Abstract
The ATP-binding cassette transporter A1 (ABCA1) facilitates the cellular release of cholesterol and choline-phospholipids to apolipoprotein A-I (apoA-I) and several studies indicate that vesicular transport is associated with ABCA1 function. Syntaxins play a major role in vesicular fusion and have also been demonstrated to interact with members of the ABC-transporter family. Therefore, we focused on the identification of syntaxins that directly interact with ABCA1. The expression of syntaxins and ABCA1 in cultured human monocytes during M-CSF differentiation and cholesterol loading was investigated and syntaxins 3, 6, and 13 were found induced in foam cells together with ABCA1. Immunoprecipitation experiments revealed a direct association of syntaxin 13 and full-length ABCA1, whereas syntaxin 3 and 6 failed to interact with ABCA1. The colocalization of ABCA1 and syntaxin 13 was also shown by immunofluorescence microscopy. Silencing of syntaxin 13 by small interfering RNA (siRNA) led to reduced ABCA1 protein levels and hence to a significant decrease in apoA-I–dependent choline-phospholipid efflux. ABCA1 is localized in Lubrol WX–insoluble raft microdomains in macrophages and syntaxin 13 and flotillin-1 were also detected in these detergent resistant microdomains along with ABCA1. Syntaxin 13, flotillin-1, and ABCA1 were identified as phagosomal proteins, indicating the involvement of the phagosomal compartment in ABCA1-mediated lipid efflux. In addition, the uptake of latex phagobeads by fibroblasts with mutated ABCA1 was enhanced when compared with control cells and the recombinant expression of functional ABCA1 normalized the phagocytosis rate in Tangier fibroblasts. It is concluded that ABCA1 forms a complex with syntaxin 13 and flotillin-1, residing at the plasma membrane and in phagosomes that are partially located in raft microdomains.
INTRODUCTION
The discovery of the ATP-binding cassette transporter 1 (ABCA1) as a regulator of choline-phospholipid and cholesterol efflux to lipid-poor pre-β-high-density lipoprotein particles (HDL), provided new insights into the metabolism of HDL subclasses. Mutations in ABCA1 are the cause of genetic HDL deficiency syndromes, which are characterized by the absence of mature α-HDL and presence of pre-β-HDL as the sole HDL particle group.
Loss of functional ABCA1 has been associated with clinical manifestations like hepatosplenomegaly and peripheral neuropathy in classical Tangier disease or atherosclerosis (Bodzioch et al., 1999; Brooks et al., 1999; Rust et al., 1999). ABCA1 facilitates the cellular release of cholesterol and choline-phospholipids to apolipoprotein A-I (apoA-I). In addition, the secretion of the lipophilic vitamins A, E, and K, (Orso et al., 2000; Oram et al., 2001), apolipoprotein E (apoE; von Eckardstein et al., 2001) and interleukin 1-β (IL-1β; Hamon et al., 1997) were found associated with ABCA1.
Several studies indicate that ABCA1 may operate in conjunction with associated heteromeric proteins. Besides the binding of apoA-I to extracellular loops of ABCA1 (Fitzgerald et al., 2002), the Rho GTPase Cdc42 directly interacts with ABCA1 and modulates ABCA1 dependent lipid efflux. Cdc42 controls cellular responses such as actin cytoskeleton rearrangement, migration (Diederich et al., 2001), phagocytosis (Dombrosky-Ferlan et al., 2003), and cell growth and is involved in intracellular lipid transport (Tsukamoto et al., 2002). Furthermore, ABCA1 was found to interact with Fas-associated death domain protein (FADD) and overexpression of a dominant negative FADD reduced choline-phospholipid release (Buechler et al., 2002a).
The PSD-95-discs-large-ZO-1(PDZ)-domain proteins β2-syntrophin, α1-syntrophin, and Lin7 were also described to bind to the extreme C-terminus of ABCA1, which contains a PDZ-interacting motif (Buechler et al., 2002b; Munehira et al., 2004).
Even though numerous studies related to ABCA1 have been published, the molecular mechanisms of ABCA1-dependent secretory pathways are not completely understood. ABCA1 is located on the plasma membrane as well as in late endosomes and lysosomes (Neufeld et al., 2001). Endocytosis is enhanced in fibroblasts with defective ABCA1 (Zha et al., 2001) and it also plays a role in the phagocytosis of apoptotic cells (Hamon et al., 2002).
Retroendocytosis of apoA-I may be involved in ABCA1-related release of cholesterol from endosomes and lysosomes (Takahashi and Smith, 1999). In addition, enhanced vesicular transport from the Golgi to the plasma membrane, in response to apoA-I/ABCA1–mediated cholesterol efflux is absent in Tangier fibroblasts (Zha et al., 2003). These findings may indicate that ABCA1 is a regulator of vesicular transport. Intracellular transport requires the fusion of membranous vesicles mediated in part by N-ethylmaleimide (NEM)-sensitive factor attachment protein receptors (SNAREs; Teng et al., 2001). The cystic fibrosis transmembrane conductance regulator (CFTR), another ABC-transporter family member, has been shown to directly interact with the SNARE proteins syntaxin 1A and SNAP-23 (Cormet-Boyaka et al., 2002) and, similar to ABCA1, its C-terminus interacts with PDZ-proteins (Kunzelmann, 2001).
The similarity of ABCA1 and CFTR and the observation of ABCA1 shuttling in endocytic vesicles prompted us to investigate whether ABCA1 may directly associate with syntaxins. Syntaxins belong to the family of SNAREs; they play a role in vesicular transport and membrane fusion. Here we have identified syntaxin 13 as an ABCA1-interacting protein and silencing of syntaxin 13 by siRNA significantly reduced choline-phospholipid efflux.
MATERIALS AND METHODS
Antibodies
Antibodies were purchased from different suppliers as follows: syntaxin 4, syntaxin 7, and syntaxin 13 (Synaptic Systems, Goettingen, Germany); flotillin-1, syntaxin 6, and syntaxin 8 (BD Biosciences, PharMingen, San Diego, CA); LAMP1, syntaxin 2, and syntaxin 3 (Calbiochem, Darmstadt, Germany); ABCA1 (Novus Biologicals, Littleton, CO); β-actin (Sigma, Taufkirchen, Germany); Rab9 and all fluorescent antibodies (ABCAM Limited, Cambridge, United Kingdom). ABCA1 antibody R1/61 for immunoprecipitation was generated in rabbits against the last 20 amino acids of the human ABCA1 protein (Szakacs et al., 2001).
Coimmunoprecipitation
For coimmunoprecipitation, cells were lysed in 1% Triton X-100 in PBS with a protease inhibitor mixture (Calbiochem, Darmstadt, Germany) for 30 min at 4°C followed by centrifugation at 14,000 rpm for 10 min. The supernatant was incubated overnight with ABCA1 or syntaxin antibodies linked to magnetic protein-A beads (Protein A-Dynabeads, Dynal, Hamburg, Germany). The beads were washed three times with the lysis buffer and were eluted by 100 mM triethylamine, pH 11, supplemented with 10% dioxane. The eluates were analyzed on immunoblots. Phagosomal extracts were lysed in 50 mM Tris, 150 mM NaCl, 1% Nonident P-40, and 0.5% Na deoxycholate in PBS with protease inhibitor (RIPA buffer) and then incubated with either ABCA1 or syntaxin 13 antibodies linked to magnetic beads for 1 h, washed with RIPA buffer three times at RT, and further processed as described above.
