Abstract
Insulin and hypertonicity each increase the content of GLUT4 glucose transporters at the surface of muscle cells. Insulin enhances GLUT4 exocytosis without diminishing its endocytosis. The insulin but not the hypertonicity response is reduced by tetanus neurotoxin, which cleaves vesicle-associated membrane protein (VAMP)2 and VAMP3, and is rescued upon introducing tetanus neurotoxin-resistant VAMP2. Here, we show that hypertonicity enhances GLUT4 recycling, compounding its previously shown ability to reduce GLUT4 endocytosis. To examine whether the canonical soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) mechanism is required for the plasma membrane fusion of the tetanus neurotoxin-insensitive GLUT4 vesicles, L6 myoblasts stably expressing myc-tagged GLUT4 (GLUT4myc) were transiently transfected with dominant negative N-ethylmaleimide-sensitive factor (NSF) (DN-NSF) or small-interfering RNA to tetanus neurotoxin-insensitive VAMP (TI-VAMP siRNA). Both strategies markedly reduced the basal level of surface GLUT4myc and the surface gain of GLUT4myc in response to hypertonicity. The insulin effect was abolished by DN-NSF, but only partly reduced by TI-VAMP siRNA. We propose that insulin and hypertonicity recruit GLUT4myc from partly overlapping, but distinct sources defined by VAMP2 and TI-VAMP, respectively.
INTRODUCTION
The GLUT4 glucose transporter is a recycling membrane protein responsible for insulin-dependent glucose uptake into skeletal muscle (Klip et al., 1987; Hirshman et al., 1988; Douen et al., 1990; Goodyear et al., 1990; Rudich et al., 2003). In the basal state, the GLUT4 protein recycles to and from the muscle cell plasma membrane, and the steady-state favors its intracellular localization (Li et al., 2001). Insulin and hypertonicity change this distribution, so that each stimulus doubles the cell surface GLUT4 within 30 min (Li et al., 2001). However, insulin mainly increases the exocytic rate of GLUT4, whereas hypertonic sucrose significantly reduces GLUT4 internalization (Li et al., 2001). The gain in surface GLUT4 in the latter condition would likely arise from the continued fusion of constitutively recycling vesicles. It is not known if in addition to inhibition of GLUT4 endocytosis, hypertonicity also enhances the recycling rate of GLUT4.
Recently, we characterized the traffic of myc-tagged GLUT4 (GLUT4myc) expressed in L6 muscle cells (Ueyama et al., 1999; Randhawa et al., 2000; Foster et al., 2001; Li et al., 2001). The insulin-dependent gain in GLUT4myc was largely (∼70%) diminished by the transient expression of tetanus neurotoxin light chain (TeNT), an enzyme that cleaves vesicle-associated membrane protein (VAMP)2 and its isoform VAMP3 (Randhawa et al., 2000). Coexpression of a TeNT-resistant VAMP2 mutant, but not of a TeNT-resistant VAMP3 mutant, rescued the decrease caused by TeNT in the insulin-induced gain of GLUT4myc at the cell surface (Randhawa et al., 2000). These results suggested that VAMP2, but not VAMP3, mediates the majority of the insulin-dependent fusion of GLUT4myc vesicles with the plasma membrane. Interestingly, the gain in GLUT4myc at the cell surface caused by hypertonic sucrose was insensitive to TeNT (Li et al., 2001). Similarly, the steady-state levels of surface GLUT4myc maintained by basal-state (constitutive) recycling and endocytosis were not affected by the neurotoxin. These results raise the possibility that two types of traffic mechanisms furnish the plasma membrane with GLUT4myc: one that is functionally dependent mostly on VAMP2 for fusion, and another one that does not use VAMP2 or VAMP3 for membrane fusion. The insulin-sensitive VAMP2-dependent GLUT4myc pool is likely the specialized GLUT4 vesicles proposed in several models (Kandror and Pilch, 1996; Hashiramoto and James, 1998; Khayat et al., 2000; Bryant et al., 2002). This scenario begs the question of whether the fusion of vesicles recruited by hypertonicity uses a soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) mechanism at all, and if so, whether it involves TeNT-insensitive v-SNAREs. Similarly, the question arises as to the v-SNARE that mediates the 30% gain in surface GLUT4 in response to insulin, which is insensitive to TeNT. A candidate v-SNARE is TeNT-insensitive VAMP (TI-VAMP), described to mediate fusion of vesicles in neurite outgrowth of differentiated PC12 cells (Martinez-Arca et al., 2000) and at the apical plasma membrane of epithelial cells (Galli et al., 1998).
SNARE-dependence is a hallmark of vesicular fusion (Rothman, 1994; Rothman and Warren, 1994). SNARE proteins from two opposing membranes contribute coiled-coil regions to a fusion-competent structure composed of four parallel α-helices (to form the trans-SNARE complex) (Fasshauer et al., 1998; Sutton et al., 1998; Weber et al., 1998; McNew et al., 2000). Cis-SNARE complexes (i.e., on the same membrane) also exist and must be disassembled to allow for trans-SNARE complex formation (Littleton et al., 2001). SNARE complex disassembly is driven by the ATPase activity of N-ethylmaleimide-sensitive factor (NSF), a protein tethered to the cis-SNARE complex via the adaptor protein synaptosome-associated protein (SNAP) (Barnard et al., 1997). In contrast to SNAREs, which are abundant and organelle specific, only one mammalian NSF protein exists (Boulianne and Trimble, 1995).
Here, we show that dominant negative NSF precludes the gain in surface GLUT4myc in response to either insulin or hypertonicity. Furthermore, in search for a v-SNARE that might mediate fusion of the GLUT4myc vesicles furnishing surface GLUT4myc in response to hyperosmotic challenge, we tested the effect of gene silencing by using small-interfering RNA to TI-VAMP. This strategy markedly reduced the steady-state level of surface GLUT4myc and the gain in surface GLUT4myc elicited by hypertonicity, while affecting less the net insulin response. We propose that different v-SNAREs may mediate the fusion of GLUT4myc vesicles responding to insulin or to hypertonicity stimulus.
MATERIALS AND METHODS
Reagents and Constructs
Bicinchoninic acid (BCA) reagent was purchased from Pierce Chemical (Rockford, IL). Cell-TAK tissue adhesive and monoclonal anti-NSF antisera were purchased from Calbiochem (San Diego, CA). Dako fluorescent mounting medium was obtained from DakoCytomation California (Carpinteria, CA). Enhanced chemiluminescence reagent was purchased from PerkinElmer Life and Analytical Sciences (Boston, MA). o-Phenylenediamine dihydrochloride was purchased from Sigma-Aldrich (St. Louis, MO). Alexa 488 (A488) or indocarbocyanine (Cy3)-conjugated goat anti-mouse and anti-rabbit IgGs and horseradish peroxidase (HRP)-coupled donkey anti-mouse secondary antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). Monoclonal anti-GLUT4 (1F8) antibody was purchased from R & D Systems (Minneapolis, MN). Monoclonal and polyclonal anti-myc antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and the latter was also a kind gift from Dr. Mike Moran (University of Toronto, Toronto, Ontario, Canada). Monoclonal anti-VAMP2 and polyclonal TI-VAMP antibodies were described previously (Alberts et al., 2003). Polyclonal anti-phospho-Akt serine473 IHC (immunohistochemical compatible) antibody and immunogenic phospho-peptide was purchased from Cell Signaling Technology (Beverly, MA).
