Abstract
TPX2 has multiple functions during mitosis, including microtubule nucleation around the chromosomes and the targeting of Xklp2 and Aurora A to the spindle. We have performed a detailed domain functional analysis of TPX2 and found that a large N-terminal domain containing the Aurora A binding peptide interacts directly with and nucleates microtubules in pure tubulin solutions. However, it cannot substitute the endogenous TPX2 to support microtubule nucleation in response to Ran guanosine triphosphate (GTP) and spindle assembly in egg extracts. By contrast, a large C-terminal domain of TPX2 that does not bind directly to pure microtubules and does not bind Aurora A kinase rescues microtubule nucleation in response to RanGTP and spindle assembly in TPX2-depleted extract. These and previous results suggest that under physiological conditions, TPX2 is essential for microtubule nucleation around chromatin and functions in a network of other molecules, some of which also are regulated by RanGTP.
INTRODUCTION
Spindle assembly involves the collective activities of multiple proteins that results in localized microtubule nucleation, dynamics, and organization (Karsenti and Vernos, 2001). One of these proteins is TPX2 (Wittmann et al., 2000). This protein was initially identified by its capacity to bind microtubules in Xenopus egg extract and to mediate the localization of the kinesin-like protein Xklp2 to microtubule minus ends during mitosis. In tissue culture cells, TPX2 is cell cycle regulated (Gruss et al., 2002). It accumulates in the nucleus during S/G2, at the spindle poles during mitosis, and is degraded in early G1.
A major breakthrough in our understanding of TPX2 function and spindle assembly came from work demonstrating the control that the small GTPase Ran exerts on microtubules during mitosis (Heald and Weis, 2000; Dasso, 2001; Kahana and Cleveland, 2001; Karsenti and Vernos, 2001). Indeed, it is now clearly established that the guanosine triphosphate (GTP)-bound form of Ran (RanGTP), locally generated around chromosomes, coordinates the nucleation, dynamics, and organization of microtubules during spindle assembly by releasing regulatory factors from importins α and β. One of these factors turns out to be TPX2 (Gruss et al., 2001) that triggers local nucleation of microtubules around chromosomes. This activity is essential for spindle assembly in the presence or absence of centrosomes, in egg extract, and in tissue culture cells (Gruss et al., 2001, 2002). TPX2 also has other activities, some of which may be related to its role in spindle pole organization (Garrett et al., 2002). A direct interaction of TPX2 with the mitotic kinase Aurora A has recently been described and seems to be required for the targeting of the kinase to spindle microtubules (Kufer et al., 2002). This interaction is particularly interesting because it leads to the activation of the kinase (Bayliss et al., 2003; Eyers et al., 2003; Tsai et al., 2003). Finally a complex functional interaction between TPX2 and the dynein–dynactin complex leads to spindle pole localization of TPX2 and the targeting of Xklp2 (Wittmann et al., 2000).
Although TPX2 has multiple activities that are essential for mitosis, very little is known about the protein domains and the mechanisms involved. We decided to undertake a detailed functional analysis of serial deletions of TPX2. Our results show that TPX2 could be described as having two distinct domains. The N-terminal domain (aa 1–480) binds directly to pure microtubules and contains the 39-aa peptide that binds and activates Aurora A. The C-terminal region that overlaps partially with the previous one (aa 319–715) is essential but not sufficient to promote microtubule nucleation in egg extracts. However, it is functional for spindle assembly and can fully rescue the depletion of TPX2 from egg extracts. These results have important implications for the understanding of how TPX2 might be involved in chromosome-dependent nucleation of microtubules and spindle assembly.
MATERIALS AND METHODS
Recombinant Proteins
To express green fluorescent protein (GFP)-tagged TPX2 fusion proteins, polymerase chain reaction-amplified fragments of interest were cloned into a modified pHAT2 vector that contained the enhanced green fluorescent protein (EGFP) sequence in frame after the histidine tag (obtained from Andrei Popov, INSERM Unité 366, Grenoble, France). For expression of the ΔNLS proteins, constructs were in the same vector were prepared by site-directed mutagenesis (Stratagene, La Jolla, CA) to change K284 and R285 to alanine. All constructs were fully sequenced. The histidine-tagged recombinant proteins were expressed in Escherichia coli BL21(DE3)pLysS and purified on TALON metal affinity resin (BD Biosciences Clontech, Palo Alto, CA) according to the manufacturer's instructions. Buffer was subsequently exchanged to 25 mM K-HEPES, pH 7.5, 250 mM KCl, 2 mM MgCl2, 10% glycerol, and 1 mM dithiothreitol (DTT) by using PD-10 desalting columns (Amersham Biosciences UK, Little Chalfont, Buckinghamshire, United Kingdom). The purified proteins were frozen in liquid nitrogen and stored at –80°C. Proteins concentrations were estimated using their A280 nm absorbance and corrected using Coomassie-stained SDS gels.
p50/dynamitin was produced as described previously (Wittmann and Hyman, 1999). The glutathione S-transferase (GST)-fusion protein containing the C-terminal 250 amino acids of Xklp2 tail was produced as described previously (Wittmann et al., 1998). GST-TPX (1–39) was expressed and purified as described previously (Bayliss et al., 2003). RanQ69L was expressed, purified, and loaded with GTP as described previously (Weis et al., 1996). Z-tagged Xenopus importin α was expressed and purified as described previously (Gorlich et al., 1994).
Antibodies
The polyclonal antibody against TPX2 was raised against bacterially expressed GST-TPX2 (Wittmann et al., 2000) and affinity purified against TPX(480–715). The 1C1 monoclonal anti-Eg2 was a gift from C. Prigent (CNRS Université de Rennes 1, Rennes, France). Polyclonal antibodies against EGFP and GST were raised in rabbits and affinity purified. Polyclonal antibodies against importin α and importin β were a gift from I. Mattaj (European Molecular Biology Laboratory, Heidelberg, Germany).
Microtubule Pelleting and Microtubule Nucleation In Vitro
Tubulin was polymerized in BRB80, 1 mM GTP, 5 mM MgCl2 for 30 min at 37°C and microtubules stabilized with 20 μM paclitaxel (Molecular Probes, Eugene, OR). Microtubules were centrifuged in a TLA 100 rotor at 200,000 × g for 20 min at 37°C. The microtubule pellet was resuspended in incubation buffer (20 mM HEPES, pH 7,5, 200 mM KCl, 2 mM MgCl2, 1 mM EGTA, 0.1% triton X-100, 1 mM DTT, 10 μM taxol) to a final tubulin concentration of 0.2 mg/ml. Each GFP-TPX2 fragment (10 μg) was incubated with 100 μl of microtubule solution for 20 min at 20°C or in incubation buffer without microtubules. Samples were centrifuged through a 10% sucrose-BRB80 cushion containing 5 μM paclitaxel in TLS55 rotor at 200,000 × g for 15 min at 20°C.