Cell Culture and Differentiation
Human monocytes were isolated from blood donors 25–45 years of age with normal lipid status by counterflow elutriation. Cells were cultivated at 37°C in 5% CO2 atmosphere in serum-free macrophage (SFM) medium (Life Technologies, Eggenstein, Germany) with M-CSF (50 ng/ml) for 4 d on plastic Petri dishes (106 cells/ml). Incubation with 30 μg/ml enzymatically modified low-density lipoprotein (E-LDL) for 24 h was performed as previously described (Buechler et al., 2001). For deloading with HDL3 the medium was removed, and cells were washed and incubated in SFM medium with M-CSF and 100 μg/ml HDL3 for 24 h.
HL-60 human leukemia cells were cultured in VLE RPMI 1640 medium supplemented with 10% fetal bovine serum and MEM (All Biochrom KG, Berlin, Germany) in six-well flat bottom cell+ plates (Sarstedt, Nuembrecht, Germany) with phorbol 12-myristate 13-acetate (PMA; Calbiochem, Darmstadt, Germany) for 24 h to induce monocytic differentiation; after that the cells were washed with PBS (Cambrex, Veviers, Belgium) and used for transfection with siRNA.
Human Fibroblasts
Human fibroblasts TD1, TD3, and TD5 were obtained from patients with documented mutations in their ABCA1 gene. Patient TD1 bears a homozygous K171X mutation, patients TD3 and TD5 display mutations that have been published previously (Bodzioch et al., 1999). As control, human fibroblasts obtained from healthy individuals were used. The fibroblasts were incubated in DMEM medium supplemented with 1% MEM and 10% FCS at 37°C in 5% CO2 atmosphere.
Isolation of Phagosomes
Human macrophages were cultivated for 4 d, and blue-dyed latex phagobeads (0.8-μm diameters, 10% aqueous suspension, Sigma, Munich, Germany) were then added to the cells at a dilution of 1:100 in SFM medium. After a 1-h pulse (internalization), the cells were washed with PBS and homogenized either directly or after further incubation for 2, 4, 6, 12, and 20 h, respectively, at 37°C. Phagosomes were then isolated by centrifugation on a discontinuous sucrose gradient as described by Desjardins et al. (1994).
Electroporation
Human fibroblasts were transfected with the human dermal fibroblast Nucleofector Kit (Amaxa GmbH, Cologne, Germany) according to manufacturer's instructions. The wild-type ABCA1-construct in pcDNA3.1D/V5-His vector was a kind gift of Dr. T. Langmann. For control, the pcDNA3.1D/V5-His vector with lacZ insert was used (Invitrogen, Carlsbad, CA). After incubation for 16 h, the transfected fibroblasts were loaded with fluorescent fluoresbrite yellow green microspheres.
Phagocytic Activity
Phagocytic capacity was determined by flow cytometry. Fibroblasts were incubated with fluoresbrite yellow green microspheres, 0.75 μm (Polysciences Europe GmbH, Eppelheim, Germany) for 3 h, washed with PBS and fluorescence was determined by FACSCalibur Flow Cytometer.
Immunoblotting
Cells were harvested, washed with PBS, frozen (-20°C), and thawed twice. Cell debris was separated by centrifugation at 10,000 × g for 10 min at 4°C. Proteins were separated by SDS-PAGE and transferred to PVDF (polyvinylidene fluoride) membranes. Incubations with antibodies were performed in 5% nonfat dry milk in PBS, 0.1% Tween. Detection of the immune complexes was carried out with the ECL Western blot detection system (Amersham Pharmacia, Deisenhofen, Germany).
Detergent Lysis and Sucrose Flotation Gradients
The cell pellets (100–300 μg of protein) were lysed for 30 min on ice in 500 μl ice-cold TNE-buffer containing 50 mM Tris, 150 mM NaCl, 5 mM EDTA, 200 μM aminoethyl benzene sulfonyl fluoride, 160 μM aprotinin, 10 μM bestatin, 3 μM E64-protase inhibitor, 4 μM leupeptin, 2 μM pepstatin A (TNE-buffer; all protease inhibitors from Calbiochem (Bad Soden, Germany) and 1% Lubrol WX (Serva, Heidelberg, Germany). The lysates were brought to 1.2 M sucrose by adding 300 μl of 2.4 M sucrose in TNE-buffer placed on the bottom of a SW55 TI tube (Beckman-Coulter, Krefeld, Germany) and overlaid with 1.2 ml of 0.9 M, 0.6 ml of 0.8 M, 1.2 ml of 0.7 M, and 1.2 ml of 0.1 M sucrose in TNE-buffer. Samples were subjected to ultracentrifugation for 16 h (335,000 × g, 4°C). After centrifugation 600 μl fractions were collected from the top to the bottom and the pellet was resuspended in Triton X-100 at RT.
Isolation of RNA and cDNA Synthesis
Harvesting of cells and RNA extraction was carried out according to the manufacturer's instructions using the TRIzol reagent (Life Technologies, Gaithersburg, MD). Purity and integrity of the RNA were determined on the Agilent 2100 Bioanalyzer with the RNA 6000 Nano LabChip reagent set (Agilent Technologies, Palo Alto, CA). RNA was quantified spectrophotometrically at 260 nm and stored at -80°C.
First-strand cDNA synthesis was performed with the Reverse Transcription System from Promega (Madison, WI) using 2 μg of total RNA in a final volume of 40 μl. The reaction mixture was incubated at 42°C for 60 min followed by heat inactivation of the enzyme at 95°C for 5 min. After cooling on ice for 5 min the cDNA was stored at -20°C.
Primers and TaqMan Probe Design
mRNA sequences for all human syntaxins were derived from the GenBank. Primers and TaqMan probes were designed using the PrimerExpress Software v2.0 (Applied Biosystems, Darmstadt, Germany; Table 1). Primers were obtained from MWG-Biotech (Ebersberg, Germany), 6-carboxyfluorescein (FAM)-labeled probes and assays-on-demand from Applied Biosystems (Darmstadt, Germany). For the normalization of the results a VIC labeled GAPDH TaqMan PDAR endogenous control kit (Applied Biosystems) was used. Each of the probes is quenched by a nonfluorescent quencher (TAMRA) at its 3′ end.
