and may serve as a compensatory mechanism to reduce uncontrolled spreading of keratinocytes at the wound edge. Therefore, it will be interesting to analyze the St2* overexpressing mice in a carcinogenesis model, where such regulatory mechanisms may no longer exist.
Does the observed phenotype reflect the role of endogenous St2 in wound healing? Assuming that the major effect of St2* overexpression is increased laminin-5 processing, it is tempting to speculate that endogenous St2 also is involved in the processing of laminin-5 required during the healing process. The tight temporal and spatial control of laminin-5 processing during keratinocyte migration that has been demonstrated in various in vitro studies (Goldfinger et al., 1999; Nguyen et al., 2000a; Frank and Carter, 2004) correlates well with the restricted expression pattern of endogenous St2 at the migrating tip of the epithelium (Madlener et al., 1998). Thus, we propose that the appropriately regulated and localized expression of St2 in keratinocytes is required for the organized migration of these cells into the wound site. By contrast, excessive expression of this enzyme causes scattering of normal keratinocytes in wounded skin and possibly invasive growth of malignant cells.
Acknowledgments
We thank Dr. M. Madlener for the generation of the St2* mutant cDNA, Dr. K. Bleuel for help with the initial analysis of the animals, Dr. C. Mauch for helpful suggestions, and C. Born-Berclaz for excellent technical assistance. This work was supported by grants from the Swiss National Science foundation (grant 31-61358.00), the German Ministry for Education and Research, and the “Bundesamt für Bildung und Wissenschaft”, Switzerland (BBW, no. 01.0139; EC grant QLG1-CT-2001-00869, to S.W.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04–02–0109. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–02–0109.
References
- Birkedal-Hansen, B., Pavelic, Z.P., Gluckman, J.L., Stambrook, P., Li, Y.Q., and Stetler-Stevenson, W.G. (2000). MMP and TIMP gene expression in head and neck squamous cell carcinomas and adjacent tissues. Oral Dis. 6, 376–382. [DOI] [PubMed] [Google Scholar]
- Bodey, B., Bodey, B., Jr., Siegel, S.E., and Kaiser, H.E. (2001). Matrix metalloproteinases in neoplasm-induced extracellular matrix remodeling in breast carcinomas. Anticancer Res. 21, 2021–2028. [PubMed] [Google Scholar]
- Caldelari, R., Suter, M.M., Baumann, D., De Bruin, A., and Muller, E. (2000). Long-term culture of murine epidermal keratinocytes. J. Investig. Dermatol. 114, 1064–1065. [DOI] [PubMed] [Google Scholar]
- Chen, C., and Okayama, H. (1987). High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 7, 2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeblad, M., and Werb, Z. (2002). New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2, 161–174. [DOI] [PubMed] [Google Scholar]
- Frank, D.E., and Carter, W.G. (2004). Laminin 5 deposition regulates keratinocyte polarization and persistent migration. J. Cell Sci. 117, 1351–1363. [DOI] [PubMed] [Google Scholar]
- Giancotti, F.G., and Ruoslahti, E. (1999). Integrin signaling. Science 285, 1028–1032. [DOI] [PubMed] [Google Scholar]
- Goldfinger, L.E., Hopkinson, S.B., deHart, G.W., Collawn, S., Couchman, J.R., and Jones, J.C. (1999). The alpha3 laminin subunit, alpha6beta4 and alpha3beta1 integrin coordinately regulate wound healing in cultured epithelial cells and in the skin. J. Cell Sci. 112, 2615–2629. [DOI] [PubMed] [Google Scholar]
- Goldfinger, L.E., Stack, M.S., and Jones, J.C. (1998). Processing of laminin-5 and its functional consequences: role of plasmin and tissue-type plasminogen activator. J. Cell Biol. 141, 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariya, Y., and Miyazaki, K. (2004). The basement membrane protein laminin-5 acts as a soluble cell motility factor. Exp. Cell Res. 297, 508–520. [DOI] [PubMed] [Google Scholar]