Mammalian expression vector for enhanced green fluorescent protein (eGFP), peGFP, was purchased from BD Biosciences Clontech (Palo Alto, CA). The pcDNA3 plasmid was purchased from Invitrogen (Carlsbad, CA). Wild-type (WT) or dominant negative (DN) NSF (with point mutation E329Q) cDNAs were kind gifts from Dr. William Trimble (Hospital for Sick Children, Toronto, Ontario, Canada) as described (Coppolino et al., 2001). All DNA constructs were prepared using Maxi-prep columns according to the manufacturer's protocol (QIAGEN, Valencia, CA). Small interfering double-stranded RNA (siRNA) molecules were generated against a rat TI-VAMP sequence, base pairs 486–506, as described previously (Alberts et al., 2003): AACCTCGTAGATTCGTCCGTC or to an unrelated sequence, NNATTCTATCACTAGCGTGAC (Dharmacon Research, Lafeyette, CO).
Cell Culture, cDNA Transfection, and siRNA Treatment
The myc epitope-tagged GLUT4 protein cDNA as well as stable L6-GLUT4myc and L6-GLUT1myc cell lines were constructed as described previously (Kanai et al., 1993; Kishi et al., 1998; Huang et al., 2002). L6-GLUT4myc and L6-GLUT1myc myoblasts were maintained in α-minimal essential medium (MEM) supplemented with 10% (vol/vol) fetal bovine serum (and also without antibiotics for cells undergoing siRNA treatment) in a humidified atmosphere containing 5% CO2 and 95% air at 37°C. For all experimental conditions, cells were serum-deprived for 3 h in serum-free α-MEM before use.
For cDNA transfection, cells were transfected for 16 h as described previously (Li et al., 2001) with the following additional exceptions. Coverslips were pretreated with 3.15 μg of Cell-TAK tissue adhesive in 200 μl of 0.1 M NaH2CO3 buffer, pH 8.0, for 30 min, followed by washing twice with double distilled H2O to remove excess salts, before plating cells on coverslips. Indirect immunofluorescence for GLUT4myc translocation was carried out on stimulated or unstimulated intact cells as described below and previously (Randhawa et al., 2000; Li et al., 2001).
For siRNA treatment, cells cultured in 24-well plates (or 12-well plates) were treated twice on two consecutive days with 1 μl (or 2 μl) of Oligofectamine reagent only or with 2 μl (or 4 μl) of 20 μM TI-VAMP or unrelated siRNA according to the manufacturer's protocol. The only exception was the use of antibiotic-free α-MEM media. Cells were lysed for immunoblot analysis or used 48 h later for GLUT4myc translocation or externalization (recycling) by o-phenylenediamine dihydrochloride detection described below.
Cell Lysates and Immunoblotting
Cells were rinsed with phosphate buffered saline containing an inhibitor cocktail (with 1 mM leupeptin, 1 mM pepstatin, 4 mM E-64, 1 mM sodium vanadate, and 10 nM okadaic acid). Cells were lysed in 100 μl of β-mercaptoethanol-free Laemmli sample buffer also containing the inhibitor cocktail described above, heated for 30 min at 65°C, and prepared for protein determination by the BCA reagent method. Lysates were syringed using a 27.5-gauge syringe five times, and 7.5% β-mercaptoethanol and 0.1% bromophenol were added to the final preparation. Equal protein samples were run on 15% SDS-PAGE gels, transferred to polyvinylidene fluoride membranes and immunoblotted for TI-VAMP, VAMP2, and GLUT4 protein expression levels. Antibody labeling was detected by the enhanced chemiluminescence reagent method. TI-VAMP was detected with the polyclonal anti-TI-VAMP antibody (1:1000) followed by a goat anti-rabbit HRP-conjugated secondary antibody (1:10,000). VAMP2 and GLUT4 were detected with the monoclonal anti-VAMP2 antibody (1:10,000) and anti-GLUT4 (1:1000) antibody, respectively, followed by a goat anti-mouse HRP-conjugated secondary antibody (1:10,000).
For determination of phospho-Akt staining, cells were serum starved for 3 h, following which they were left unstimulated or stimulated with 100 nM insulin for 10 min or 0.45 M hypertonic sucrose for 30 min. Cells were then lysed as described above, and phosphorylated Akt was detected using a polyclonal anti-phospho-Ser473 Akt antibody (1:1000) followed by a goat anti-rabbit HRP-conjugated secondary antibody (1:10,000).
GLUT4myc Externalization (Recycling) and Steady-State Levels of Surface GLUT4myc
The rate of GLUT4myc externalization (i.e., recycling) was determined in live cells essentially as described by Foster et al. (2001). Here, cells incubated in the absence or presence of 0.45 M hypertonic sucrose in α-MEM were exposed to anti-myc antibody for the times indicated. At each time point, cells were then fixed, permeabilized, and reacted with HRP-conjugated IgG to determine the amount of anti-myc antibody bound at the cell surface as well as that which had become labeled at the surface and subsequently internalized during the incubation time.
Steady-state levels of cell surface GLUT4myc were detected as described previously (Li et al., 2001). Briefly, cells were exposed to the various treatments as indicated, following which they were cooled on ice, fixed, and surface GLUT4myc was detected with anti-myc antibody without cellular permeabilization, followed by reaction with HRP-conjugated IgG or where indicated with fluorophore-conjugated IgG as described previously (Randhawa et al., 2000; Li et al., 2001). In all instances, the amount of bound HRP-conjugated IgG was detected from the oxidation of o-phenylenediamine dihydrochloride reagent, as described previously (Wang et al., 1998). Background levels of optical absorbance were determined in parallel wells omitting the primary antibody and were subtracted from all samples.
Immunofluorescence Quantification and Statistical Analyses
Indirect immunofluorescence was carried out in permeabilized cells as outlined to detect subcellular GLUT4myc (1:100) or NSF (1:150) expression (Randhawa et al., 2000). Images were acquired using a Leica epifluorescence microscope (Leica Mikroscopie Systeme, Wetzlar, Germany) at identical exposure and gain settings and processed using ImageJ software for quantification (National Institutes of Health, Bethesda, MD). Indirect immunofluorescence for pSer473 Akt (1:250) was carried out as described above, with 1 mM sodium vanadate and 10 nM okadaic acid during fixing and subsequent washes. The specificity of the antibody was confirmed by the absence of signal when the antibody was preincubated for 1 h at room temperature in a 1:10 ratio with the immunogenic phospho-peptide. Images were obtained using an Axiovert 100M laser scanning confocal 510 microscope (Carl Zeiss, Thornwood, NY) as indicated. In all instances, images were taken from at least 10 fields of view from at least three separate experiments. Quantification of fluorescence pixel intensity was carried out as described previously (Randhawa et al., 2000).
All statistical analyses were carried out with the analysis of variance test (Fisher, multiple comparisons) as indicated.