Table 1.
mRNA sequences for all human syntaxins
| Gene name | Forward primer | Reverse primer | Probe | ||
|---|---|---|---|---|---|
| Syntaxin 2 | TCGGTGCTGTCTCGGAAGTT | TCCGCTCCCGAAACAGAGT | CCATGGCGGAGTACAATGAGGCACA | ||
| Syntaxin 4 | TGCCATCTGTGTGTCCATCAC | TGCGACATTATCCAACCACTGT | CGTCCTCCTAGCAGTCATCATTGGCG | ||
| Syntaxin 8 | GGGCGGAGTCTGCAGGAT | TTGGGCAATTTGACAAGTAGAATC | CACCGGACCCCTGGTTCTCCACAT | ||
| Syntaxin 13 | CGTGCAGCGGCAACATC | CCTAGCTGGCTCATCAAATTCTTT | CGGATCAGCCAAGCCACTGCTCA | ||
| Syntaxin 3 | Assay-On-Demand Applied Biosystems context sequence: GAGTTCTTTTCTGAGATTGAGGAAA | ||||
| Syntaxin 6 | Assay-On-Demand Applied Biosystems context sequence: ATGACCAGTGATCGGCGCCAATGGT | ||||
Generation of Standard Curves
For quantification of the results obtained by real-time PCR we used the standard curve method. For this purpose a stock of total RNA from macrophages was serially diluted. A standard curve with 50, 25, 12.5, 6.25, and 3.125 ng total RNA was generated for syntaxins and GAPDH. The standard curves were used to determine the relative expression of syntaxin and GAPDH mRNA in each sample. Finally the results were normalized to the endogenous control GAPDH.
TaqMan PCR
TaqMan PCR assays were performed on the ABI Prism 7900 HT Sequence Detection System (Perkin Elmer-Cetus-Applied Biosystems, Darmstadt, Germany). For quantitation of each syntaxin gene, a mastermix containing 10 μl 2× TaqMan Universal PCR Master Mix, 1 μl of both gene specific forward and reverse primer (each 18 μM), 1 μl gene specific probe (3 μM), and 2 μl sterile water was prepared and aliquoted in a 384-well optical plate. A mastermix for the endogenous control GAPDH containing 10 μl 2× TaqMan Universal PCR Master Mix, 1 μl predeveloped TaqMan assay reagents (PDAR) endogenous control kit and 4 μl sterile water was treated similarly. Finally triplicates of cDNA templates equivalent to 50 ng of RNA were added to a final volume of 20 μl. The thermal cycling was performed for 2 min at 50°C and 10 min at 95°C followed by 45 cycles of 15 s at 95°C and 1 min at 60°C.
Small Interference RNA (siRNA)
Knockdown of the syntaxin 13 expression was achieved by transfection of cells with siRNA. The siRNA was designed and supplied by Ambion (Austin, TX). A sequence of 19 nucleotides starting at position 421 of syntaxin 13 (NM_177424) was targeted; the sense sequence was 5′-ggguaucugaaaaggaaaatt-3′, and the antisense sequence was 5′-uuuuccuuuucagauaccctt-3′. Transfection was done using RNAiFect reagents (Qiagen, Hilden, Germany), according to the manufacturer's instructions. As control a nonsilencing siRNA was used (Qiagen). The knockdown in syntaxin 13 expression reached its maximum 3 d after transfection.
Phospholipid Efflux
Efflux of [3H]choline phospholipids from cells was measured by the appearance of radioactive label in the medium after incubation with either apoA-I or HDL3. Lipids in the medium were extracted according to the method of Bligh and Dyer (1959), and the radioactivity was measured by liquid scintillation counting. Lipid efflux was calculated by subtracting the radioactivity secreted in the supernatant from total radioactivity. Specific lipid efflux was determined as lipid efflux in the presence of apoA-I or HDL3 subtracted by the efflux in the absence of a nonspecific acceptor (BSA).
Immunofluorescence Microscopy
Human macrophages and HL-60 cells were seeded on Lab-Tek II glass chamber slides (Nalge Nunc Intl., Naperville, IN) at a concentration of 2.5 × 105 cells per well. After differentiation for 4 d cells were washed with PBS and fixed with 4% paraformaldehyde and 0.02% of glutaraldehyde. All monoclonal and polyclonal antibodies were diluted with PBS to the recommended concentrations. Incubation with both primary and secondary antibodies was for 2 h at 37°C in a moist chamber under a glass coverslip. Slides were washed with PBS, stained with DAPI, and mounted in Vectashield (Vector Laboratories, Burlingame, CA). Nonconfocal fluorescent images were collected on a Zeiss Axiovert S100 spectral microscope (Carl Zeiss GmbH, Goettingen, Germany) using a CCD camera from Princeton Instruments MicroMax RTE/ CCD-1317-K/1 (Roper Scientific, Trenton, NJ) controlled by Metamorph software (Universal Imaging, Downingtown, PA) with excitation and emission conditions chosen to clearly resolve Texas Red– and FITC-labeled secondary antibodies. Nuclear shape was determined from the DAPI staining.
RESULTS
To identify ABCA1 interactive syntaxins, we first examined the expression of syntaxins in monocytes and macrophages. Fifteen members of the syntaxin family are present in the human genome with different subcellular locations of the corresponding proteins (Teng et al., 2001). We focused on syntaxins relevant for endocytosis, phagocytosis, and secretion, namely the syntaxins 1, 2, 3, and 4 located at the plasma membrane; syntaxin 8 and 13 associated with early endosomes; syntaxin 7, which plays a role in the biogenesis of lysosomes; and syntaxin 6, which is involved in the vesicle release from the trans-Golgi Network (TGN). Syntaxin 1 is not expressed in macrophages (Hackam et al., 1996) and therefore was not analyzed.
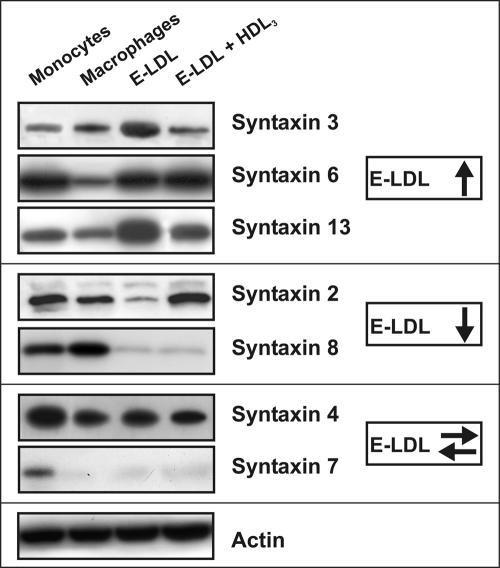
ABCA1 is induced during monocytic differentiation and further up-regulated by sterol loading of these cells (Langmann et al., 1999). We aimed to investigate the expression of syntaxins in monocytes, in in vitro–differentiated macrophages, in macrophages incubated with E-LDL for 24 h, and in E-LDL loaded cells subsequently treated with HDL3 for 24 h to allow lipid efflux. Syntaxin 4, 6, and 7 are downregulated during phagocytic differentiation, whereas the expression of syntaxin 2, 3, 8, and 13 was unchanged (Figure 1). Incubation with E-LDL induced syntaxin 3, 6, and 13 and the subsequent addition of HDL3 reversed this up-regulation for syntaxin 3 and 13. Syntaxin 2 and 8 were found reduced in E-LDL–loaded macrophages and HDL3 treatment again up-regulated syntaxin 2 expression, whereas syntaxin 8 levels were not altered (Figure 1).
Figure 1.