To test for their microtubule nucleation activity, GFP-TPX2 fragments (0.8 μM) were mixed with 10 μM tubulin containing 5% rhodamine-labeled tubulin and 1 mM GTP in BRB80 and incubated for 20 min at 25°C. Structures were fixed and centrifuged onto coverslips.
Immunoprecipitation
For immunoprecipitation from 50 μl of extract, protein A-conjugated Dynabeads 280 (Dynal Biotech, Lake Success, NY) were coated with anti-GFP (6 μg/20 μl of beads) in a total volume of 300 μl of phosphate-buffered saline, 0.1%Triton X-100 (PBS-T), for at least 1 h at 4°C on a rotating wheel. Beads were washed twice with PBS-T and twice with CSF-XB (10 mM K-HEPES, pH 7,7, 50 mM sucrose, 100 mM KCl, 2 mM MgCl2, 0,1 mM CaCl2, 5 mM EGTA). GFP-tagged TPX2 fusion proteins (200 nM), GFP-Eg2 (200 nM), or GST-TPX (1–39) were incubated in the extract for 20 min at 20°C before adding the extract to the beads. After incubating the beads in the extract on ice for 30 min, beads were retrieved on a magnet for 10 min and then washed twice with CSF-XB-T (CSF-XB, 0.1% Triton X-100) and twice with PBS-T. TPX2-associated proteins were eluted with 0.5 M NaCl in PBS-T for 15 min on ice or directly with SDS-PAGE sample buffer. The samples were then subjected to gel electrophoresis and analyzed by Western blotting with anti-TPX (480-715), anti-importin α, anti-importin β, anti-GST, or anti-Eg2.
Egg Extracts, Spindle Assembly, Asters Formation, and Immunodepletion
CSF egg extracts were prepared according to Wittmann et al. (1998). For cycled spindle assembly around sperm nuclei, rhodamine-labeled tubulin (Hyman et al., 1991) was added at 0.2 mg/ml and sperm at a concentration of ∼500 nuclei/μl to 20 μl of extract on ice. The extract was then released into interphase by the addition of 0.4 mM Ca2+ (Desai et al., 1999) and incubated for 90 min at 20°C. This extract was cycled back to mitosis by the addition of 1 volume of CSF-arrested extract. After 45 min, spindles were fixed in 1 ml of BRB80 (80 mM K-PIPES, pH 6.8, 1 mM EGTA, 1 mM MgCl2) containing 30% glycerol, 0.25% glutaraldehyde, and 0.1% Triton X-100 and centrifuged (HB6 rotor; 12,000 rpm, 12 min, 18°C) through a 40% glycerol cushion in BRB80 onto coverslips. Coverslips were washed with PBS, briefly incubated in Hoechst 33342 (Sigma-Aldrich, St. Louis, MO) (2 μg/ml), washed in PBS, and mounted in Mowiol (Hoechst).
Spindle assembly around chromatin beads was performed as described previously (Heald et al., 1996). Chromatin beads were prepared by incubating DNA-coated beads in TPX2-depleted or mock-depleted CSF-arrested extracts cycled through interphase and back into mitosis with mock-depleted or TPX2-depleted CSF-extract containing the different TPX2 recombinant proteins.
TPX2- and RanGTP-dependent asters were formed by adding the indicated amounts of recombinant proteins to 20 μl of CSF extract supplemented with rhodamine-labeled tubulin at 0.2 mg/ml. After a 20- to 30-min incubation at 20°C, several aliquots of 1 μl were mixed with fixative solution (11% folmaldehyde, 48% glycerol) and squashed. All the structures formed in at least two squashes were counted. Alternatively, 10 μl of the reaction mixture were fixed and centrifuged onto a coverslip. Structures present in at least 10 randomly selected fields were counted.
Human KE37 cell line centrosomes were prepared as described previously (Bornens and Moudjou, 1999). Centrosomes at a concentration of 2 × 108/ml were added in a 1-μl volume to 20 μl of CSF extract complemented with Cy5- or rhodamine-labeled tubulin at 0.2 mg/ml. Preparations were incubated at 20°C for 20 min, fixed, and centrifuged onto coverslips.
For immunodepletion, 1 volume of protein A-conjugated Dynabeads 280 (Dynal Biotech) coupled to antibodies was used to deplete three volumes of extract. Antibodies were coupled to beads (0.4 μg of antibody per microliter of beads) in PBS-T and incubated at 4°C for 1 h on a rotating wheel. After coupling, beads were washed twice with PBS-T and twice with CSF-XB. The required volume of extract was added on the beads and mixed by pipetting up and down. The mixture was then placed on ice for 60 min and mixed by pipetting once during this time. The beads were retrieved on a magnet on ice for ∼10 min, and the depleted extract was used for the different assays.
Fluorescence Loss after Photoconversion
Glass bottomed 96-wells microplates (polyfiltronics) were sonicated for 10 min in [60:40 (vol/vol) ethanol/5 M NaOH], rinsed intensively with water, coated overnight with 80 μl of poly-l-lysine solution [0.1% (wt/vol); Sigma-Aldrich], and rinsed with water. Nonlabeled and Cy5-labeled tubulin (30 and 1.5 μM, respectively) were polymerized in 1 mM GTP, 5 mM MgCl2 in BRB80 for 30 min at 37°C and subsequently stabilized by a 1:20 dilution in BRB80 containing 20 μM paclitaxel (Molecular Probes) and 0.15 M KCl. Microtubules (100 μl) were centrifuged (Megafuge 1.0 R centrifuge; Heraeus, Berlin, Germany) (3800 rpm, 25 min, 18°C) through 100 μl of a 20% glycerol cushion in BRB80 containing 20 μM paclitaxel and 0.15 M KCl onto the wells bottom. Wells bottom were subsequently rinsed with BRB80 containing paclitaxel (20 μM). GFP-fusion proteins of interest were incubated in the wells in modified CSF XB (0.1M KCl, 3 mM MgCl2, 0.1 mM CaCl2, 10 mM K-HEPES, pH 7.7, 10% glycerol) containing β-mercaptoethanol [0.1% (vol/vol)], casein (1 mg/ml), and hemoglobin (Sigma-Aldrich) [10% (vol/vol) of a saturated solution] as oxygen scavenger. Fragments were incubated at 5 μM with the exception of TPX (1-480) incubated at 3 μM, TPX (319-715) and TPX (481-715) incubated at 5 and 8 μM. The preparation was layered with 500 μl of mineral oil (Sigma-Aldrich).
Photoconversion and images processing were performed on a Leica TCS SP2 AOBS confocal microscope.
Microscopy
Spindles and asters were processed for immunofluorescence as described previously (Wittmann et al., 1998). Immunolocalization of the GST-Xklp2 tail on asters was performed as described previously (Wittmann et al., 1998), by using an Alexa red-coupled secondary antibody (Molecular Probes) and visualizing microtubules with Cy-5–labeled tubulin (Hyman et al., 1991). For each experiment, pictures were taken on a Leica TCS Sp2 AOBS confocal microscope at 63× magnification with identical illumination settings and processed identically with Adobe Photoshop.