RESULTS
Hypertonicity Increases GLUT4myc Externalization in L6-GLUT4myc Myoblasts
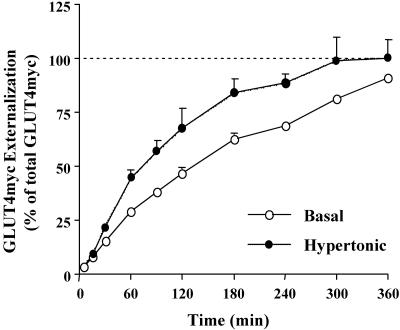
We have previously shown that insulin increases the recycling of GLUT4myc to the cell surface in L6-GLUT4myc myoblasts (Foster et al., 2001). Hypertonic sucrose also elevates the steady-state levels of GLUT4myc at the plasma membrane, in part, by retention of GLUT4myc at the cell surface (Li et al., 2001). To determine whether hypertonicity also enhances the externalization rate of GLUT4myc in L6 myoblasts, we measured its rate of appearance at the cell surface (i.e., recycling) in living cells. For this purpose, anti-myc antibody present within the culture medium during incubation at 37°C was used to label GLUT4myc molecules arriving at the cell surface in the presence or absence of hypertonic sucrose. At various times post-incubation with or without hypertonic sucrose, the amount of antibody-bound GLUT4myc was quantified (see Materials and Methods). The total cellular GLUT4myc protein was measured in parallel in myoblasts permeabilized before addition of anti-myc antibody. The amount of GLUT4myc labeled at the cell surface at each time point was expressed as a percentage of the total GLUT4myc. In the basal state, approximately one-half of the total GLUT4myc was labeled by anti-myc antibody in 120 min (Figure 1). In the presence of hypertonic sucrose, the half-time for GLUT4myc labeling was reduced to 70 min (Figure 1). By comparison, in the presence of insulin, one-half of the total cellular GLUT4myc molecules cycled to the plasma membrane by 40 min (Foster et al., 2001). The time required to label the entire cellular GLUT4myc content was reduced to 5 h by hypertonic sucrose, from >6 h in the basal state (Figure 1). By comparison, all of the GLUT4myc was labeled by 3 h in the presence of insulin. These results are summarized in Table 1. Therefore, in addition to abating GLUT4myc endocytosis (Li et al., 2001), hypertonicity also enhanced the rate of GLUT4myc exocytosis (recycling) to the plasma membrane relative to the basal state.
Figure 1.
Hypertonicity increases the rate of GLUT4myc externalization. L6-GLUT4myc myoblasts were incubated with anti-myc (9E10) antibody at 37°C in the presence (•) or absence (○) of 0.45 M hypertonic sucrose. At the indicated times, cells were fixed and permeabilized to determine the amount of GLUT4myc protein bound by the 9E10 antibody at the cell surface by using an enzyme-linked o-phenylenediamine dihydrochloride absorbance assay. Total cellular GLUT4myc was measured by labeling permeabilized myoblasts with 9E10 antibody. The amount of GLUT4myc labeled at the cell surface for each time point was expressed as a percent of total GLUT4myc content. Background o-phenylenediamine dihydrochloride absorbance (measured by secondary antibody labeling only) was subtracted from all values. Data are shown as mean ± SE of at least three separate experiments.
Table 1.
Kinetics of externalization of GLUT4myc
| Condition | Externalization | Externalization |
|---|---|---|
| t1/2 | Total time | |
| Basal | 2 h | 6 h |
| Insulin | 0.7 h | 3 h |
| Hypertonic sucrose | 1.2 h | 5 h |
L6-GLUT4myc myoblasts were incubated with anti-myc (9E10) antibody at 37°C in the presence or absence of 100 nM insulin or 0.45 M hypertonic sucrose. The amount of GLUT4myc labeled at the cell surface over time was expressed as a percentage of total GLUT4myc content (see Materials and Methods). The externalization t1/2 denotes the time (hours) taken for one-half of the total GLUT4myc protein to be labeled at the plasma membrane by 9E10 antibody. The total externalization time denotes the time (hours) taken for all of the cellular GLUT4myc protein to be labeled by 9E10 antibody.
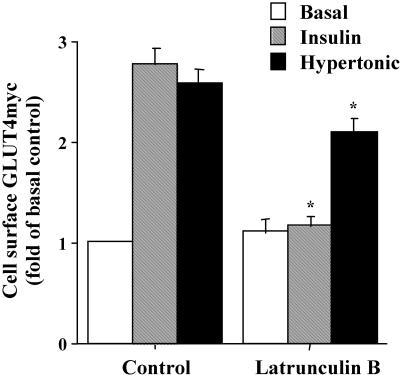
Because GLUT4 externalization by insulin involves actin remodeling in muscle cells (Khayat et al., 2000; Tong et al., 2001), we determined whether the gain in surface GLUT4myc induced by hypertonicity was similarly inhibited by disrupting the actin cytoskeleton (Figure 2). As before, in the absence of latrunculin B, the steady-state level of surface GLUT4myc increased twofold in response to 30 min of exposure to insulin or hypertonic sucrose relative to the basal state (Figure 2). In the presence of latrunculin B the insulin-mediated response was completely inhibited, whereas the hypertonic sucrose-stimulated response was only reduced by 20%. In contrast, the surface levels of GLUT4myc in the basal state were not altered by pretreatment with latrunculin B (Figure 2). Therefore, as with insulin, an intact actin cytoskeleton also may facilitate the small exocytic component of the hypertonic sucrose-mediated gain in surface GLUT4myc.
Figure 2.
Latrunculin B abrogates the insulin response but only slightly reduces the hypertonic response of GLUT4myc translocation to the plasma membrane. L6-GLUT4myc myoblasts were serum starved for 3 h and pretreated with 1 μM latrunculin B for 1 h, followed by the addition of 100 nM insulin or 0.45 M hypertonic sucrose for 30 min only as indicated. Intact cells were labeled with anti-myc IgG. Cell surface GLUT4myc was determined by measuring bound HRP-conjugated secondary antibody by using the enzyme-linked o-phenylenediamine dihydrochloride detection assay, by following the optical absorbance at 492 nm. Background o-phenylenediamine dihydrochloride absorbance (measured by secondary antibody labeling only) was subtracted from all values. Data are shown as fold increase over basal ± SE for each experimental condition; p < 0.01 relative to basal (*) control.
N-Ethylmaleimide-sensitive Factor Is Required for GLUT4myc Arrival at the Cell Surface
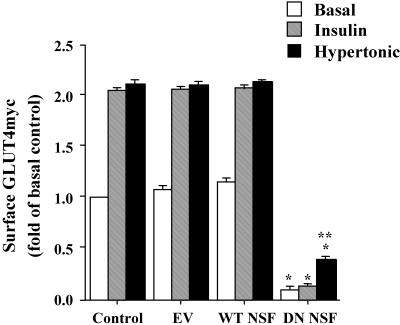
Because the hypertonicity-induced gain in surface GLUT4myc was unaffected by TeNT-induced cleavage of VAMP2/VAMP3 (Randhawa et al., 2000; Li et al., 2001), we asked whether GLUT4myc arrival at the plasma membrane in response to this stimulus depends on vesicle fusion mechanisms mediated by SNAREs at all. To this end, we used an E329Q mutant NSF protein that functions in a dominant negative manner to some, but not all known functions of NSF (Muller et al., 1999) because it can no longer hydrolyze ATP (Coppolino et al., 2001). cDNA encoding EV, WT-NSF, or DN-NSF was transiently cotransfected into L6-GLUT4myc myoblasts. Overexpression of WT-NSF or DN-NSF proteins appreciably increased the total immunodetectable levels of NSF protein within the cells by ∼1.5-fold relative to untransfected myoblasts. DN-NSF expression markedly reduced the plasma membrane GLUT4myc in the basal state, obliterated the insulin response, and markedly diminished the hypertonicity response (Figure 3). Thus, all GLUT4myc vesicle traffic events seem to require an NSF-dependent mechanism. These results further suggest that the entire insulin-dependent gain in surface GLUT4myc uses a SNARE mechanism for fusion, of which ∼70% depends on the v-SNARE VAMP2.
Figure 3.