Analysis of syntaxin expression in primary human monocytes and macrophages. The expression of syntaxin 2, 3, 4, 6, 7, 8, and 13 was investigated in monocytes, M-CSF differentiated macrophages, macrophages incubated for 24 h with 40 μg/ml ELDL, and E-LDL–loaded cells subsequently treated with 100 μg/ml HDL3 for additional 24 h using immunoblots. Syntaxin 3, 6, and 13 were induced by E-LDL, Syntaxin 2 and 8 were down-regulated by E-LDL and Syntaxin 4 and 7 are not regulated by E-LDL. β-actin was used as a loading control.
To determine whether these changes in protein expression are transcriptionally or posttranscriptionally regulated, we established TaqMan assays for syntaxin 2, 3, 4, 6, 8, and 13. The mRNA expression was determined in monocytes, macrophages, foam cells, and E-LDL–loaded cells deloaded with HDL3. The mRNA abundance of the syntaxins investigated was unchanged under the conditions described above and therefore the differential expression of syntaxins is most likely related to altered protein stability and is regulated posttranscriptionally.
ABCA1 is up-regulated by E-LDL loading and downregulated by further HDL3 deloading (Langmann et al., 1999). Syntaxins 3, 6, and 13 follow the same regulation pattern except for syntaxin 6, which is not down-regulated by HDL3 deloading. This coregulation may indicate a functional association of these syntaxins and ABCA1.
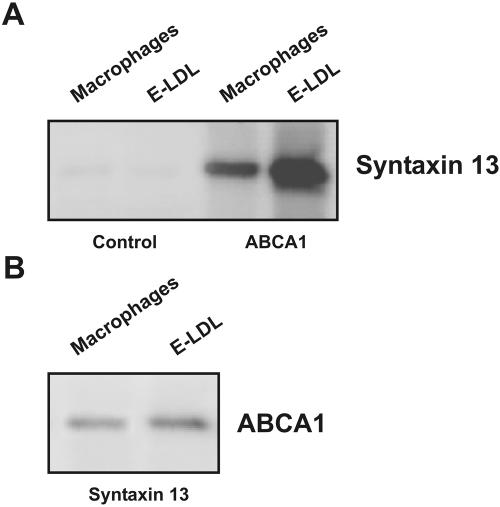
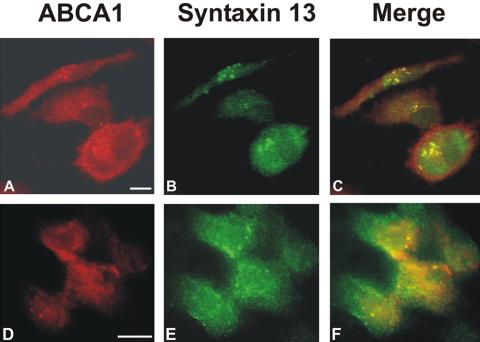
Immunoprecipitation experiments using cell lysates from differentiated and E-LDL–treated macrophages were performed. ABCA1 antibodies coimmunoprecipitated syntaxin 13 (Figure 2A) and, for confirmation, syntaxin 13 antibodies also coimmunoprecipitated ABCA1 from the lysates (Figure 2B). Although syntaxin 3 and 6 and ABCA1 were coordinately regulated, we could not identify an interaction in human monocyte derived macrophages or foam cells. In E-LDL–loaded cells, an increased amount of ABCA1-interactive syntaxin 13 was detected when compared with macrophages (Figure 2). To confirm this interaction in living cells, immunofluorescence microscopy was performed and ABCA1 was found to colocalize with syntaxin 13 in HL-60 cells and macrophages (Figure 3).
Figure 2.
Interaction of ABCA1 with syntaxin 13 demonstrated by immunoprecipitation. (A) Immunoblot of syntaxin 13 precipitated with control (lanes 1 and 2) and ABCA1 antibody (lane 3 and 4) from cell lysates prepared from 4-d differentiated macrophages and E-LDL–loaded cells. (B) Immunoblot of ABCA1 precipitated with syntaxin 13 antibody from cell lysates prepared from 4-d differentiated macrophages and E-LDL–loaded cells.
Figure 3.
Syntaxin 13 colocalizes with ABCA1. Human macrophages (A–C) and PMA-differentiated HL-60 cells (D–F) were fixed with 4% paraformaldehyde and 0.02% glutaraldehyde, immunostained with primary antibodies for syntaxin 13 (mouse) and ABCA1 (rabbit) and then incubated with Texas Red–labeled antirabbit and FITC-labeled anti-mouse secondary antibodies and processed for immunofluorescent microscopy. ABCA1 (A and D), syntaxin 13 (B and E); merged images (C and F). Yellow: area of overlap. Bars, 10 μm.
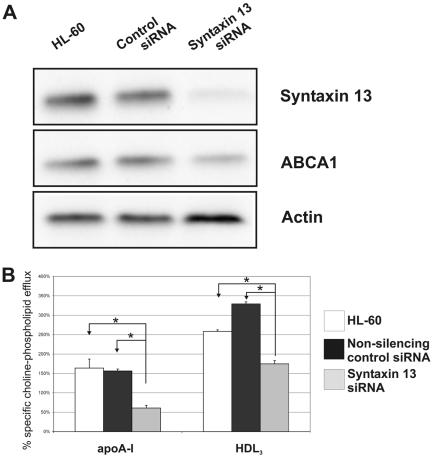
The myelomonocytic HL-60 cell line, which can be induced to differentiate to macrophages by PMA (Chou et al., 2002), was selected as a suitable model for transfection experiments. Transfection of HL-60 cells with syntaxin 13–specific siRNA not only led to a significantly reduced syntaxin 13 protein level but also to a decreased ABCA1 protein expression. Cells transfected with nonsilencing control siRNA did not show any alteration in syntaxin 13 and ABCA1 when compared with untransfected cells (Figure 4A). The HL-60 cell model was also used to determine choline-phospholipid efflux. The silencing of syntaxin 13 protein in HL-60 cells with the syntaxin 13–specific siRNA led to a significant decrease in choline-phospholipid efflux to apoA-I and HDL3, whereas lipid efflux was not affected in control transfected cells (Figure 4B). These findings may indicate that the expression of syntaxin 13 stabilizes ABCA1 protein. Reduced abundance of syntaxin 13 is associated with a decrease in ABCA1 protein and, as a consequence, reduced lipid efflux to apoA-I.
Figure 4.
Silencing of syntaxin 13 and choline-phospholipid efflux in siRNA-treated HL-60 cells. (A) PMA-differentiated HL-60 cells and PMA-differentiated HL-60 cells transfected with nonsilencing siRNA as control or transfected with syntaxin 13 siRNA were analyzed. Protein was isolated and the expression of syntaxin 13 and ABCA1 was analyzed by immunoblot. (B) Choline-phospholipid efflux was determined in the PMA-differentiated HL-60 cells, PMA-differentiated HL-60 cells transfected with nonsilencing siRNA as control, or HL-60 cells transfected with syntaxin 13 siRNA. The apoA-I and HDL3-mediated lipid efflux relative to the unspecific efflux from three independent experiments is given in percent. The significance of the decrease in efflux between syntaxin 13–silenced cells and control cells was calculated using t test; *p < 0.05.