RESULTS
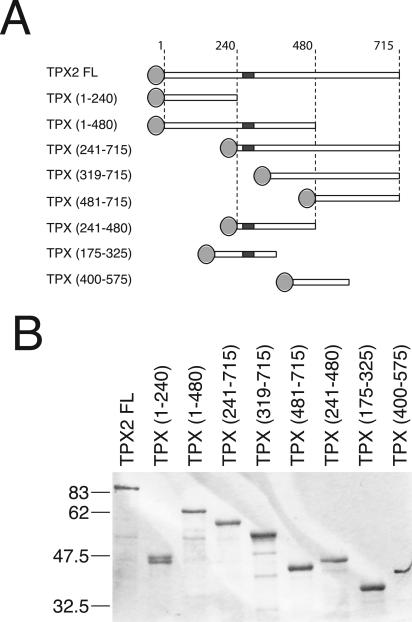
The analysis of the TPX2 protein sequence did not reveal the presence of any conserved protein domains. However, several putative nuclear localization signal (NLS) sequences, two short sequences with high probability to form coiled coils and several consensus sites for phosphorylation were found (Wittmann et al., 2000). Recently, one of the putative NLS (NLS284) was reported to be the functional importin α binding site and to be directly involved in the regulation of TPX2 microtubule nucleation activity (Schatz et al., 2003). We designed a series of deletion constructs to express overlapping TPX2 fragments (Figure 1). Because the full-length protein tagged at its N terminus with GFP is fully functional for rescue of spindle assembly in TPX2-depleted Xenopus egg extracts (Wittmann et al., 2000), we tagged all the fragments with an N-terminal EGFP. We then examined the activity of these fragments in pure tubulin solutions and in egg extract.
Figure 1.
TPX2 recombinant proteins used in this study. (A) Schematic representation of the GFP-fusion TPX2 fragments generated for this study. Light gray discs represent EGFP tag. TPX2 functional NLS (NLS284) is a dark gray square. (B) Coomassie-stained gel of the GFP-fusion TPX2 fragments purified from E. coli (see Materials and Methods). Molecular masses of marker proteins are indicated on the left.
Microtubule Binding Properties and Nucleating Activities of TPX2 Domains In Vitro
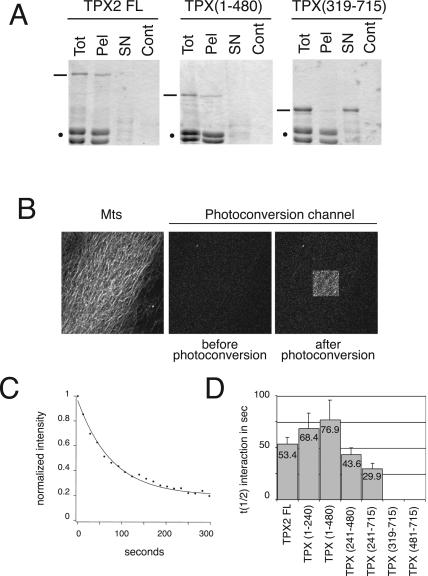
To determine which domain(s) of TPX2 were involved in its binding to microtubules, we examined the ability of GFP-TPX2 full-length and of the different fragments to sediment with pure prepolymerized and taxol-stabilized microtubules (Figure 2A; our unpublished data). These experiments indicated the presence of a microtubule binding activity in the N-terminal half of TPX2 as previously found for human TPX2 (Trieselmann et al., 2003). We then decided to use a novel method called fluorescence loss after photoconversion (FLAP; our unpublished data) to measure the affinities of the different GFP-fragments for microtubules. This method is based on a property of the green fluorescent protein that becomes stably photoconverted upon excitation with blue light in the absence of oxygen and emits red fluorescence upon excitation with green light (Elowitz et al., 1997; Sawin and Nurse, 1997). GFP-TPX2 fragments were introduced in an oxygen-depleted chamber previously coated with a layer of stabilized microtubules. On a confocal microscope, a defined area of the microtubule layer was then illuminated at 488 nm to induce the photoconversion of GFP-proteins within this region. After a short time, the emission of the photoconverted GFP proteins was recorded. Photoconverted proteins not bound to microtubules diffused away and no signal was detected. By contrast photoconverted proteins bound to microtubules emitted a red fluorescent signal in the illuminated region (Figure 2B). With time, the bound proteins dissociated from the microtubules and the intensity of the red signal decayed (Figure 2C), providing an accurate measurement of the off rate of the fusion protein independently of the protein concentration (our unpublished data). We therefore used the decay kinetics of the photoconversion signal to calculate the interaction half-life (t1/2 int) and determine the affinity of each GFP-protein for microtubules.
Figure 2.
Microtubule binding of GFP-TPX2 fragments in vitro. (A) Behavior of TPX2 FL, TPX (1-480), and TPX (319-715) in a microtubule-pelleting assay. Equivalent volumes of the reaction before sedimentation (Tot), the microtubule pellet (Pel), the microtubule supernatant (SN), and the control pellet (without microtubules) (Cont) were analyzed by SDS-PAGE. Proteins were visualized with Coomassie Brilliant Blue (TPX fragments are indicated with a bar and tubulin dimers with a spot). (B) Microtubule binding properties of the GFP-TPX2 fragments studied by FLAP. Glass bottomed wells were coated with taxol-stabilized microtubules labeled with Cy5 (left). GFP-TPX2 fragments were added to the wells in an oxygen-depleted medium. Before photoconversion, no signal is detected in the photoconversion channel. After illumination of a restricted area (100-μm2 square), GFP-fragments that bind to the microtubules (here GFP-TPX2 full length) emit a signal in the photoconversion channel. (C) Typical decay curve of the photoconversion signal (here measured for GFP-TPX2 full length). (D) Half-life of the interaction of GFP-TPX2 fragments with microtubules. Ten curves were analyzed for GFP-TPX2 full length, 21 for TPX (1-240), 30 for TPX (1-480), 13 for TPX (241-480), and 15 for TPX (241-715). The average half-life value for each fragment is indicated on the corresponding bar. Error bars represent SD.
A half-life of 53.4 s was obtained for the full-length protein (Figure 2D). Fragments covering the N-terminal domain of TPX2 exhibited the highest values: 68.4 s for TPX (1-240) and 76.9 s for TPX (1-480). By contrast, the half-life values decreased dramatically for fragments lacking the N-terminal domain with a value of 43.6 s for TPX (241-480) and 29.9 s for TPX (241-715). No photoconversion signal was detected for TPX (481-715) and TPX (319-715), indicating that these two fragments did not interact with microtubules (Figure 2D).
These results suggest the existence of one or more microtubule-binding domain(s) at the N-terminal half of TPX2, whereas its C-terminal domain (from aa 319) has no affinity for pure microtubules.