Dominant negative NSF inhibits the gain in surface GLUT4myc by insulin or hypertonicity. L6-GLUT4myc myoblasts were left untransfected (Control) or cotransfected with 0.45 μg of eGFP and either pcDNA3 (EV), WT NSF or dominant negative (DN) NSF cDNAs. After 16 h, cells were serum starved for 3 h and left untreated or stimulated with 100 nM insulin or 0.45 M hypertonic sucrose for 30 min as shown. Intact cells were processed for indirect surface immunofluorescence with anti-myc IgG to detect surface GLUT4myc staining. The fluorescence intensity of surface GLUT4myc staining in transfected and untransfected cells from at least 10 fields of view from three separate experiments was quantitated using ImageJ software. The surface fluorescence of untransfected cells in the basal state (basal control) was arbitrarily assigned a value of 1. Data are shown as fold above basal control ± SE for each experimental condition; p < 0.01 relative to basal control (*) or relative to basal DN-NSF–expressing cells (**).
Interference with NSF function could alter multiple vesicle traffic events in the cell that may non-specifically affect GLUT4 traffic by disrupting intracellular GLUT4 donor pools and/or by interfering with required signaling processes. Therefore, it was important to confirm that within the time-frame of the experiments with DN-NSF, the intracellular GLUT4myc compartment(s), and major signaling events were not disrupted. Neither insulin signaling to Akt nor insulin-induced actin remodeling was affected by transient expression of EV, WT-NSF, or DN-NSF (our unpublished observations). We next examined the effect of DN-NSF expression on the intracellular GLUT4myc distribution upon permeabilization of L6-GLUT4myc myoblasts. In the absence of any transfection, the GLUT4myc signal is concentrated largely within the perinuclear region, but it is also found within punctate elements dispersed throughout the cytosol in the basal steady state as reported recently (Sweeney et al., 2004). The transient expression of DN-NSF did not seem to affect the perinuclear or the punctate cytosolic staining of the intracellular GLUT4myc in comparison with untransfected cells (our unpublished observations). This is reminiscent of observations that the ATPase activity of NSF is not functionally required for some of its functions, such as Golgi membrane fusion (Muller et al., 1999). As well, the overall fluorescence intensity of immunodetected GLUT4myc was quantitatively unaltered by DN-NSF overexpression (1.04 ± 0.05, relative to untransfected controls assigned a value of 1.0). Because the proportion of GLUT4 present at the surface of control cells represents only 10% of the total GLUT4 (Li et al., 2001), the reduction in surface GLUT4myc that was observed in DN-NSF–expressing cells (Figure 3) would not be expected to contribute noticeably to the net intracellular GLUT4myc content. Hence, DN-NSF overexpression did not appreciably alter the intracellular localization of the GLUT4myc pool(s) in the time-frame studied.
Toxin-insensitive VAMP Is Required for GLUT4myc Arrival at the Cell Surface
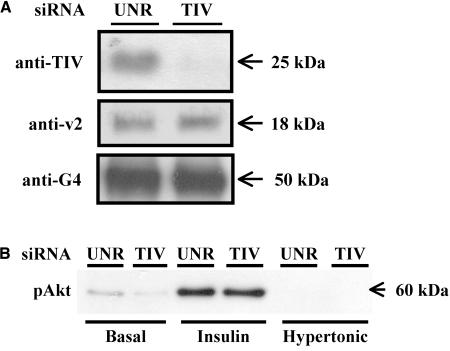
The above-mentioned results combined with our studies with TeNT forecast the requirement of a v-SNARE that is TeNT-insensitive in the traffic of GLUT4 in response to hypertonicity. We have recently shown that a TeNT-insensitive VAMP, TI-VAMP, is expressed in L6 cells and mainly resides in the perinuclear region (Li et al., 2001). To determine whether TI-VAMP might mediate the fusion of the TeNT-insensitive GLUT4 vesicles, we made use of small interfering RNA molecules directed against rat TI-VAMP (TI-VAMP siRNA) to silence the expression of this protein. This approach was recently used successfully to inhibit the cell surface presence of the L1 adhesion molecule, its intracellular transport and L1-dependent adhesion (Alberts et al., 2003). The level of TI-VAMP protein was markedly decreased by 72 h after transfection with TI-VAMP siRNA compared with transfection with unrelated siRNA by immunoblot analysis by using anti-TI-VAMP antibody (Figure 4A). In contrast, neither GLUT4 nor VAMP2 protein levels were altered by TI-VAMP siRNA-treatment in comparison with cells treated with unrelated siRNA, determined by immunoblot analysis by using anti-GLUT4 and anti-VAMP2 antibodies, respectively (Figure 4A). Thus, TI-VAMP siRNA treatment specifically reduced the expression of TI-VAMP protein and not that of GLUT4 or other vesicle-associated proteins such as VAMP2.
Figure 4.
TI-VAMP siRNA treatment reduces TI-VAMP but not GLUT4 or VAMP2 expression and does not alter the insulin-induced phosphorylation of Akt. L6-GLUT4myc myoblasts were treated with TI-VAMP siRNA (TIV) or unrelated siRNA (UNR) as described in Materials and Methods. (A) After 48 h, cells were lysed and immunoblotted for TI-VAMP, VAMP2, and GLUT4 protein expression by using anti-TI-VAMP, anti-VAMP2 or anti-GLUT4 IgGs, respectively, as indicated in Materials and Methods. Shown are representative immunoblots for TI-VAMP, VAMP2, and GLUT4 detection. (B) Alternatively, cells were serum starved for 3 h and left untreated or stimulated with 100 nM insulin or 0.45 M hypertonic sucrose for 10 or 30 min, respectively, as shown. Cells were lysed and immunoblotted for phosphorylated Akt by using anti-phospho-Akt IgG as described in Materials and Methods. Shown is a representative immunoblot for phosphorylated Akt detection.
To exclude the possibility that the TI-VAMP siRNA inhibited GLUT4 externalization by interfering with signaling events, we examined the effect of TI-VAMP siRNA expression on the phosphorylation of Akt. The insulin-induced phosphorylation of Akt, detected by immunoblot analysis by using a polyclonal anti-phospho-Akt antibody, was unaltered in cells expressing TI-VAMP siRNA relative to cells expressing unrelated siRNA (Figure 4B). Similarly, TI-VAMP siRNA did not affect the phosphorylation of p38 MAPK in response to hypertonic sucrose (our unpublished observations). Therefore, signaling events resulting in Akt or p38 MAPK phosphorylation in response to insulin or hypertonic sucrose treatment, respectively, were unaffected by expression of TI-VAMP siRNA.