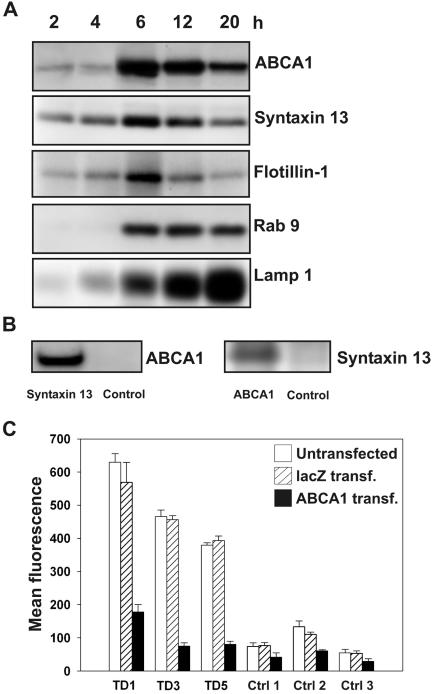
Syntaxin 13 mediates endosomal cycling of plasma membrane proteins (Prekeris et al., 1998) and is required for the interaction of endosomes with the phagosome (Collins et al., 2002). ABCA1 has been found on endosomes (Neufeld et al., 2001) but its association with phagosomes has never been investigated. To induce phagosome formation, macrophages were loaded with 0.8-μm blue-dyed latex phagobeads for 1 h and washed, and intracellular processing of the phagobeads in the phagosome compartment was followed for 2, 4, 6, 12, and 20 h.
Immunoblots using purified phagosomal proteins were performed. Both ABCA1 and syntaxin 13 were similarly detectable in the 2- and 4-h fraction, reaching its peak after 6 h and decreasing afterward (Figure 5A). Low amounts of syntaxin 4, a plasma membrane–associated syntaxin, were also detected in the phagosomal protein lysates (unpublished data).
Figure 5.
Phagosomal localization of ABCA1 interactive proteins. (A) Phagosomes were purified from 4-d differentiated macrophages incubated with latex beads for 2, 4, 6, 12, and 20 h. Purified phagosomal proteins were used for immunoblotting. The expression of ABCA1, syntaxin 13, syntaxin 4, flotillin-1, Rab 9, and LAMP1 was analyzed. (B) Immunoblot of ABCA1 precipitated with syntaxin 13 antibody from phagosomal protein fraction (6 h) and syntaxin 13 precipitated with ABCA1. Control rabbit antibody was used as negative control. (C) The phagocytic activity of fibroblasts was determined in human fibroblasts with functional, recombinant ABCA1 or lacZ as control after 4-h incubation with fluoresbrite yellow green microspheres by flow cytometry. Both, the increased phagocytosis of Tangier compared with normal fibroblasts as well as the reduced phagocytic activity for ABCA1-transfected Tangier when compared with lacZ-transfected fibroblasts were statistically significant (p < 0.05). TD1, 3, and 5: fibroblasts obtained from Tangier disease patients with documented mutations in their ABCA1 gene as described in Materials and Methods. Ctrl1, 2, and 3: fibroblasts obtained from healthy control individuals.
Flotillin-1, known to accumulate on maturing phagosomes (Dermine et al., 2001), reached maximum expression after 6 h, afterward decreasing in fractions 12 and 20 h. Rab 9, an additional phagosomal protein, was detectable and unchanged after 6 h, whereas LAMP1, associated with phagosomes and lysosomes, continuously increased, indicating the gradient incorporation of phagosomes into lysosomes (Figure 5A).
Nevertheless, the purity of the isolated phagosomes cannot be ascertained because there is no specific phagosomal marker protein. Flotillin-1, recently described as a phagosomal protein (Garin et al., 2001), could also be detected at the plasma membrane (Edgar and Polak, 2001).
To investigate whether the interaction between ABCA1 and syntaxin 13 can be reproduced in phagosomes, we performed additional coimmunoprecipitation assays with stringent RIPAbuffer (Figure 5B) and found that syntaxin 13 antibodies coimmunoprecipitated ABCA1 and, for confirmation, ABCA1 antibodies coimmunoprecipitated syntaxin 13 from phagosomal lysates. For negative control a rabbit antibody was used.
To further support the hypothesis that ABCA1 may regulate phagocytosis, the phagocytic activity of fibroblasts from control donors and fibroblasts isolated from the Tangier patients TD1 (yielding a mutation at K171X), TD3 II:4 and TD5 III:4 (Bodzioch et al., 1999) was measured by flow cytometry using fluorescent yellow green-labeled phagobeads. Fibroblasts from Tangier patients with ABCA1 mutations had a significantly higher internalization rate compared with controls, further supporting a function of ABCA1 in the phagocytic uptake of particles (Figure 5C). Recombinant expression of wild-type ABCA1 in Tangier fibroblasts led to a decreased phagocytosis rate as was seen in control fibroblasts (Figure 5C).
These findings indicate that ABCA1 is located at the plasma membrane and in the phagosome. Both compartments are involved in phagocytosis and may also be important in ABCA1-dependent lipid efflux.
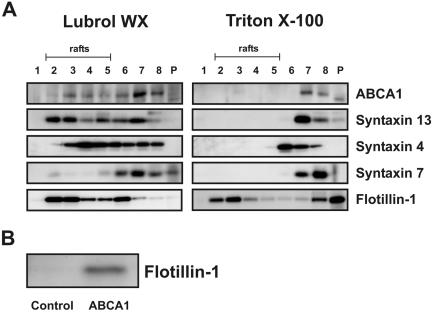
Flotillin-1 (Dermine et al., 2001) and ABCA1 (Drobnik et al., 2002) are raft-associated proteins. A wide variety of detergents have been used to isolate rafts and significant differences exist among the preparations (Pike, 2003). Flotillin-1 is enriched in Triton X-114–insoluble microdomains, whereas 5–10% of ABCA1 are located in Lubrol WX rafts, whereas it is absent from Triton X-100 rafts (Drobnik et al., 2002). Using whole cell lysates, we examined whether flotillin-1 and syntaxin 13 colocalize in Lubrol WX–insoluble microdomains in E-LDL–loaded macrophages solubilized in 1% Lubrol WX and fractionated by sucrose gradient centrifugation. Immunoblots using ABCA1 antibodies confirmed our previous data reported by Drobnik et al. (2002) (Figure 6A). Syntaxin 13 was highly abundant in Lubrol WX rafts but not detectable in Triton X-100 rafts and flotillin-1 copurifies with Triton X-100 and Lubrol WX rafts (Figure 6A).
Figure 6.
Microdomain localization of ABCA1-associated proteins and association of ABCA1 with flotillin-1. (A) E-LDL–loaded macrophages were solubilized in 1% Lubrol WX or Triton X-100 and fractionated by sucrose gradient centrifugation. Immunoblots were performed with ABCA1, syntaxin 13, syntaxin 4, syntaxin 7, and flotillin-1. The fractions 2–5 indicate rafts, whereas lanes 6–8 are not associated with rafts. Fraction 8 represents soluble membrane proteins and P is the insoluble pellet. (B) Immunoblot of flotillin-1 precipitated with control and ABCA1 antibody from cell lysates prepared from 4-d differentiated macrophages.