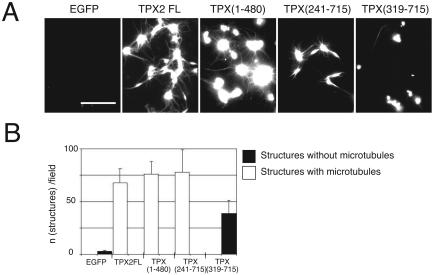
We then examined which fragment(s) could nucleate microtubules in pure tubulin solutions. Purified proteins (800 nM) were mixed with soluble tubulin (10 μM) and incubated for 20 min at 20°C before fixing and pelleting onto coverslips. As previously reported, GFP-TPX2 formed aggregates with tubulin from which microtubules emanated forming asters (Schatz et al., 2003). We found that the N-terminal domain TPX (1-480) also formed aggregates and microtubule asters (Figure 3). TPX (241-715) formed smaller aggregates from which short bundled microtubules grew. The C-terminal domain: TPX (319-715) formed aggregates, but none of these aggregates nucleated microtubules.
Figure 3.
Microtubule nucleation activity of GFP-TPX2 fragments in vitro. (A) Representative images of microtubules and aggregates formed after incubation of GFP-TPX2 fragments (as indicated) with tubulin. Bar, 10 μm. (B) Quantification of the structures observed as in A. For each TPX2 fragment, the number of structures associated with microtubules (white bars) or not associated with microtubules (black bars) was counted in at least 10 fields in two independent experiments. Error bars correspond to the variance between the two experiments.
Therefore, both the microtubule-binding assay and the nucleation assay point to the N-terminal domain of TPX2 as the region for microtubule and/or tubulin interaction in vitro.
Identification of the Domain in TPX2 Required for Microtubule Nucleation in Egg Extracts
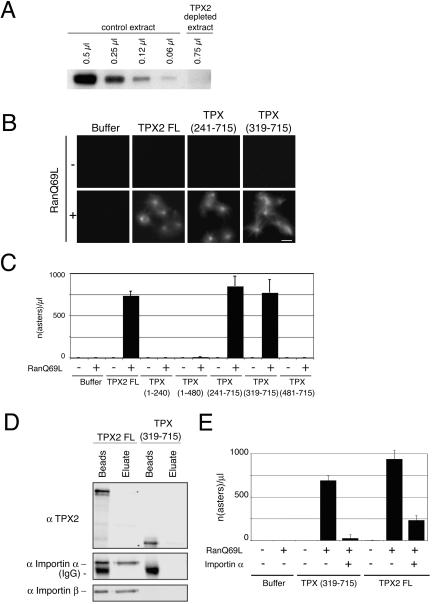
In mitotic egg extract, TPX2 nucleates microtubules in a RanGTP-dependent manner (Gruss et al., 2001, 2002). This activity can be revealed by addition of the mutant form of Ran Q69L locked in the GTP form that releases TPX2 from the inhibitory interaction with importins leading to the formation of microtubule asters (Gruss et al., 2001). We investigated the ability of the different TPX2 fragments to nucleate microtubules when added at 0.1 μM (physiological concentration) to a TPX2-depleted CSF egg extract in the presence of Ran Q69L (Figure 4, A–C). Under these conditions, full-length GFP-TPX2 induced microtubule nucleation as expected. None of the fragments lacking the C-terminal part of TPX2 were able to support microtubule nucleation (Figure 4, B and C). By contrast, fragments lacking the N-terminal part of TPX2 were competent for microtubule nucleation in the presence of Ran Q69L. The shortest fragment retaining this activity was TPX (319-715). Further deletions at the amino terminus of this fragment eliminated the activity (Figure 4, B and C). These results were in sharp contrast to those obtained in pure tubulin and showed that in the egg extract the portion of TPX2 involved in microtubule nucleation is the C terminus.
Figure 4.
Microtubule nucleation activity of GFP-TPX2 fragments in Xenopus egg extract. (A) Western blot probed with the anti-TPX2 antibody showing the efficiency of TPX2 depletion from the egg extract. Indicated volumes of TPX2 depleted and control extracts were loaded on SDS-PAGE gel. (B) Representative images of microtubule asters formed upon addition of GFP-TPX2 FL or GFP-TPX2 fragments to TPX2 depleted extract, in the presence or absence of RanQ69L. Bar, 10 μm. (C) Quantification of the experiment shown in B. The values represent the averages from three independent experiments. Error bars represent SD. (D) GFP-TPX2 FL and GFP-TPX (319-715) were incubated in mitotic egg extract and immunoprecipitated with an anti GFP antibody. Immunoprecipitated proteins were eluted with a high salt buffer (eluate) and sample buffer (beads) successively. The blot was probed with anti-importin α and β antibodies. (E) Quantification of microtubule asters formed in TPX2-depleted extract containing RanQ69L after addition of either TPX2 full-length or TPX (319-715) in the presence or absence of exogenous importin α. Importin α inhibits microtubule aster formation induced by TPX2 full length and TPX (319-715). The averages from two independent experiments are shown. Error bars represent the variance between the two experiments.
Interestingly, although TPX (319-715) did not contain the functional NLS284, its ability to induce microtubule nucleation was regulated by Ran. To investigate whether importins α and β could interact with some of the putative NLS present in this fragment, GFP-TPX2-FL or GFP-TPX (319-715) was added to an egg extract and incubated for 20 min at 20°C before immunoprecipitation with anti-GFP antibodies. The presence of importins α and β in the immunoprecipitate was then monitored by Western blot. We found that both importins coimmunoprecipitated with TPX2-FL as expected but not with TPX (319-715) (Figure 4D). This result was in agreement with the identification of a single functional NLS in TPX2 at position 284 (Schatz et al., 2003). It suggested strongly that in addition to TPX2 other factor(s) regulated by RanGTP were required for microtubule nucleation. To check whether such factor(s) were regulated through their binding to importin α, we repeated the nucleation assay after addition of importin α (Figure 4E). Indeed, this blocked nucleation. Thus, the C-terminal portion of TPX2 is essential but not sufficient for microtubule nucleation. One or more other importin α binding protein(s) are involved as well.