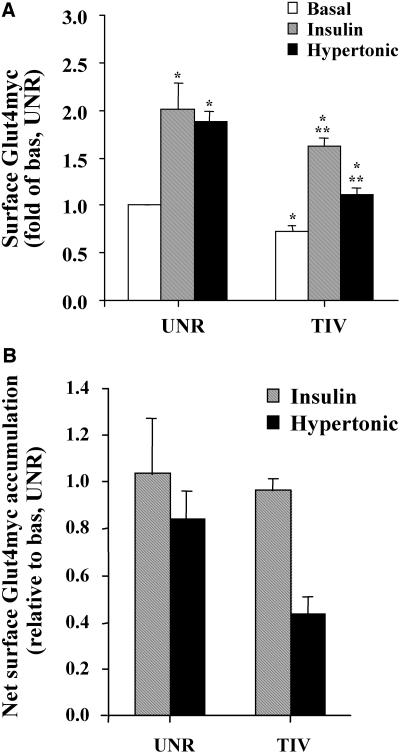
We next investigated the effect of TI-VAMP siRNA on cell surface GLUT4myc in the basal, insulin- and hypertonic sucrose-stimulated states. The level of cell surface GLUT4myc in unstimulated cells transfected with unrelated siRNA was assigned a value of 1.0 (Figure 5A). Insulin and hypertonicity increased surface GLUT4myc levels each by twofold in cells transfected with unrelated siRNA relative to the basal state (Figure 5A). Treatment of cells with TI-VAMP siRNA diminished surface GLUT4myc in the basal state, as well as the gains caused by insulin or hypertonic sucrose compared with the respective level of surface GLUT4myc in cells transfected with unrelated siRNA (Figure 5A). However, the insulin response was more mildly affected than the hypertonic response. These differences are further noted by comparing the net accumulation in surface GLUT4myc in response to both stimuli in cells expressing TI-VAMP or unrelated siRNA (Figure 5B). The net insulin-dependent accumulation of surface GLUT4myc was barely affected whereas the hypertonicity-dependent response was reduced by 50%. Thus, we propose that TI-VAMP may mediate to a larger extent the fusion of toxin-insensitive GLUT4 vesicles in the basal and hypertonicity-stimulated states and contributes to a lesser extent to the fusion of insulin-sensitive GLUT4 vesicles. To further examine this differential behavior, we determined the effect of siRNA to TI-VAMP on GLUT4myc recycling. TI-VAMP siRNA increased the t1/2 of GLUT4myc by only 15% in insulin-stimulated cells, but it more markedly retarded recycling in unstimulated cells and hypertonic-treated cells (t1/2 increased by 40 and 50%, respectively). These results further suggest that GLUT4myc vesicles that recycle to the plasma membrane in the basal state and under hypertonic stimulation rely more markedly on TI-VAMP than do GLUT4myc vesicles that recycle in response to insulin.
Figure 5.
TI-VAMP siRNA treatment reduces the gain in surface GLUT4myc. L6-GLUT4myc myoblasts were treated with TI-VAMP siRNA (TIV) or unrelated siRNA (UNR) as described in Materials and Methods. After 48 h, cells were serum-starved for 3 h and left untreated or stimulated with 100 nM insulin or 0.45 M hypertonic sucrose for 30 min as shown. Intact cells were labeled with anti-myc IgG. Cell surface GLUT4myc was determined by measuring bound HRP-conjugated secondary antibody using the enzyme-linked o-phenylenediamine dihydrochloride detection assay, by following the optical absorbance at 492 nm. The surface detection of GLUT4myc in unrelated siRNA treated cells in the basal state (basal unr) was arbitrarily assigned a value of 1. (A) Data are shown as fold increase over basal unr ± SE for each experimental condition (n = 6); p < 0.01 relative to basal unr (*) or to basal tiv (**). (B) Data also are expressed as net accumulation of surface GLUT4myc above basal UNR or basal TIV, respectively, for each experimental condition.
DISCUSSION
Stimulation with insulin or hypertonic sucrose for 30 min causes a 2-fold gain in GLUT4 at the cell surface relative to the basal state, but TeNT interferes only with the GLUT4 gain in response to insulin by 70% (Li et al., 2001). The objective of the present study was to explore whether GLUT4 mobilized by each stimulus require the canonical SNARE mechanism, and more specifically whether they functionally rely on distinct v-SNAREs.
Differential Recycling Kinetics of GLUT4myc and NSF Dependence in Response to Insulin and Hypertonicity
We have previously shown that insulin enhances surface GLUT4myc levels through an increase of GLUT4myc recycling to the cell surface (Table 1; Foster et al., 2001). Because insulin did not reduce the rate of internalization of GLUT4myc from the cell surface, the enhanced recycling is solely due to stimulation of the exocytic arm of GLUT4myc traffic. In contrast, hypertonic sucrose causes significant retention of GLUT4myc at the cell surface (Li et al., 2001) presumably as a result of interfering with clathrin-cage formation (Heuser and Anderson, 1989; Hansen et al., 1993). Here, we show that, in addition, hypertonic sucrose enhances the rate of externalization (recycling) of GLUT4myc (Figure 1 and Table 1). Thus, hypertonic sucrose also actively engages the exocytic arm of GLUT4myc traffic, compounding the effect of reduced internalization of the transporter. Indeed, the steady-state levels of surface GLUT4myc attained in the presence of hypertonic sucrose were reduced by ∼20% in the presence of tyrosine kinase inhibitors (Li et al., 2001) or cytoskeleton disruptors (Figure 2), which do not enhance GLUT4myc endocytosis (our unpublished observations). Collectively, these findings suggest that the exocytic arm of GLUT4myc traffic in response to hypertonicity is regulated and seems to require input from tyrosine kinases. Insulin stimulation followed by hypertonic sucrose causes a higher gain in surface GLUT4myc levels compared with those achieved by either stimulus alone (Li et al., 2001). Hence, the question arises whether GLUT4 vesicles cycling to the plasma membrane in response to insulin or hypertonic sucrose functionally rely on distinct fusion mechanisms and thus, may be drawn from distinct intracellular compartment(s).
We recently showed that TeNT eliminated ∼70% of the insulin-stimulated, but not the hypertonicity-mediated GLUT4myc translocation, by cleaving toxin-sensitive v-SNARE proteins (Randhawa et al., 2000; Li et al., 2001). Thus, ∼30% of the insulin-responding GLUT4 vesicles and all of the hypertonicity-responding vesicles seem to be mobilized independently of VAMP2 (Randhawa et al., 2000; Li et al., 2001). Because distinct SNARE proteins populate the various subcellular compartments (McNew et al., 2000), we hypothesized that if the TeNT-insensitive GLUT4 vesicles depend on the canonical SNARE mechanism for fusion with the plasma membrane, they would use “tetanus neurotoxin-insensitive” v-SNAREs as fusogens. However, it also was plausible that a novel “SNARE-less” mechanism could mediate the fusion of these neurotoxin-resistant GLUT4 vesicles (Scales et al., 2001; Whalley et al., 2004). For instance, hypertonicity could drive the fusion of GLUT4 vesicles by physically distorting the membrane (Rosenmund and Stevens, 1996), thereby increasing the cell surface GLUT4myc content. NSF is a key regulator of SNARE-dependent vesicle fusion (Rothman, 1994; Yu et al., 1999) and has been found along with the SNAP adaptor molecules to associate with intracellularly derived GLUT4 vesicles (Mastick and Falick, 1997). Here, we show that transient transfection of L6-GLUT4myc myoblasts with DN-NSF cDNA completely abrogates the gain in surface GLUT4myc in response to insulin or hypertonicity (Figure 3). Moreover, the basal steady-state level of surface GLUT4myc also was markedly reduced (Figure 3). Therefore, all recycling GLUT4 vesicles (i.e., those recycling in the basal state or upon insulin or hyperosmolar stimulus) seem to require an NSF-dependent SNARE mechanism for fusion with the plasma membrane. It is very possible that intracellular fusion events also are halted by DN-NSF.