Syntaxin 4, localized at the plasma membrane, was also identified in Lubrol WX microdomains, whereas syntaxin 7, a marker of late endosomes, was not detected.
The abundance of ABCA1 and flotillin-1 at the plasma membrane and phagosomes together with their colocalization in Lubrol WX rafts may indicate a direct interaction of ABCA1 and flotillin-1. Subsequent immunoprecipitations confirmed that flotillin-1 is directly associated with ABCA1 (Figure 6B).
In conclusion, we have shown that ABCA1 interacts with syntaxin 13 and flotillin-1.
Syntaxin 13 may be involved in ABCA1 trafficking and disturbed syntaxin 13 is associated with diminished ABCA1 expression and reduced lipid efflux.
DISCUSSION
Recent publications suggest that ABCA1 associates with heteromeric proteins (Tsukamoto et al., 2001; Buechler et al., 2002a, 2002b; Munehira et al., 2004). We aimed to identify ABCA1 interactive syntaxins. The SNARE protein syntaxin 1A is well described to directly bind to CFTR and to modulate its activity (Cormet-Boyaka et al., 2002). Homologies in mechanism and structure between ABCA1 and CFTR (Schmitz and Buechler, 2002) raised the possibility that ABCA1 may interact with syntaxins as well.
The regulation of syntaxins 2, 3, 4, 6, 7, 8, and 13 was investigated in macrophages loaded with E-LDL and syntaxins 3, 6, and 13 were found induced in lipid-loaded cells. Because ABCA1 is similarly regulated by atherogenic lipoproteins (Langmann et al., 1999) the direct interaction of ABCA1 with syntaxin 3, 6, and 13 was analyzed. Syntaxin 13 turned out to directly associate with ABCA1, whereas syntaxin 3 and 6 failed to bind to ABCA1.
To gain further insights into the functionality, we silenced syntaxin 13 in PMA-differentiated HL-60 using specific siRNA probes. This led to a decrease of both syntaxin 13 and ABCA1 protein expression and reduced apoA-I–mediated choline-phospholipid efflux, indicating that syntaxin 13 stabilizes ABCA1 expression and thereby regulates ABCA1-mediated lipid release.
ABCA1 copurifies with Lubrol WX rafts and apoA-I preferentially depletes lipids from these microdomains (Drobnik et al., 2002). Flotillin-1, recently described to copurify with Triton X-114 rafts, and syntaxin 13 were demonstrated in this work to be also associated with Lubrol WX rafts. Although flotillin-1 expression is not regulated during macrophage differentiation or incubation with atherogenic lipoproteins (own unpublished observation), flotillin-1 was found to directly interact with ABCA1 and therefore, ABCA1 forms a complex with either syntaxin 13, or flotillin-1 or both. Besides being localized at the plasma membrane, detergent insoluble microdomains have been detected in phagosomes (Dermine et al., 2001) and endosomes (Sharma et al., 2003) and may control internalization pathways.
ABCA1 was detected in endosomes (Neufeld et al., 2001) and may also associate with phagosomes. The phagosome proteome was recently described and flotillin-1 is highly abundant in this cellular compartment (Garin et al., 2001). Purification of phagosomal proteins using the method described by Desjardin et al. (1994) and subsequent immunoblot analysis confirmed this finding and, in addition, identified ABCA1 as a phagosomal protein. Syntaxin 13 was also copurified and coimmunoprecipitation revealed a direct association of phagosomal ABCA1 and syntaxin 13.
In this study we show that ABCA1-deficient fibroblasts reveal increased phagocytosis compared with normal fibroblasts. Recombinant expression of ABCA1 in Tangier fibroblasts normalized the uptake of phagobeads by Tangier cells providing additional evidence that ABCA1 is involved in phagosome regulation. Type II phagocytosis occurs through inward bending of the membrane and sinking of the particles into the cell (Kaplan, 1977). This maybe related to the impaired phosphatidylserine content of the outer leaflet of the plasma membrane in the absence of functional ABCA1 (Zha et al., 2001). An alternative attractive scenario would be that apo-AI binds to the β-chain of the ATP synthase complex at the plasma membrane, which promotes HDL uptake via type II phagocytosis (Martinez et al., 2003), a mechanism that may operate even in the absence of functional ABCA1.
Furthermore the effect of type II phagocytosis includes the reorganization of the cytoskeleton and Rho A (Caron and Hall, 1998), and Rho A was found accumulated in Tangier fibroblasts (Utech et al., 2001). ABCA1 also promotes the engulfment of apoptotic cells via type I phagocytosis, involving Fcγ-receptors and pseudopodia extension initiated by outward bending of the membrane (Hamon et al., 2002). Ex vivo data from our laboratory, that Fcγ-receptor expression on CD14+ monocyte subsets positively correlates with plasma cholesterol and triglycerides and negatively correlates with HDL cholesterol levels, support both types of phagocytosis (Rothe et al., 1996).
Syntaxin 13 is a soluble NEM-sensitive factor-attachment protein receptor (SNARE) protein, a family of proteins that mediates the coordinated fusion of membranes through the formation of four-helical bundle structure protein complexes (Teng et al., 2001). Overexpression of syntaxin 13 blocks CFTR trafficking through the nonconventional pathway from the ER to the Golgi, indicating a role of syntaxin 13 in CFTR maturation (Yoo et al., 2002). Furthermore, syntaxin 13 functions in membrane fusion events during the recycling of plasma membrane proteins (Prekeris et al., 1998) as well as in lysosome and endosome fusion with the phagosome (Collins et al., 2002). Deficiency of syntaxin 13 binding protein (pallidin) leads to a Hermansky-Pudlak syndrome (HPS)-like phenotype in mice, which is associated with severe vesicular trafficking defects in secretory lysosomes (Starcevic et al., 2002). Secretory lysosomes are organelles that can undergo regulated secretion in response to external stimuli and syntaxins involved in lysosomal secretion vary between cell types (Stinchcombe et al., 2004). Initial studies in our group point to reduced plasma apoA-I and HDL in the pallid mouse, but additional experiments are currently performed to further strengthen this unpublished observation. These findings raise the possibility that syntaxin 13 plays a role in phago-lysosomal release of lipids in macrophages.
HDL is mainly internalized by a receptor-mediated pathway not associated with clathrin-coated pits (Schmitz et al., 1985) and apoA-I was identified in phagosomes isolated with latex beads, further supporting an internalization pathway different from classical coated pit endocytosis (Garin et al., 2001). The identification of ABCA1 as a regulator of phagocytosis may indicate a cross-talk of phagocytic and endocytic pathways involved in ABCA1-mediated efflux with cholesterol deposited in late endosomes and lysosomes as the preferred source (Chen et al., 2001). The enhanced uptake of apoA-I by endocytic and phagocytic ingestion may also explain the increased catabolism of apoA-I in Tangier patients (Bojanovski et al., 1987).
In conclusion, our results indicate that syntaxin 13 forms a heteromeric complex with ABCA1 and flotillin-1. This complex is already formed in differentiated monocyte-derived macrophages and does not require induction by ELDL loading. Syntaxin 13 deficiency causes ABCA1 protein degradation and therefore syntaxin 13 may be important in ABCA1 maturation and vesicular transport. These findings indicate that besides retroendocytosis of apoA-I and HDL, the phagosomal and lysosomal compartment may be involved in ABCA1-dependent choline-phospholipid efflux.