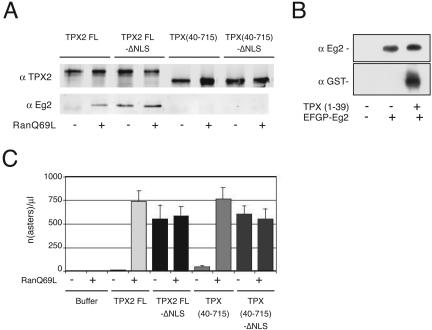
Aurora A Activation by TPX2 Is Not Required for TPX2-dependent Microtubule Nucleation in Egg Extracts
TPX2 interacts with and activates the mitotic kinase Aurora A (Eg2 in Xenopus) in the presence of RanGTP (Eyers et al., 2003; Tsai et al., 2003). We wondered whether the activation of Eg2 was required for the microtubule nucleation activity of TPX2. Based on the results from Bayliss et al. (2003) that defined the first N-terminal 43 amino acids of human TPX2 as the Aurora A binding domain, we checked whether Aurora A/Eg2 would bind to a truncated TPX2 protein lacking the first 39 amino acids (equivalent to the first 43 amino acids from human TPX2). As shown in Figure 5A, TPX (40-715) was unable to interact with Aurora A even in the presence of RanGTP. Because Aurora A may be activated by RanGTP through a mechanism independent of TPX2 binding, we introduced two point mutations in the NLS 284 to abolish importin α binding and the regulation by RanGTP as described previously (Schatz et al., 2003) to prepare TPX2 FL-ΔNLS and TPX (40-715)-ΔNLS. TPX2 FL-ΔNLS was found to interact with Aurora A/Eg2 in the presence or absence of RanGTP, whereas TPX (40-715)-ΔNLS did not in any of the two conditions (Figure 5A). We also verified that the first 39 amino acids were sufficient to interact with Aurora A in the extract (Figure 5B). We then compared the microtubule nucleation activities of TPX2 FL and GFP-TPX2 FL-ΔNLS with those of the equivalent proteins lacking the N-terminal 39 aa. We reasoned that if the activation of Aurora A by TPX2 was required for microtubule nucleation, TPX2 FL-ΔNLS would nucleate microtubules in the absence of RanGTP but TPX2 (40-715)-ΔNLS should not. We found that this was not the case and that this protein was able to nucleate microtubules in the absence of RanGTP as efficiently as the full-length protein (Figure 5C). These results indicated that the microtubule nucleation induced by TPX2 does not require Aurora A/Eg2 activation by TPX2.
Figure 5.
The interaction of TPX2 with Aurora A/Eg2 is not required for TPX2 directed microtubule nucleation in the egg extract. (A) GFP-TPX2 FL, GFP-TPX (40-715), and the corresponding proteins mutated in the NLS [GFP-TPX2 FL-ΔNLS and GFP-TPX (40-715)-ΔNLS] were incubated in mitotic egg extract in the presence or absence of RanQ69L and immunoprecipitated with an anti-GFP antibody. Immunoprecipitated proteins were analyzed by Western blot by using anti-GFP and anti-Eg2 antibodies. TPX (40-715) does not interact with Aurora A/Eg2 even in the presence of RanQ69L. (B) GST-TPX (1-39) and GFP-Eg2 were added to a mitotic egg extract as indicated. Immunoprecipitation with an anti-GFP antibody revealed that the first N-terminal amino acids of TPX2 are sufficient for interaction with GFP-Eg2. Western blots probed with anti-Eg2 and anti-GST are shown. (C) Quantification of asters formed in TPX2-depleted extract upon addition of buffer, GFP-TPX2 FL, GFP-TPX (40-715), GFP-TPX2 FL-ΔNLS, and GFP-TPX (40-715)-ΔNLS. The graph shows the data obtained in one representative experiment counting the number of asters in ten randomly selected microscope fields (the experiment was repeated twice). The bars represent the internal SD of the experiment.
The conclusion of this analysis is that the C-terminal part of TPX2 contains the essential domain involved in microtubule nucleation but it is not sufficient. The complementing activity(ies) can be provided by the N-terminal part of TPX2 itself or by other factors themselves regulated by RanGTP or both under physiological conditions.
Identification of the Domain Required for TPX2 Localization to Spindle Poles and Microtubules in Egg Extract
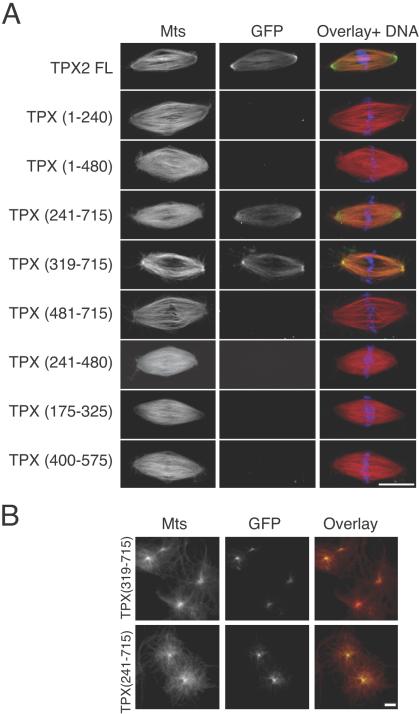
In the spindle, TPX2 is localized on microtubules and enriched at the poles. This localization depends on the activity of the dynein–dynactin complex (Wittmann et al., 2000). To determine which domains of TPX2 are involved in the enrichment of the protein at spindle poles, we investigated the localization of the different GFP-fusion proteins on spindles assembled around sperm nuclei in Xenopus egg extracts (Sawin and Mitchison, 1991). Fusion proteins were added at 100 nM to egg extracts as they were cycled back into mitosis. Samples were fixed after 45 min and centrifuged onto coverslips. As previously described, GFP-TPX2 FL was present on spindle microtubules and concentrated at the spindle poles (Figure 6A). Only two of the fragments tested localized to spindle poles like the full-length protein, whereas the others did not localize anywhere. These fragments did not correspond to those found to interact with taxol-stabilized microtubules in vitro. The smallest fragment found to localize to spindle microtubules and spindle poles was the C-terminal fragment missing the NLS284 TPX (319-715) whose localization was very similar to that of the full-length protein (Figure 6A). This fragment did also accumulate at the center of centrosomal asters assembled in TPX2 depleted extract (Figure 6B), indicating that its localization was not dependent on the endogenous TPX2 protein. These observations indicated that the C-terminal half of TPX2 (from aa 319) is necessary and sufficient for TPX2 localization to the spindle in Xenopus mitotic extracts.
Figure 6.
Localization of GFP-TPX2 fragments on spindles and asters assembled in Xenopus egg extracts. (A) GFP-TPX2 fragments (0.1 μM) were added to egg extracts as they were cycled back into mitosis. Their localization was examined on spindles assembled around sperm nuclei. In the overlays, microtubules are in red, GFP-fragments in green, and chromatin in blue. Bar, 10 μm. (B) TPX (241-715) and TPX (319-715) (0.1 μM) were added to a TPX2-depleted extract. Microtubule asters formation was induced by addition of purified centrosomes. In the overlays, microtubules are in red, and GFP-fragments in green.
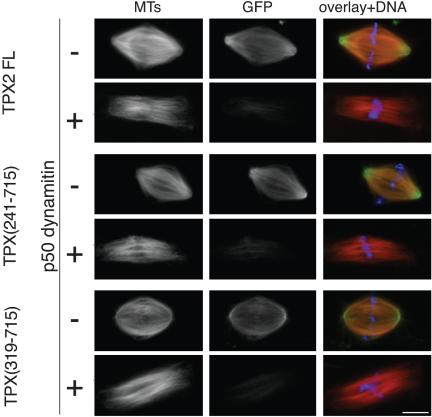
We then investigated whether the localization to spindle poles of the C-terminal fragment of TPX (319-715) was dependent on the activity of the dynein–dynactin complex. Spindles were assembled in interphase-to-mitotic Xenopus egg extracts in the presence of an excess of recombinant p50 dynamitin to disrupt the dynactin complex and inhibit the dynein–dynactin activity (Wittmann and Hyman, 1999). As described previously, in the presence of p50 dynamitin, spindles assembled but had unfocused poles and were more elongated than in control reactions (Figure 7). Under these conditions, full-length GFP-TPX2 failed to accumulate at the poles (Wittmann et al., 2000). The same effect was observed for GFP-TPX (319-715) (Figure 7).