- Kerkela, E., Ala-aho, R., Lohi, J., Grenman, R., M-Kahari, V., and Saarialho-Kere, U. (2001). Differential patterns of stromelysin-2 (MMP-10) and MT1-MMP (MMP-14) expression in epithelial skin cancers. Br J Cancer 84, 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshikawa, N., Giannelli, G., Cirulli, V., Miyazaki, K., and Quaranta, V. (2000). Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J. Cell Biol. 148, 615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund, L.R., Romer, J., Bugge, T.H., Nielsen, B.S., Frandsen, T.L., Degen, J.L., Stephens, R.W., and Dano, K. (1999). Functional overlap between two classes of matrix-degrading proteases in wound healing. EMBO J 18, 4645–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madlener, M., Mauch, C., Conca, W., Brauchle, M., Parks, W.C., and Werner, S. (1996). Regulation of the expression of stromelysin-2 by growth factors in keratinocytes: implications for normal and impaired wound healing. Biochem. J. 320, 659–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madlener, M., Parks, W.C., and Werner, S. (1998). Matrix metalloproteinases (MMPs) and their physiological inhibitors (TIMPs) are differentially expressed during excisional skin wound repair. Exp. Cell Res. 242, 201–210. [DOI] [PubMed] [Google Scholar]
- Madlener, M., and Werner, S. (1997). cDNA cloning and expression of the gene encoding murine stromelysin-2 (MMP-10). Gene 202, 75–81. [DOI] [PubMed] [Google Scholar]
- Martin, P. (1997). Wound healing–aiming for perfect skin regeneration. Science 276, 75–81. [DOI] [PubMed] [Google Scholar]
- Mathew, R., Khanna, R., Kumar, R., Mathur, M., Shukla, N.K., and Ralhan, R. (2002). Stromelysin-2 overexpression in human esophageal squamous cell carcinoma: potential clinical implications. Cancer Detect. Prev. 26, 222–228. [DOI] [PubMed] [Google Scholar]
- McCawley, L.J., and Matrisian, L.M. (2001). Matrix metalloproteinases: they're not just for matrix anymore! Curr. Opin. Cell Biol. 13, 534–540. [DOI] [PubMed] [Google Scholar]
- Miranti, C.K., and Brugge, J.S. (2002). Sensing the environment: a historical perspective on integrin signal transduction. Nat. Cell Biol. 4, E83–E90. [DOI] [PubMed] [Google Scholar]
- Miyazaki, K., Kikkawa, Y., Nakamura, A., Yasumitsu, H., and Umeda, M. (1993). A large cell-adhesive scatter factor secreted by human gastric carcinoma cells. Proc. Natl. Acad. Sci. USA 90, 11767–11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munz, B., Smola, H., Engelhardt, F., Bleuel, K., Brauchle, M., Lein, I., Evans, L.W., Huylebroeck, D., Balling, R., and Werner, S. (1999). Overexpression of activin A in the skin of transgenic mice reveals new activities of activin in epidermal morphogenesis, dermal fibrosis and wound repair. EMBO J. 18, 5205–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, G., Cockett, M.I., Ward, R.V., and Docherty, A.J. (1991). Matrix metalloproteinase degradation of elastin, type IV collagen and proteoglycan. A quantitative comparison of the activities of 95 kDa and 72 kDa gelatinases, stromelysins-1 and -2 and punctuated metalloproteinase (PUMP). Biochem. J. 277, 277–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, B.P., Gil, S.G., and Carter, W.G. (2000a). Deposition of laminin 5 by keratinocytes regulates integrin adhesion and signaling. J. Biol. Chem. 275, 31896–31907. [DOI] [PubMed] [Google Scholar]
- Nguyen, B.P., Ryan, M.C., Gil, S.G., and Carter, W.G. (2000b). Deposition of laminin 5 in epidermal wounds regulates integrin signaling and adhesion. Curr. Opin. Cell Biol. 12, 554–562. [DOI] [PubMed] [Google Scholar]
- Nicholson, R., Murphy, G., and Breathnach, R. (1989). Human and rat malignant-tumor-associated mRNAs encode stromelysin-like metalloproteinases. Biochemistry 28, 5195–5203. [DOI] [PubMed] [Google Scholar]