Segregation of SNARE Proteins in GLUT4myc Traffic
The insulin-responsive toxin-sensitive GLUT4 vesicles can be defined by their need for VAMP2 as a fusogen (Randhawa et al., 2000). However, this applies to only 70% of the total insulin-mediated GLUT4 traffic response. Which v-SNARE then mediates the SNARE-dependent fusion of the remaining insulin-responsive GLUT4 vesicles with the plasma membrane? And, which v-SNARE mediates the fusion of the hypertonicity-driven GLUT4myc vesicles? VAMP3 is a resident molecule of recycling endosomes (Galli et al., 1994; Hashiramoto and James, 1998). However, VAMP3 knockout mice show normal levels of glucose uptake in the basal state and in response to insulin or exercise (Yang et al., 2001). Yet, VAMP3 facilitated the fusion of GLUT4 vesicles in response to GTPγS and also was required for the insulin-responsive traffic of another glucose transporter, GLUT1, in 3T3-L1 adipocytes (Millar et al., 1999). GTPγS, like hypertonic sucrose, engages an “alternate,” phosphatidylinositol 3-kinase (PI 3-kinase)–independent signaling mechanism to elicit a GLUT4 translocation event (Millar et al., 1999; Saltiel and Pessin, 2002). However, we consider it unlikely that VAMP3 mediates the 30% VAMP2-independent GLUT4 exocytosis caused by insulin, or the exocytosis in response to hypertonic sucrose, because these responses were unaffected by TeNT (Li et al., 2001). Alternatively, it is more likely that a TeNT-resistant GLUT4 vesicle fusion mechanism is required for the VAMP2-independent portion of the insulin-induced gain in surface GLUT4, and for the hypertonicity-induced gain.
A potential candidate would be the neurotoxin-insensitive v-SNARE TI-VAMP, which is expressed in L6 myoblasts as shown previously by immunofluorescence (Li et al., 2001), and in this study by immunoblotting (Figure 4A). Interestingly, TI-VAMP forms productive fusion complexes with syntaxin4 and SNAP23 in other cell types (Coco et al., 1999; Prekeris et al., 1999). In addition, this v-SNARE has been recently implicated in mediating TeNT-resistant exocytic and endocytic vesicle traffic events that includes neurite outgrowth (Martinez-Arca et al., 2000) and plasma membrane recycling of a cell adhesion molecule L1 through the recycling endocytic pathway (Alberts et al., 2003). Last, TI-VAMP has been localized within the perinuclear region of several neuronal cell types (Alberts et al., 2003) as well as L6 myoblasts (Li et al., 2001), where the majority of GLUT4 also lodges.
To examine the role of TI-VAMP in toxin-resistant GLUT4 traffic, we used small interfering RNA molecules directed against rat TI-VAMP to silence TI-VAMP expression. TI-VAMP siRNA considerably reduced TI-VAMP levels in L6 myoblasts (Figure 4A). Correspondingly, TI-VAMP siRNA-treated cells showed a marked reduction in the surface content of GLUT4myc in the basal state and under stimulation by hypertonic sucrose, but only partially under insulin stimulation (Figure 5, A and B). These results are consistent with the idea that TI-VAMP is functionally required for ∼30% of the gain in surface GLUT4myc in response to insulin, the rest of which can be attributed to the TeNT-sensitive VAMP2. On the other hand, it is more difficult to estimate the magnitude of the functional reliance on TI-VAMP of the constitutive GLUT4myc recycling in the basal state and of the response to hypertonic sucrose. siRNA-mediated TI-VAMP gene silencing significantly, although incompletely, decreased both the response to hypertonic sucrose and surface GLUT4myc in the basal state. Moreover, the ablation of TI-VAMP expression had minimal effect on GLUT4myc recycling in insulin-stimulated cells but retarded recycling in the basal and hypertonicity states. These results suggest that TI-VAMP is more relevant for recycling in the hypertonic-stimulated and basal states, than in the insulin-stimulated state. The incomplete elimination of GLUT4myc recycling and the residual gain in surface GLUT4myc in response to hypertonicity may be due to any or all of the following possibilities: 1) Because the siRNA approach did not completely deplete the cells of TI-VAMP, the remaining TI-VAMP molecules suffice to mediate effective if delayed GLUT4 vesicle recycling under these conditions. 2) An additional TeNT-insensitive VAMP also is involved in these processes, such as VAMP8. 3) Under conditions of decreased TI-VAMP expression, alternate VAMPs can functionally compensate for this loss. In this context, the interplay between TI-VAMP and VAMP8 is not well known, but a recent study shows that TI-VAMP determines heterotypic fusion in contrast to VAMP8 which is required for homotypic fusion of late endosomes (Pryor et al., 2004). TI-VAMP is also known to participate in fusion events with the plasma membrane (Rao et al., 2004). We envisage that the siRNA-dependent reduction in TI-VAMP diminishes the ability of a subset of GLUT4myc vesicles to fuse with the plasma membrane, and/or the dynamics of intracellular compartments that ultimately furnish the surface-bound vesicles. This would affect GLUT4myc that recycles under the basal and hypertonic conditions.
Regardless of these scenarios, future studies should focus on the identity of cognate SNARE-binding partners for TI-VAMP in L6 muscle cells, and on their relative contribution to TI-VAMP–mediated fusion of GLUT4myc vesicles with the plasma membrane. As indicated previously, syntaxin4 and SNAP23 have been shown to form a complex with TI-VAMP (Martinez-Arca et al., 2003). Recently, this particular pairing of SNARE proteins has been shown to facilitate lysosomal secretion in normal rat kidney cells (Rao et al., 2004). It would be interesting to determine whether TI-VAMP could pair with syntaxin4 and SNAP23 in L6 muscle cells to mediate the fusion of the TeNT-insensitive GLUT4 vesicles with the plasma membrane.
In conclusion, we propose the possibility that distinct GLUT4 vesicles or GLUT4 vesicle fusion mechanisms are engaged by insulin and hypertonicity, both requiring NSF. These can be differentiated on the basis of their requirement of v-SNAREs. Our findings are summarized in Table 2, and support the notion that GLUT4 may be recruited from distinct but partly overlapping intracellular donor compartments in L6 muscle cells. Because all of the GLUT4 molecules recycle to the cell surface in the basal, insulin and hypertonic sucrose-stimulated states (Figure 1), it is likely that these donor compartments of GLUT4 are interrelated (Zeigerer et al., 2002; Karylowski et al., 2004). Future work will focus on the localization of these compartments within the muscle cell.
Table 2.
Characterization of the effects of insulin and hypertonicity
| Parameter | Insulin | Hypertonic sucrose | Reference |
|---|---|---|---|
| Gain in surface GLUT4 | Yes | Yes | Li et al. (2001) |
| Increased GLUT4 recycling | Yes | Yes | Foster et al. (2001), present study |
| Reduced GLUT4 internalization | No | Yes | Li et al. (2001) |
| PI 3-kinase activation | Yes | No | Li et al. (2001) |
| Inhibition by WM, LY294002 | Yes | No | Li et al. (2001) |
| p-Akt | Yes | No | Li et al. (2001) |
| p-Tyr | Yes | Yes | Li et al. (2001) |
| Actin remodeling | Yes | No | Khayat et al. (2000), Tong et al. (2001), our unpublished data |
| GLUT4 colocalization with actin mesh | Yes | nd | Khayat et al. (2000) |
| Inhibition by actin filament disrupting agents | Full (100%) | Partial (20%) | Present study |
| Tetanus toxin sensitivity | Yes | No | Randhawa et al. (2000) Li et al. (2001) |
| VAMP2 rescue | Yes | nd | Randhawa et al. (2000) |
| VAMP3 rescue | No | nd | Randhawa et al. (2000) |
| NSF-dependence | Yes | Yes | Present study |
| TI-VAMP requirement | Partial (30%) | Yes | Present study |
nd = not determined.