Acknowledgments
We thank Dr. C. Aslanidis, Dr. W. Kaminski, Dr. T. Langmann, and Dr. B. Stoelcker for their helpful comments. This work was supported by the Deutsche Forschungsgemeinschaft (grant PO 708/1-2) and the Transregional Collaborative Research Centre 13: “Membrane microdomains and their role in human disease.”
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04–03–0182. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–03–0182.
References
- Bligh, E.G., and Dyer, W.J. (1959). A rapid method of total lipid extraction and purification. Can. J. Med. Sci. 37, 911-917. [DOI] [PubMed] [Google Scholar]
- Bodzioch, M. et al. (1999). The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat. Genet. 22, 347-351. [DOI] [PubMed] [Google Scholar]
- Bojanovski, D., Gregg, R.E., Zech, L.A., Meng, M.S., Bishop, C., Ronan, R., and Brewer, H.B., Jr. (1987). In vivo metabolism of proapolipoprotein A-I in Tangier disease. J. Clin. Invest. 80, 1742-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, W.A. et al. (1999). Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency [see comments]. Nat. Genet. 22, 336-345. [DOI] [PubMed] [Google Scholar]
- Buechler, C., Bared, S.M., Aslanidis, C., Ritter, M., Drobnik, W., and Schmitz, G. (2002a). Molecular and functional interaction of the ATP-binding cassette transporter A1 with Fas-associated death domain protein. J. Biol. Chem. 277, 41307-41310. [DOI] [PubMed] [Google Scholar]
- Buechler, C., Boettcher, A., Bared, S.M., Probst, M.C., and Schmitz, G. (2002b). The carboxyterminus of the ATP-binding cassette transporter A1 interacts with a beta2-syntrophin/utrophin complex. Biochem. Biophys. Res. Commun. 293, 759-765. [DOI] [PubMed] [Google Scholar]
- Buechler, C., Ritter, M., Duong, C.Q., Orso, E., Kapinsky, M., and Schmitz, G. (2001). Adipophilin is a sensitive marker for lipid loading in human blood monocytes. Biochim. Biophys. Acta 1532, 97-104. [DOI] [PubMed] [Google Scholar]
- Caron, E., and Hall, A. (1998). Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282, 1717-1721. [DOI] [PubMed] [Google Scholar]
- Chen, W., Sun, Y., Welch, C., Gorelik, A., Leventhal, A.R., Tabas, I., and Tall, A.R. (2001). Preferential ATP-binding cassette transporter A1-mediated cholesterol efflux from late endosomes/lysosomes. J. Biol. Chem. 276, 43564-43569. [DOI] [PubMed] [Google Scholar]
- Chou, S.F., Chen, H.L., and Lu, S.C. (2002). Up-regulation of human deoxyribonuclease II gene expression during myelomonocytic differentiation of HL-60 and THP-1 cells. Biochem. Biophys. Res. Commun. 296, 48-53. [DOI] [PubMed] [Google Scholar]
- Collins, R.F., Schreiber, A.D., Grinstein, S., and Trimble, W.S. (2002). Syntaxins 13 and 7 function at distinct steps during phagocytosis. J Immunol. 169, 3250-3256. [DOI] [PubMed] [Google Scholar]
- Cormet-Boyaka, E., Di, A., Chang, S.Y., Naren, A.P., Tousson, A., Nelson, D.J., and Kirk, K.L. (2002). CFTR chloride channels are regulated by a SNAP-23/ syntaxin 1A complex. Proc. Natl. Acad. Sci. USA 99, 12477-12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermine, J.F., Duclos, S., Garin, J., St. Louis, F., Rea, S., Parton, R.G., and Desjardins, M. (2001). Flotillin-1-enriched lipid raft domains accumulate on maturing phagosomes. J. Biol. Chem. 276, 18507-18512. [DOI] [PubMed] [Google Scholar]
- Desjardins, M., Huber, L.A., Parton, R.G., and Griffiths, G. (1994). Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. J. Cell Biol. 124, 677-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederich, W., Orso, E., Drobnik, W., and Schmitz, G. (2001). Apolipoprotein AI and HDL(3) inhibit spreading of primary human monocytes through a mechanism that involves cholesterol depletion and regulation of CDC42. Atherosclerosis 159, 313-324. [DOI] [PubMed] [Google Scholar]
- Dombrosky-Ferlan, P., Grishin, A., Botelho, R.J., Sampson, M., Wang, L., Rudert, W.A., Grinstein, S., and Corey, S.J. (2003). Felic (CIP4b), a novel binding partner with the Src kinase Lyn and Cdc42, localizes to the phagocytic cup. Blood 101, 2804-2809. [DOI] [PubMed] [Google Scholar]
- Drobnik, W., Borsukova, H., Bottcher, A., Pfeiffer, A., Liebisch, G., Schutz, G.J., Schindler, H., and Schmitz, G. (2002). Apo AI/ABCA1-dependent and HDL3-mediated lipid efflux from compositionally distinct cholesterol-based microdomains. Traffic 3, 268-278. [DOI] [PubMed] [Google Scholar]
- Edgar, A.J., and Polak, J.M. (2001). Flotillin-1,gene structure: cDNA cloning from human lung and the identification of alternative polyadenylation signals. Int. J. Biochem. Cell Biol. 33, 53-64. [DOI] [PubMed] [Google Scholar]
- Fitzgerald, M.L., Morris, A.L., Rhee, J.S., Andersson, L.P., Mendez, A.J., and Freeman, M.W. (2002). Naturally occurring mutations in the largest extracellular loops of ABCA1 can disrupt its direct interaction with apolipoprotein A-I. J. Biol. Chem. 277, 33178-33187. [DOI] [PubMed] [Google Scholar]
- Garin, J., Diez, R., Kieffer, S., Dermine, J.F., Duclos, S., Gagnon, E., Sadoul, R., Rondeau, C., and Desjardins, M. (2001). The phagosome proteome: insight into phagosome functions. J. Cell Biol. 152, 165-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackam, D.J., Rotstein, O.D., Bennett, M.K., Klip, A., Grinstein, S., and Manolson, M.F. (1996). Characterization and subcellular localization of target membrane soluble NSF attachment protein receptors (t-SNAREs) in macrophages. Syntaxins 2, 3, and 4 are present on phagosomal membranes. J. Immunol. 156, 4377-4383. [PubMed] [Google Scholar]
- Hamon, Y., Chambenoit, O., and Chimini, G. (2002). ABCA1 and the engulfment of apoptotic cells. Biochim. Biophys. Acta 1585, 64-71. [DOI] [PubMed] [Google Scholar]
- Hamon, Y., Luciani, M.F., Becq, F., Verrier, B., Rubartelli, A., and Chimini, G. (1997). Interleukin-1beta secretion is impaired by inhibitors of the Atp binding cassette transporter, ABC1. Blood 90, 2911-2915. [PubMed] [Google Scholar]
- Kaplan, G. (1977). Differences in the mode of phagocytosis with Fc and C3 receptors in macrophages. Scand. J. Immunol. 6, 797-807. [DOI] [PubMed] [Google Scholar]