Figure 7.
P50 dynamitin disrupts GFP-TPX2 full length, GFP-TPX (241-715), and GFP-TPX (319-715) association with the spindle. Recombinant p50 dynamitin (0.7 mg/ml) and TPX (241-715) or TPX (319-715) or TPX2 full length (0.1 μM) were added to cycled spindle reactions. In overlays, microtubules are in red, GFP-fragments in green, and chromatin in blue. Bar, 10 μm.
Identification of the TPX2 Domain Required for Xklp2 Targeting to Spindle Poles
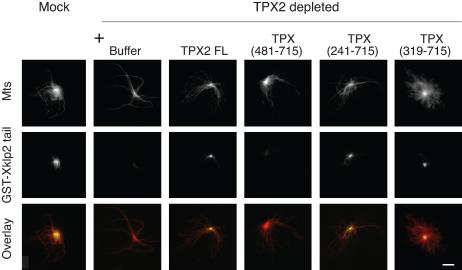
TPX2 is required for the targeting of the kinesin-like protein Xklp2 to spindle poles (Wittmann et al., 1998). We therefore examined whether the C-terminal fragments of TPX2 that localized to spindle poles also were sufficient to target Xklp2 to microtubule minus ends. To this end, we followed the localization of a recombinant GST-fusion protein containing the C-terminal 250 amino acids of Xklp2 tail (GST-Xklp2-tail) (Wittmann et al., 1998) on centrosomal asters assembled in TPX2-depleted mitotic extracts (Figure 8). As described previously, GST-Xklp2-tail protein did not localize to the center of centrosomal asters formed in TPX2 depleted extracts but its localization was fully restored upon addition of the full-length GFP-TPX2 protein. We found that TPX (241-715) and TPX (319-715) that localized to spindle poles also were able to rescue the targeting of GST-Xklp2-tail to the center of asters (Figure 8), whereas a shorter fragment, TPX (480-715), did not.
Figure 8.
GFP-TPX (319-715) is sufficient to localize GST-Xklp2-tail to the center of centrosomal asters. Purified centrosomes were incubated for 30 min in TPX2-depleted or mock-depleted mitotic egg extracts containing GST-Xklp2 tail (2 μM) and GFP-TPX2 fragments (0.1 μM). GST-Xklp2-tail was detected with an anti-GST antibody. Microtubules are in red, and the GST-Xklp2 tail in green. Bar, 10 μm.
We thus conclude that in egg extracts, a C-terminal part of TPX2 starting at aa 319 retains the functionality of TPX2 concerning localization to spindle poles, functional interaction with the dynein–dynactin complex, and targeting of Xklp2 to microtubule minus ends.
Functionality of the C-Terminal Domain of TPX2 for Spindle Assembly in Egg Extract
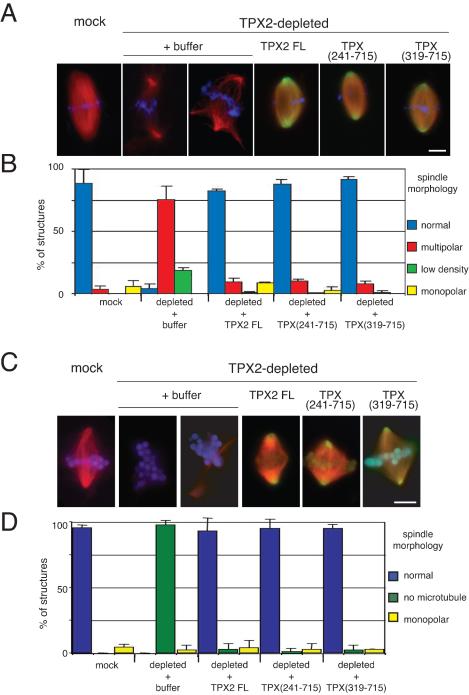
The different experiments performed indicated that the C-terminal part of TPX2 (aa 319-715) was the minimal domain required for most of the activities of the full-length protein. However this fragment was not able to interact with and activate Aurora A/Eg2. We thus wondered whether it would be able to rescue spindle formation in TPX2-depleted extracts. We used the two types of spindle assembly assays in egg extract: with sperm nuclei in the presence of centrosomes and around DNA-coated beads in the absence of centrosomes. In both cases, TPX2 full-length, TPX (241-715) and TPX (319-715) were added at 0.2 μM to a TPX2-depleted egg extract as it was cycled back into mitosis. The reaction was incubated for 45 min at 20°C, fixed, and centrifuged onto coverslips. As described previously, TPX2 depletion (>92%) led to the assembly of multipolar and bipolar spindles characterized by a low density of microtubules (Wittmann et al., 2000) around sperm nuclei and no microtubules at all around chromatin beads (Gruss et al., 2001) (Figure 9). These phenotypes were fully rescued by the addition of GFP-TPX2, TPX (241-715) or TPX (319-715) (Figure 9). These results suggest a strong correlation between the nucleation inducing activity of the C-terminal TPX2 fragment and its requirement for spindle assembly.
Figure 9.
GFP-TPX (319-715) rescues spindle assembly around sperm nuclei and DNA-coated beads in TPX2-depleted extract. (A) Morphology of spindles assembled around sperm nuclei in mock-depleted extract and in TPX2-depleted extract to which buffer, GFP-TPX2 full length, GFP-TPX (241-715), or GFP-TPX (319-715) was added. Microtubules are in red, chromatin in blue, and the GFP-proteins in green. Bar, 10 μm. (B) Quantification of the different types of structures formed under various depletion and add-back conditions as in A. Blue bars, normal spindles; red bars, multipolar spindles; green bars, bipolar spindles with low microtubule density; and yellow bars, monopolar spindles. The experiment was performed in three different egg extracts. The bars represent the SD. (C) Morphology of spindles assembled around DNA-coated beads in mock-depleted extract and in TPX2-depleted extract to which buffer, GFP-TPX2 full length, GFP-TPX (241-715), or GFP-TPX (319-715) was added. Microtubules are in red, chromatin in blue, and the GFP-proteins in green. Bar, 10 μm. (D) Quantification of different types of structures formed under the various depletion and addback conditions as in B. Magenta bars, normal spindles; green bars, beads without microtubules; and yellow bars, monopolar spindles. The experiment was performed in two different egg extracts. The bars correspond to the variance between the two experiments.