Acknowledgments
We thank Dr. Yousuke Ebina, Dr. Philip J. Bilan, Dr. Carol Huang, Dr. Zhi Liu, Dr. William S. Trimble, and Dr. Sergio Grinstein for reagents, technical assistance, and/or useful discussion. This work was supported by a grant to A.K. from the Canadian Institutes of Health Research (MT7307). V.K.R. was funded by a Canadian Institutes of Health Doctoral Award studentship. F.S.L.T. was funded by a Natural Science and Engineering Research Council of Canada fellowship. D.L. was funded by a Canadian Diabetes Association fellowship. A.R. was funded by the Albert Renold Career Development Award of the European Foundation for the Study of Diabetes.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04–03–0266. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–03–0266.
Abbreviations used: DN, dominant negative; eGFP, enhanced green fluorescent protein; GLUT, glucose transporter; GLUT1myc, GLUT1 protein with an exofacial myc epitope; GLUT4myc, GLUT4 protein with an exofacial myc epitope; L6-GLUT1myc, L6 myoblasts stably expressing GLUT1myc protein; L6-GLUT4myc, L6 myoblasts stably expressing GLUT4myc protein; PI 3-kinase, phosphatidylinositol 3-kinase; NSF, N-ethylmaleimide-sensitive factor; siRNA, small interfering RNA; SNAP, synaptosome-associated protein; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor; t-SNARE, target membrane SNARE; v-SNARE, vesicle SNARE; TeNT, tetanus neurotoxin light chain; TI-VAMP, TeNT-insensitive VAMP; VAMP, vesicle-associated membrane protein; VW, toxin-resistant/insensitive; WT, wild type.
References
- Alberts, P., et al. (2003). Cross talk between tetanus neurotoxin-insensitive vesicle-associated membrane protein-mediated transport and L1-mediated adhesion. Mol. Biol. Cell 14, 4207-4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard, R.J., Morgan, A., and Burgoyne, R.D. (1997). Stimulation of NSF ATPase activity by alpha-SNAP is required for SNARE complex disassembly and exocytosis. J. Cell Biol. 139, 875-8838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulianne, G.L., and Trimble, W.S. (1995). Identification of a second homolog of N-ethylmaleimide-sensitive fusion protein that is expressed in the nervous system and secretory tissues of Drosophila. Proc. Natl. Acad. Sci. USA 92, 7095-7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant, N.J., Govers, R., and James, D.E. (2002). Regulated transport of the glucose transporter GLUT4. Nat. Rev. Mol. Cell. Biol. 3, 267-277. [DOI] [PubMed] [Google Scholar]
- Coco, S., Raposo, G., Martinez, S., Fontaine, J.J., Takamori, S., Zahraoui, A., Jahn, R., Matteoli, M., Louvard, D., and Galli, T. (1999). Subcellular localization of tetanus neurotoxin-insensitive vesicle-associated membrane protein (VAMP)/VAMP7 in neuronal cells: evidence for a novel membrane compartment. J. Neurosci. 19, 9803-9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppolino, M.G., Kong, C., Mohtashami, M., Schreiber, A.D., Brumell, J.H., Finlay, B.B., Grinstein, S., and Trimble, W.S. (2001). Requirement for N-ethylmaleimide-sensitive factor activity at different stages of bacterial invasion and phagocytosis. J. Biol. Chem. 276, 4772-4780. [DOI] [PubMed] [Google Scholar]
- Douen, A.G., Ramlal, T., Rastogi, S., Bilan, P.J., Cartee, G.D., Vranic, M., Holloszy, J.O., and Klip, A. (1990). Exercise induces recruitment of the “insulin-responsive glucose transporter”. Evidence for distinct intracellular insulin- and exercise-recruitable transporter pools in skeletal muscle. J. Biol. Chem. 265, 13427-13430. [PubMed] [Google Scholar]
- Fasshauer, D., Sutton, R.B., Brunger, A.T., and Jahn, R. (1998). Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc. Natl. Acad. Sci. USA 95, 15781-15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, L.J., Li, D., Randhawa, V.K., and Klip, A. (2001). Insulin accelerates inter-endosomal GLUT4 traffic via phosphatidylinositol 3-kinase and protein kinase B. J. Biol. Chem. 276, 44212-44221. [DOI] [PubMed] [Google Scholar]
- Galli, T., Chilcote, T., Mundigl, O., Binz, T., Niemann, H., and De Camilli, P. (1994). Tetanus toxin-mediated cleavage of cellubrevin impairs exocytosis of transferrin receptor-containing vesicles in CHO cells. J. Cell Biol. 125, 1015-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli, T., Zahraoui, A., Vaidyanathan, V.V., Raposo, G., Tian, J.M., Karin, M., Niemann, H., and Louvard, D. (1998). A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells. Mol. Biol. Cell 9, 1437-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear, L.J., Hirshman, M.F., King, P.A., Horton, E.D., Thompson, C.M., and Horton, E.S. (1990). Skeletal muscle plasma membrane glucose transport and glucose transporters after exercise. J. Appl. Physiol. 68, 193-198. [DOI] [PubMed] [Google Scholar]
- Hansen, S.H., Sandvig, K., and van Deurs, B. (1993). Clathrin and HA2 adaptors: effects of potassium depletion, hypertonic medium and cytosol acidification. J. Cell Biol. 121, 61-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiramoto, M., and James, D.E. (1998). Snareing GLUT4 at the plasma membrane in muscle and fat. Adv. Exp. Med. Biol. 441, 47-61. [DOI] [PubMed] [Google Scholar]
- Heuser, J.E., and Anderson, R.G. (1989). Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 108, 877-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshman, M.F., Wallberg-Henriksson, H., Wardzala, L.J., Horton, E.D., and Horton, E.S. (1988). Acute exercise increases the number of plasma membrane glucose transporters in rat skeletal muscle. FEBS Lett. 238, 235-239. [DOI] [PubMed] [Google Scholar]
- Huang, C., Somwar, R., Patel, N., Niu, W., Torok, D., and Klip, A. (2002). Sustained exposure of L6 myotubes to high glucose and insulin decreases insulin-stimulated GLUT4 translocation but upregulates GLUT4 activity. Diabetes 51, 2090-2098. [DOI] [PubMed] [Google Scholar]
- Kanai, F., Nishioka, Y., Hayashi, H., Kamohara, S., Todaka, M., and Ebina, Y. (1993). Direct demonstration of insulin-induced GLUT4 translocation to the surface of intact cells by insertion of a c-myc epitope into an exofacial GLUT4 domain. J. Biol. Chem. 268, 14523-14526. [PubMed] [Google Scholar]
- Kandror, K.V., and Pilch, P.F. (1996). Compartmentalization of protein traffic in insulin-sensitive cells. Am. J. Physiol. 271, E1-E14. [DOI] [PubMed] [Google Scholar]
- Karylowski, O., Zeigerer, A., Cohen, A., and McGraw, T.E. (2004). GLUT4 is retained by an intracellular cycle of vesicle formation and fusion with endosomes. Mol. Biol. Cell 15, 870-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayat, Z.A., Tong, P., Yaworsky, K., Bloch, R.J., and Klip, A. (2000). Insulin-induced actin filament remodeling colocalizes actin with phosphatidylinositol 3-kinase and GLUT4 in L6 myotubes. J. Cell Sci. 113, 279-290. [DOI] [PubMed] [Google Scholar]