- Ogawa, T., Tsubota, Y., Maeda, M., Kariya, Y., and Miyazaki, K. (2004). Regulation of biological activity of laminin-5 by proteolytic processing of gamma2 chain. J. Cell Biochem. 92, 701–714. [DOI] [PubMed] [Google Scholar]
- Okada, A., Tomasetto, C., Lutz, Y., Bellocq, J.P., Rio, M.C., and Basset, P. (1997). Expression of matrix metalloproteinases during rat skin wound healing: evidence that membrane type-1 matrix metalloproteinase is a stromal activator of pro-gelatinase A. J. Cell Biol. 137, 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, A.J., Matrisian, L.M., Kells, A.F., Pearson, R., Yuan, Z.Y., and Navre, M. (1991). Mutational analysis of the transin (rat stromelysin) autoinhibitor region demonstrates a role for residues surrounding the “cysteine switch”. J. Biol. Chem. 266, 1584–1590. [PubMed] [Google Scholar]
- Parks, W.C. (1999). Matrix metalloproteinases in repair. Wound Repair Regen. 7, 423–432. [DOI] [PubMed] [Google Scholar]
- Parks, W.C., Wilson, C.L., and Lopez-Boado, Y.S. (2004). Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 4, 617–629. [DOI] [PubMed] [Google Scholar]
- Pilcher, B.K., Wang, M., Qin, X.J., Parks, W.C., Senior, R.M., and Welgus, H.G. (1999). Role of matrix metalloproteinases and their inhibition in cutaneous wound healing and allergic contact hypersensitivity. Ann. N.Y. Acad. Sci. 878, 12–24. [DOI] [PubMed] [Google Scholar]
- Pirila, E., Sharabi, A., Salo, T., Quaranta, V., Tu, H., Heljasvaara, R., Koshikawa, N., Sorsa, T., and Maisi, P. (2003). Matrix metalloproteinases process the laminin-5 gamma 2-chain and regulate epithelial cell migration. Biochem. Biophys. Res. Commun. 303, 1012–1017. [DOI] [PubMed] [Google Scholar]
- Rechardt, O., Elomaa, O., Vaalamo, M., Paakkonen, K., Jahkola, T., Hook-Nikanne, J., Hembry, R.M., Hakkinen, L., Kere, J., and Saarialho-Kere, U. (2000). Stromelysin-2 is upregulated during normal wound repair and is induced by cytokines. J. Investig. Dermatol. 115, 778–787. [DOI] [PubMed] [Google Scholar]
- Rheinwald, J.G., and Green, H. (1975). Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6, 331–343. [DOI] [PubMed] [Google Scholar]
- Rousselle, P., and Aumailley, M. (1994). Kalinin is more efficient than laminin in promoting adhesion of primary keratinocytes and some other epithelial cells and has a different requirement for integrin receptors. J. Cell Biol. 125, 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarialho-Kere, U.K., Pentland, A.P., Birkedal-Hansen, H., Parks, W.C., and Welgus, H.G. (1994). Distinct populations of basal keratinocytes express stromelysin-1 and stromelysin-2 in chronic wounds. J. Clin. Investig. 94, 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh, M., et al. (2001). Overexpression of matrix metalloproteinase-10 and matrix metalloproteinase-3 in human diabetic corneas: a possible mechanism of basement membrane and integrin alterations. Am. J. Pathol. 158, 723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Lopez, R., Nicholson, R., Gesnel, M.C., Matrisian, L.M., and Breathnach, R. (1988). Structure-function relationships in the collagenase family member transin. J. Biol. Chem. 263, 11892–11899. [PubMed] [Google Scholar]
- Sasaki, T., Gohring, W., Mann, K., Brakebusch, C., Yamada, Y., Fassler, R., and Timpl, R. (2001). Short arm region of laminin-5 gamma2 chain: structure, mechanism of processing and binding to heparin and proteins. J. Mol. Biol. 314, 751–763. [DOI] [PubMed] [Google Scholar]
- Scheid, M.P., and Woodgett, J.R. (2003). Unravelling the activation mechanisms of protein kinase B/Akt. FEBS Lett. 546, 108–112. [DOI] [PubMed] [Google Scholar]
- Sternlicht, M.D., and Werb, Z. (2001). How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 17, 463–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupack, D.G., and Cheresh, D.A. (2002). Get a ligand, get a life: integrins, signaling and cell survival. J. Cell Sci. 115, 3729–3738. [DOI] [PubMed] [Google Scholar]