DISCUSSION
TPX2 and Microtubule Nucleation In Vitro and in Extracts
It has been clearly established that in Xenopus egg extracts, several molecules are released from importin α in response to RanGTP generated by chromosomes during mitosis. One of them, TPX2, seems to be involved in the nucleation of spindle microtubules (Gruss et al., 2001). However, the exact mechanism by which this protein triggers microtubule nucleation remains unclear. In particular, although recent data have shown that TPX2 can nucleate microtubules in pure tubulin solutions and that this activity is inhibited by importin α (Schatz et al., 2003), we do not know whether it nucleates microtubules in extracts through a direct effect on the tubulin dimer itself or through a complex pathway. The fact that the full-length TPX2 molecule mutated on the functional NLS triggers microtubule nucleation in egg extracts in the absence of RanGTP demonstrates that the importin-mediated regulation is essential. This result also has lead to the conclusion that TPX2 might be the only NLS molecule involved in the induction of microtubule nucleation in egg extracts (Schatz et al., 2003). The present results complicate this interpretation, however, and suggest the existence of a more complex mechanism. Indeed, the TPX2 N-terminal domain that binds and nucleates microtubules in pure tubulin solutions is unable to promote microtubule nucleation in egg extracts even in large excess. By contrast, the C-terminal domain (319-715) that does not bind to and does not nucleate microtubules in pure tubulin solutions is essential for triggering microtubule nucleation in egg extracts. Surprisingly, although this domain does not interact with importin α, it is unable to trigger microtubule nucleation in the absence of RanGTP or when added in large excess. In the presence of Ran GTP, this fragment induces microtubule nucleation and this effect is blocked by importin α. This clearly indicates that the C-terminal domain (319-715) functions in concert with another factor itself activated by Ran-GTP. This could be NuMa, LGN (Dasso, 2002), or other importin α binding factors. The model that emerges now is that, under physiological conditions, TPX2 is part of a RanGTP-dependent pathway regulating microtubule nucleation around chromosomes and involving other molecules some of which also regulated, somewhere in this pathway γ-tubulin and/or microtubule-associated proteins are most probably involved.
TPX2 and Aurora A in Microtubule Nucleation and Spindle Assembly
Aurora A is required for bipolar spindle formation and stability (Glover et al., 1995; Roghi et al., 1998). However, it is still unclear how the kinase participates in these events. Few partners and/or substrates for the kinase have been identified so far. Two of them, the kinesin-like Eg5 (Giet et al., 1999) and the Drosophila protein D-TACC (Giet et al., 2002), are involved in spindle assembly. Interestingly, the effect of RanGTP on microtubule organization seems to be mediated at least in part by the activation of Eg5 (Wilde et al., 2001). More recently, TPX2 was found to interact with AuroraA/Eg2 and to target it to spindle microtubules (Kufer et al., 2002; Tsai et al., 2003). Furthermore, TPX2–AuroraA/Eg2 interaction is stimulated by RanGTP and leads to a strong activation of the kinase (Tsai et al., 2003). Our results show that in the egg extract a direct interaction between TPX2 and Aurora A is not essential for Ran-dependent microtubule nucleation and spindle assembly because TPX2 lacking the Aurora A binding domain is fully functional for microtubule nucleation or spindle assembly. This does not mean obviously that Aurora A activity is not required for spindle assembly.
Targeting Activities of TPX2 and Spindle Assembly
TPX2 was initially identified as a protein required for targeting the kinesin-like protein Xklp2 to microtubule minus ends during mitosis (Wittmann et al., 1998). More recently, human TPX2 was shown to target the kinase Aurora-A to spindle microtubules in human cells (Kufer et al., 2002). Here, we have shown that the C-terminal half of TPX2 starting at aa 319 is sufficient for targeting Xklp2 to the spindle poles. Concerning Aurora A/Eg2, we could not test for a possible role of TPX2 in targeting it to spindle microtubules because we could not detect the Xenopus Aurora A/Eg2 on the microtubules of spindles assembled in the egg extract either by immunofluorescence with a specific anti-Aurora A/Eg2 antibody that detects the kinase on spindle microtubules in Xenopus tissue culture cells or by adding GFP-tagged Eg2 to the egg extract. Because a direct interaction between TPX2 and Aurora A/Eg2 is not essential for spindle assembly in egg extract, we conclude that, at least in this system, Aurora A/Eg2 may not need to localize to spindle microtubules to be functional.
Initially, we undertook this study to determine whether different domains of TPX2 would be required for its different activities. It turns out that accumulation of TPX2 at spindle poles in a dynein-dependent manner, targeting of Xklp2 to spindle poles, Ran-dependent microtubule nucleation and spindle assembly all require the same large C-terminal portion of the protein and could not be dissected into smaller separate domains. This suggests that microtubule nucleation, microtubule binding, interaction with the dynein–dynactin complex and Xklp2 targeting may be intimately related. The dynein–dynactin-dependent interaction of TPX2 with spindle microtubules may allow a spatial regulation of TPX2 activities during spindle assembly. Under high RanGTP concentrations, the TPX2–importin complex dissociates. The released TPX2 nucleates microtubules and can associate to Aurora A/Eg2 and possibly other proteins, including Xklp2. TPX2 is then transported toward the minus ends of microtubules in a dynein–dynactin-dependent manner and concentrates Xklp2 to the spindle poles. We do not know whether during this process its nucleating activity could become switched off by rebinding to importins. The regulation of TPX2–importins interaction by the RanGTP gradient along the spindle (i.e., from chromosomes to spindle poles) remains to be clarified but in any case, this represents an amazing example of concentration of activities on one single molecular domain that coordinates many aspects of spindle assembly, ranging from microtubule nucleation to protein targeting to spindle poles. TPX2 seems more and more to be a key node in the complex network of protein interactions that is required for spindle assembly.
Acknowledgments
We thank J. Seiler for preparing the construct for bacterial expression of EGFP-Eg2 and excellent technical assistance. We also thank R. Valsdottir for the gift of some GFP-TPX2, A. Popov for the gift of centrosomes, I. Mattaj for the gift of anti-importin α and β antibodies, C. Prigent for the gift of anti-Eg2 antibody, M. Takagi for sharing crucial egg extracts, and O. Gruss and I. Mattaj for the critical reading of the manuscript. S.B. was supported by a European Molecular Biology Organization long-term fellowship and “Ligue Nationale contre le Cancer” fellowship. T.S. is supported by the European Union Research Training Network grant to I.V. (HPRN-CT-2000-00079).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04–05–0385. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–05–0385.
Abbreviations used: aa, amino acid; CSF, cytostatic factor; GTP, guanosine triphosphate; MAP, microtubule-associated protein; NLS, nuclear localization signal; TPX2, targeting protein for Xklp2; Xklp2, Xenopus kinesin-like protein 2.