- Kishi, K., Muromoto, N., Nakaya, Y., Miyata, I., Hagi, A., Hayashi, H., and Ebina, Y. (1998). Bradykinin directly triggers GLUT4 translocation via an insulin-independent pathway. Diabetes 47, 550-558. [DOI] [PubMed] [Google Scholar]
- Klip, A., Ramlal, T., Young, D.A., and Holloszy, J.O. (1987). Insulin-induced translocation of glucose transporters in rat hindlimb muscles. FEBS Lett. 224, 224-230. [DOI] [PubMed] [Google Scholar]
- Li, D., Randhawa, V.K., Patel, N., Hayashi, M., and Klip, A. (2001). Hyperosmolarity reduces GLUT4 endocytosis and increases its exocytosis from a VAMP2-independent pool in l6 muscle cells. J. Biol. Chem. 276, 22883-22891. [DOI] [PubMed] [Google Scholar]
- Littleton, J.T., Barnard, R.J., Titus, S.A., Slind, J., Chapman, E.R., and Ganetzky, B. (2001). SNARE-complex disassembly by NSF follows synaptic-vesicle fusion. Proc. Natl. Acad. Sci. USA 98, 12233-12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arca, S., Alberts, P., Zahraoui, A., Louvard, D., and Galli, T. (2000). Role of tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth. J. Cell Biol. 149, 889-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arca, S., et al. (2003). A dual mechanism controlling the localization and function of exocytic v-SNAREs. Proc. Natl. Acad. Sci. USA 100, 9011-9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastick, C.C., and Falick, A.L. (1997). Association of N-ethylmaleimide sensitive fusion (NSF) protein and soluble NSF attachment proteins-α and -gamma with glucose transporter-4-containing vesicles in primary rat adipocytes. Endocrinology 138, 2391-2397. [DOI] [PubMed] [Google Scholar]
- McNew, J.A., Parlati, F., Fukuda, R., Johnston, R.J., Paz, K., Paumet, F., Söllner, T.H., and Rothman, J.E. (2000). Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature 407, 153-159. [DOI] [PubMed] [Google Scholar]
- Millar, C.A., Shewan, A., Hickson, G.R., James, D.E., and Gould, G.W. (1999). Differential regulation of secretory compartments containing the insulin-responsive glucose transporter 4 in 3T3–L1 adipocytes. Mol. Biol. Cell 10, 3675-3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, J.M., Rabouille, C., Newman, R., Shorter, J., Freemont, P., Schiavo, G., Warren, G., and Shima, D.T. (1999). An NSF function distinct from ATPase-dependent SNARE disassembly is essential for Golgi membrane fusion. Nat. Cell Biol. 1, 335-340. [DOI] [PubMed] [Google Scholar]
- Prekeris, R., Yang, B., Oorschot, V., Klumperman, J., and Scheller, R.H. (1999). Differential roles of syntaxin 7 and syntaxin 8 in endosomal trafficking. Mol. Biol. Cell 10, 3891-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor, P.R., Mullock, B.M., Bright, N.A., Lindsay, M.R., Gray, S.R., Richardson, S.C., Stewart, A., James, D.E., Piper, R.C., and Luzio, J.P. (2004). Combinatorial SNARE complexes with VAMP7 or VAMP8 define different late endocytic fusion events. EMBO Rep. 5, 590-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randhawa, V.K., et al. (2000). VAMP2, but not VAMP3/Cellubrevin, mediates insulin-dependent incorporation of GLUT4 into the plasma membrane of L6 myoblasts. Mol. Biol. Cell 11, 2403-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, S.K., Huynh, C., Proux-Gillardeaux, V., Galli, T., and Andrews, N.W. (2004). Identification of SNAREs involved in synaptotagmin VII-regulated lysosomal exocytosis. J. Biol. Chem. 279, 20471-20479. [DOI] [PubMed] [Google Scholar]
- Rosenmund, C., and Stevens, C.F. (1996). Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron 16, 1197-1207. [DOI] [PubMed] [Google Scholar]
- Rothman, J.E. (1994). Mechanisms of intracellular protein transport. Nature 372, 55-63. [DOI] [PubMed] [Google Scholar]
- Rothman, J.E., and Warren, G. (1994). Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr. Biol. 4, 220-233. [DOI] [PubMed] [Google Scholar]
- Rudich, A., et al. (2003). Indinavir uncovers different contributions of GLUT4 and GLUT1 towards glucose uptake in muscle and fat cells and tissues. Diabetologia 46, 649-658. [DOI] [PubMed] [Google Scholar]
- Saltiel, A.R., and Pessin, J.E. (2002). Insulin signaling pathways in time and space. Trends Cell Biol. 12, 65-71. [DOI] [PubMed] [Google Scholar]
- Scales, S.J., Finley, M.F., and Scheller, R.H. (2001). Cell biology. Fusion without SNAREs? Science 294, 1015-1016. [DOI] [PubMed] [Google Scholar]
- Sutton, R.B., Fasshauer, D., Jahn, R., and Brunger, A.T. (1998). Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature 395, 347-353. [DOI] [PubMed] [Google Scholar]
- Sweeney, G., et al. (2004). Intracellular delivery of phosphatidylinositol (3,4,5)-trisphosphate causes incorporation of glucose transporter 4 into the plasma membrane of muscle and fat cells without increasing glucose uptake. J. Biol. Chem. 279, 32233-32242. [DOI] [PubMed] [Google Scholar]
- Tong, P., Khayat, Z.A., Huang, C., Patel, N., Ueyama, A., and Klip, A. (2001). Insulin-induced cortical actin remodeling promotes GLUT4 insertion at muscle cell membrane ruffles. J. Clin. Investig. 108, 371-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama, A., Yaworsky, K.L., Wang, Q., Ebina, Y., and Klip, A. (1999). GLUT-4myc ectopic expression in L6 myoblasts generates a GLUT-4-specific pool conferring insulin sensitivity. Am. J. Physiol. 277, E572-E578. [DOI] [PubMed] [Google Scholar]
- Wang, Q., Khayat, Z., Kishi, K., Ebina, Y., and Klip, A. (1998). GLUT4 translocation by insulin in intact muscle cells: detection by a fast and quantitative assay. FEBS Lett. 427, 193-197. [DOI] [PubMed] [Google Scholar]
- Weber, T., Zemelman, B.V., McNew, J.A., Westermann, B., Gmachl, M., Parlati, F., Söllner, T.H., and Rothman, J.E. (1998). SNAREpins: minimal machinery for membrane fusion. Cell 92, 759-772. [DOI] [PubMed] [Google Scholar]
- Whalley, T., Timmers, K., Coorssen, J., Bezrukov, L., Kingsley, D.H., and Zimmerberg, J. (2004). Membrane fusion of secretory vesicles of the sea urchin egg in the absence of NSF. J. Cell Sci. 117, 2345-2356. [DOI] [PubMed] [Google Scholar]
- Yang, C., Mora, S., Ryder, J.W., Coker, K.J., Hansen, P., Allen, L.A., and Pessin, J.E. (2001). VAMP3 null mice display normal constitutive, insulin- and exercise-regulated vesicle trafficking. Mol. Cell. Biol. 21, 1573-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, R.C., Jahn, R., and Brunger, A.T. (1999). NSF N-terminal domain crystal structure: models of NSF function. Mol. Cell 4, 97-107. [DOI] [PubMed] [Google Scholar]
- Zeigerer, A., Lampson, M.A., Karylowski, O., Sabatini, D.D., Adesnik, M., Ren, M., and McGraw, T.E. (2002). GLUT4 retention in adipocytes requires two intracellular insulin-regulated transport steps. Mol. Biol. Cell 13, 2421-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]