- Sympson, C.J., Talhouk, R.S., Alexander, C.M., Chin, J.R., Clift, S.M., Bissell, M.J., and Werb, Z. (1994). Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J. Cell Biol. 125, 681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasanen, K., Tunggal, L., Chometon, G., Bruckner-Tuderman, L., and Aumailley, M. (2004). Keratinocytes from patients lacking collagen XVII display a migratory phenotype. Am. J. Pathol. 164, 2027–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl, R., Martin, G.R., Bruckner, P., Wick, G., and Wiedemann, H. (1978). Nature of the collagenous protein in a tumor basement membrane. Eur. J. Biochem. 84, 43–52. [DOI] [PubMed] [Google Scholar]
- Udayakumar, T.S., Chen, M.L., Bair, E.L., Von Bredow, D.C., Cress, A.E., Nagle, R.B., and Bowden, G.T. (2003). Membrane type-1-matrix metalloproteinase expressed by prostate carcinoma cells cleaves human laminin-5 beta3 chain and induces cell migration. Cancer Res. 63, 2292–2299. [PubMed] [Google Scholar]
- Vaalamo, M., Karjalainen-Lindsberg, M.L., Puolakkainen, P., Kere, J., and Saarialho-Kere, U. (1998). Distinct expression profiles of stromelysin-2 (MMP-10), collagenase-3 (MMP-13), macrophage metalloelastase (MMP-12), and tissue inhibitor of metalloproteinases-3 (TIMP-3) in intestinal ulcerations. Am. J. Pathol. 152, 1005–1014. [PMC free article] [PubMed] [Google Scholar]
- Vaalamo, M., Weckroth, M., Puolakkainen, P., Kere, J., Saarinen, P., Lauharanta, J., and Saarialho-Kere, U.K. (1996). Patterns of matrix metalloproteinase and TIMP-1 expression in chronic and normally healing human cutaneous wounds. Br. J. Dermatol. 135, 52–59. [PubMed] [Google Scholar]
- Vassar, R., Rosenberg, M., Ross, S., Tyner, A., and Fuchs, E. (1989). Tissue-specific and differentiation-specific expression of a human K14 keratin gene in transgenic mice. Proc. Natl. Acad. Sci. USA 86, 1563–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visse, R., and Nagase, H. (2003). Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 92, 827–839. [DOI] [PubMed] [Google Scholar]
- Vu, T.H., and Werb, Z. (2000). Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 14, 2123–2133. [DOI] [PubMed] [Google Scholar]
- Wankell, M., Munz, B., Hubner, G., Hans, W., Wolf, E., Goppelt, A., and Werner, S. (2001). Impaired wound healing in transgenic mice overexpressing the activin antagonist follistatin in the epidermis. EMBO J. 20, 5361–5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner, S., Smola, H., Liao, X., Longaker, M.T., Krieg, T., Hofschneider, P.H., and Williams, L.T. (1994). The function of KGF in morphogenesis of epithelium and reepithelialization of wounds. Science 266, 819–822. [DOI] [PubMed] [Google Scholar]
- Werner, S., Weinberg, W., Liao, X., Peters, K.G., Blessing, M., Yuspa, S.H., Weiner, R.L., and Williams, L.T. (1993). Targeted expression of a dominant-negative FGF receptor mutant in the epidermis of transgenic mice reveals a role of FGF in keratinocyte organization and differentiation. EMBO J. 12, 2635–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson, D.G., Bailes, J.A., Champion, J.E., and McMahon, A.P. (1987). A molecular analysis of mouse development from 8 to 10 days post coitum detects changes only in embryonic globin expression. Development 99, 493–500. [DOI] [PubMed] [Google Scholar]
- Witty, J.P., McDonnell, S., Newell, K.J., Cannon, P., Navre, M., Tressler, R.J., and Matrisian, L.M. (1994). Modulation of matrilysin levels in colon carcinoma cell lines affects tumorigenicity in vivo. Cancer Res. 54, 4805–4812. [PubMed] [Google Scholar]
- Wysocki, A.B., Staiano-Coico, L., and Grinnell, F. (1993). Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J. Investig. Dermatol. 101, 64–68. [DOI] [PubMed] [Google Scholar]