References
- Bayliss, R., Sardon, T., Vernos, I., and Conti, E. (2003). Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Mol. Cell 12, 851–862. [DOI] [PubMed] [Google Scholar]
- Bornens, M., and Moudjou, M. (1999). Studying the composition and function of centrosomes in vertebrates. Methods Cell Biol. 61, 13–34. [DOI] [PubMed] [Google Scholar]
- Dasso, M. (2001). Running on Ran: nuclear transport and the mitotic spindle. Cell 104, 321–324. [DOI] [PubMed] [Google Scholar]
- Dasso, M. (2002). The Ran GTPase: theme and variations. Curr. Biol. 12, R502–R508. [DOI] [PubMed] [Google Scholar]
- Desai, A., Murray, A., Mitchison, T.J., and Walczak, C.E. (1999). The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol. 61, 385–412. [DOI] [PubMed] [Google Scholar]
- Elowitz, M.B., Surette, M.G., Wolf, P.E., Stock, J., and Leibler, S. (1997). Photoactivation turns green fluorescent protein red. Curr. Biol. 7, 809–812. [DOI] [PubMed] [Google Scholar]
- Eyers, P.A., Erikson, E., Chen, L.G., and Maller, J.L. (2003). A novel mechanism for activation of the protein kinase aurora a. Curr. Biol. 13, 691–697. [DOI] [PubMed] [Google Scholar]
- Garrett, S., Auer, K., Compton, D.A., and Kapoor, T.M. (2002). hTPX2 is required for normal spindle morphology and centrosome integrity during vertebrate cell division. Curr. Biol. 12, 2055–2059. [DOI] [PubMed] [Google Scholar]
- Giet, R., McLean, D., Descamps, S., Lee, M.J., Raff, J.W., Prigent, C., and Glover, D.M. (2002). Drosophila Aurora A kinase is required to localize D-TACC to centrosomes and to regulate astral microtubules. J. Cell Biol. 156, 437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giet, R., Uzbekov, R., Cubizolles, F., Le Guellec, K., and Prigent, C. (1999). The Xenopus laevis aurora-related protein kinase pEg2 associates with and phosphorylates the kinesin-related protein XlEg5. J. Biol. Chem. 274, 15005–15013. [DOI] [PubMed] [Google Scholar]
- Glover, D.M., Leibowitz, M.H., McLean, D.A., and Parry, H. (1995). Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell 81, 95–105. [DOI] [PubMed] [Google Scholar]
- Gorlich, D., Prehn, S., Laskey, R.A., and Hartmann, E. (1994). Isolation of a protein that is essential for the first step of nuclear protein import. Cell 79, 767–778. [DOI] [PubMed] [Google Scholar]
- Gruss, O.J., Carazo-Salas, R.E., Schatz, C.A., Guarguaglini, G., Kast, J., Wilm, M., Le Bot, N., Vernos, I., Karsenti, E., and Mattaj, I.W. (2001). Ran induces spindle assembly by reversing the inhibitory effect of importin alpha on TPX2 activity. Cell 104, 83–93. [DOI] [PubMed] [Google Scholar]
- Gruss, O.J., Wittmann, M., Yokoyama, H., Pepperkok, R., Kufer, T., Sillje, H., Karsenti, E., Mattaj, I.W., and Vernos, I. (2002). Chromosome-induced microtubule assembly mediated by TPX2 is required for spindle formation in HeLa cells. Nat. Cell Biol. 4, 871–879. [DOI] [PubMed] [Google Scholar]
- Heald, R., Tournebize, R., Blank, T., Sandaltzopoulos, R., Becker, P., Hyman, A., and Karsenti, E. (1996). Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts [see comments]. Nature 382, 420–425. [DOI] [PubMed] [Google Scholar]
- Heald, R., and Weis, K. (2000). Spindles get the ran around. Trends Cell Biol. 10, 1–4. [DOI] [PubMed] [Google Scholar]
- Hyman, A., Drechsel, D., Kellogg, D., Salser, S., Sawin, K., Steffen, P., Wordeman, L., and Mitchison, T. (1991). Preparation of modified tubulins. Methods Enzymol. 196, 478–485. [DOI] [PubMed] [Google Scholar]
- Kahana, J.A., and Cleveland, D.W. (2001). Cell cycle. Some importin news about spindle assembly. Science 291, 1718–1719. [DOI] [PubMed] [Google Scholar]
- Karsenti, E., and Vernos, I. (2001). The mitotic spindle: a self-made machine. Science 294, 543–547. [DOI] [PubMed] [Google Scholar]
- Kufer, T.A., Sillje, H.H., Korner, R., Gruss, O.J., Meraldi, P., and Nigg, E.A. (2002). Human TPX2 is required for targeting Aurora-A kinase to the spindle. J. Cell Biol. 158, 617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roghi, C., Giet, R., Uzbekov, R., Morin, N., Chartrain, I., Le Guellec, R., Couturier, A., Doree, M., Philippe, M., and Prigent, C. (1998). The Xenopus protein kinase pEg2 associates with the centrosome in a cell cycle-dependent manner, binds to the spindle microtubules and is involved in bipolar mitotic spindle assembly. J. Cell Sci. 111, 557–572. [DOI] [PubMed] [Google Scholar]
- Sawin, K.E., and Mitchison, T.J. (1991). Mitotic spindle assembly by two different pathways in vitro. J. Cell Biol. 112, 925–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin, K.E., and Nurse, P. (1997). Photoactivation of green fluorescent protein. Curr. Biol. 7, R606–607. [DOI] [PubMed] [Google Scholar]
- Schatz, C.A., Santarella, R., Hoenger, A., Karsenti, E., Mattaj, I.W., Gruss, O.J., and Carazo-Salas, R.E. (2003). Importin alpha-regulated nucleation of microtubules by TPX2. EMBO J. 22, 2060–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieselmann, N., Armstrong, S., Rauw, J., and Wilde, A. (2003). Ran modulates spindle assembly by regulating a subset of TPX2 and Kid activities including Aurora A activation. J. Cell Sci. 116, 4791–4798. [DOI] [PubMed] [Google Scholar]
- Tsai, M.Y., Wiese, C., Cao, K., Martin, O., Donovan, P., Ruderman, J., Prigent, C., and Zheng, Y. (2003). A Ran signalling pathway mediated by the mitotic kinase Aurora A in spindle assembly. Nat. Cell Biol. 5, 242–248. [DOI] [PubMed] [Google Scholar]
- Weis, K., Dingwall, C., and Lamond, A.I. (1996). Characterization of the nuclear protein import mechanism using Ran mutants with altered nucleotide binding specificities. EMBO J. 15, 7120–7128. [PMC free article] [PubMed] [Google Scholar]
- Wilde, A., Lizarraga, S.B., Zhang, L., Wiese, C., Gliksman, N.R., Walczak, C.E., and Zheng, Y. (2001). Ran stimulates spindle assembly by altering microtubule dynamics and the balance of motor activities. Nat. Cell Biol. 3, 221–227. [DOI] [PubMed] [Google Scholar]
- Wittmann, T., Boleti, H., Antony, C., Karsenti, E., and Vernos, I. (1998). Localization of the kinesin-like protein Xklp2 to spindle poles requires a leucine zipper, a microtubule-associated protein, and dynein. J. Cell Biol. 143, 673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann, T., and Hyman, T. (1999). Recombinant p50/dynamitin as a tool to examine the role of dynactin in intracellular processes. Methods Cell Biol. 61, 137–143. [DOI] [PubMed] [Google Scholar]
- Wittmann, T., Wilm, M., Karsenti, E., and Vernos, I. (2000). TPX2, A novel Xenopus MAP involved in spindle pole organization. J. Cell Biol. 149, 1405–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]