- Kunzelmann, K. (2001). CFTR: interacting with everything? News Physiol. Sci. 16, 167-170. [DOI] [PubMed] [Google Scholar]
- Langmann, T., Klucken, J., Reil, M., Liebisch, G., Luciani, M.F., Chimini, G., Kaminski, W.E., and Schmitz, G. (1999). Molecular cloning of the human ATP-binding cassette transporter 1 (hABC1): evidence for sterol-dependent regulation in macrophages. Biochem. Biophys. Res. Commun. 257, 29-33. [DOI] [PubMed] [Google Scholar]
- Martinez, L.O. et al. (2003). Ectopic beta-chain of ATP synthase is an apolipoprotein A-I receptor in hepatic HDL endocytosis. Nature 421, 75-79. [DOI] [PubMed] [Google Scholar]
- Munehira, Y. et al. (2004). alpha 1-syntrophin modulates turnover of ABCA1. J. Biol. Chem. 279, 15091-15095. [DOI] [PubMed] [Google Scholar]
- Neufeld, E.B. et al. (2001). Cellular localization and trafficking of the human ABCA1 transporter. J. Biol. Chem. 276, 27584-27590. [DOI] [PubMed] [Google Scholar]
- Oram, J.F., Vaughan, A.M., and Stocker, R. (2001). ATP-binding cassette transporter A1 mediates cellular secretion of alpha-tocopherol. J. Biol. Chem. 276, 39898-39902. [DOI] [PubMed] [Google Scholar]
- Orso, E. et al. (2000). Transport of lipids from golgi to plasma membrane is defective in tangier disease patients and Abc1-deficient mice. Nat. Genet. 24, 192-196. [DOI] [PubMed] [Google Scholar]
- Pike, L.J. (2003). Lipid rafts: bringing order to chaos. J. Lipid Res. 44, 655-667. [DOI] [PubMed] [Google Scholar]
- Prekeris, R., Klumperman, J., Chen, Y.A., and Scheller, R.H. (1998). Syntaxin 13 mediates cycling of plasma membrane proteins via tubulovesicular recycling endosomes. J. Cell Biol. 143, 957-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe, G., Gabriel, H., Kovacs, E., Klucken, J., Stohr, J., Kindermann, W., and Schmitz, G. (1996). Peripheral blood mononuclear phagocyte subpopulations as cellular markers in hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 16, 1437-1447. [DOI] [PubMed] [Google Scholar]
- Rust, S. et al. (1999). Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1 [see comments]. Nat. Genet. 22, 352-355. [DOI] [PubMed] [Google Scholar]
- Schmitz, G., and Buechler, C. (2002). ABCA1, regulation, trafficking and association with heteromeric proteins. Ann. Med. 34, 334-347. [DOI] [PubMed] [Google Scholar]
- Schmitz, G., Robenek, H., Lohmann, U., and Assmann, G. (1985). Interaction of high density lipoproteins with cholesteryl ester-laden macrophages: biochemical and morphological characterization of cell surface receptor binding, endocytosis and resecretion of high density lipoproteins by macrophages. EMBO J. 4, 613-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, D.K., Choudhury, A., Singh, R.D., Wheatley, C.L., Marks, D.L., and Pagano, R.E. (2003). Glycosphingolipids internalized via caveolar-related endocytosis rapidly merge with the clathrin pathway in early endosomes and form microdomains for recycling. J. Biol. Chem. 278, 7564-7572. [DOI] [PubMed] [Google Scholar]
- Starcevic, M., Nazarian, R., Dell'Angelica, E.C. (2002). The molecular machinery for the biogenesis of lysosome-related organelles: lessons from Hermansky-Pudlak syndrome. Semin. Cell Dev. Biol. 13, 271-278. [DOI] [PubMed] [Google Scholar]
- Stinchcombe, J., Bossi, G., Griffiths, G.M. (2003). Linking albinism and immunity: the secret of secretory lysosomes. Science 305, 55-59. [DOI] [PubMed] [Google Scholar]
- Szakacs, G., Langmann, T., Ozvegy, C., Orso, E., Schmitz, G., Varadi, A., and Sarkadi, B. (2001). Characterization of the ATPase cycle of human ABCA1, implications for its function as a regulator rather than an active transporter. Biochem. Biophys. Res. Commun. 288, 1258-1264. [DOI] [PubMed] [Google Scholar]
- Takahashi, Y., and Smith, J.D. (1999). Cholesterol efflux to apolipoprotein AI involves endocytosis and resecretion in a calcium-dependent pathway. Proc. Natl. Acad. Sci. USA 96, 11358-11363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng, F.Y., Wang, Y., and Tang, B.L. (2001). The syntaxins. Genome Biol. 2, REVIEWS3012. [DOI] [PMC free article] [PubMed]
- Tsukamoto, K. et al. (2002). Retarded intracellular lipid transport associated with reduced expression of Cdc42, a member of Rho-GTPases, in human aged skin fibroblasts: a possible function of Cdc42 in mediating intracellular lipid transport. Arterioscler. Thromb. Vasc. Biol. 22, 1899-1904. [DOI] [PubMed] [Google Scholar]
- Tsukamoto, K. et al. (2001). ATP-binding cassette transporter-1induces rearrangement of actin cytoskeletons possibly through Cdc42/N-WASP. Biochem. Biophys. Res. Commun. 287, 757-765. [DOI] [PubMed] [Google Scholar]
- Utech, M., Hobbel, G., Rust, S., Reinecke, H., Assmann, G., Walter, M. (2001). Accumulation of RhoA, RhoB, RhoG, and Rac1 in fibroblasts from Tangier disease subjects suggests a regulatory role of Rho family proteins in cholesterol efflux. Biochem. Biophys. Res. Commun. 280, 229-236. [DOI] [PubMed] [Google Scholar]
- von Eckardstein, A. et al. (2001). ATP binding cassette transporter ABCA1 modulates the secretion of apolipoprotein E from human monocyte-derived macrophages. FASEB J. 15, 1555-1561. [DOI] [PubMed] [Google Scholar]
- Yoo, J.S., Moyer, B.D., Bannykh, S., Yoo, H.M., Riordan, J.R., and Balch, W.E. (2002). Non-conventional trafficking of the cystic fibrosis transmembrane conductance regulator through the early secretory pathway. J. Biol. Chem. 277, 11401-11409. [DOI] [PubMed] [Google Scholar]
- Zha, X., Gauthier, A., Genest, J., and McPherson, R. (2003). Secretory vesicular transport from the Golgi is altered during ATP-binding cassette protein A1 (ABCA1)-mediated cholesterol efflux. J. Biol. Chem. 278, 10002-10005. [DOI] [PubMed] [Google Scholar]
- Zha, X., Genest, J., Jr., and McPherson, R. (2001). Endocytosis is enhanced in Tangier fibroblasts: possible role of ATP-binding cassette protein A1 in endosomal vesicular transport. J. Biol. Chem. 276, 39476-39483. [DOI] [PubMed] [Google Scholar]