Abstract
Background: During development of cardiovascular disease (CVD), interferon-γ–mediated inflammation accelerates degradation of tryptophan into downstream metabolites. A Mediterranean diet (MedDiet) consisting of a high intake of extra-virgin olive oil (EVOO), nuts, fruits, vegetables, and cereals has been demonstrated to lower the risk of CVD. The longitudinal relation between tryptophan and its downstream metabolites and CVD in the context of a MedDiet is unstudied.
Objective: We sought to investigate the relation between metabolites in the tryptophan-kynurenine pathway and CVD in the context of a MedDiet pattern.
Methods: We used a case-cohort design nested in the Prevención con Dieta Mediterránea randomized controlled trial. There were 231 CVD cases (stroke, myocardial infarction, cardiovascular death) among 985 participants over a median of 4.7 y of follow-up [mean ± SD age: 67.6 ± 6.1 y; 53.7% women; mean ± SD body mass index (in kg/m2): 29.7 ± 3.7]. We assessed plasma tryptophan, kynurenine, kynurenic acid, 3-hydroxyanthranilic acid, and quinolinic acid concentrations at baseline and after 1 y of intervention with a MedDiet. We combined these metabolites in a kynurenine risk score (KRS) by weighting each metabolite by the adjusted coefficient of its associations with CVD. Cox models were used in the primary analysis.
Results: Increases in tryptophan after 1 y were associated with a lower risk of composite CVD (HR per SD: 0.79; 95% CI: 0.63, 0.98). The baseline kynurenic acid concentration was associated with a higher risk of myocardial infarction and coronary artery disease death but not stroke. A higher KRS was more strongly associated with CVD in the control group than in the 2 intervention groups (P-interaction = 0.003). Adjustment for changes in plasma tryptophan attenuated the inverse association between MedDiet+EVOO and CVD.
Conclusions: An increase in the plasma tryptophan concentration was significantly associated with a decreased risk of CVD. A MedDiet may counteract the deleterious effects of a high tryptophan risk score.
Keywords: metabolomics, cardiovascular disease, Mediterranean diet, tryptophan, epidemiology, nutrition
Introduction
Inflammation has long been identified as an important component in the pathophysiology of cardiovascular disease (CVD)22 (1). IFN-γ is a key molecule that orchestrates many pathways involved in inflammation by acting as a transcriptional regulator for immune-related genes (2). Its excessive release by activated T-lymphocytes has been implicated in the pathogenesis of chronic inflammation and autoimmune disorders (3). IFN-γ has also been shown to activate macrophages, which are intimately involved in plaque formation, and can also trigger the release of reactive oxygen species, a hallmark of subsequent atherogenesis and progression of CVD (4). Another well-known consequence of IFN-γ release is the activation of the enzyme indoleamine 2,3-dioxygenase (IDO), which catabolizes tryptophan into kynurenine (5). The classic kynurenine pathway involves the breakdown of tryptophan into downstream products, such as kynurenine, kynurenic acid, and 3-hydroxyanthranilic acid (3-HAA), the last of which is eventually converted into quinolinic acid and picolinic acid (6). The final result is the production of one equivalent of NAD for every processed tryptophan molecule (7). To date, most scientific literature regarding this pathway has focused on its relation to neurological disorders, such as Alzheimer’s disease, Huntington’s disease, and depression (8). These publications stem from the early finding that tryptophan is an essential precursor to brain serotonin and melatonin (9). Some studies relating tryptophan metabolites to CVD have reported inverse associations with tryptophan and the risk of CVD (10–16). However, to our knowledge, no studies have examined whether changes in tryptophan levels are prospectively associated with the incidence of CVD. Furthermore, the effects of diet on metabolites of this pathway in relation to CVD have not been investigated.
In the present case-cohort study nested in the PREDIMED (PREvención con DIeta MEDiterránea) trial, we used repeated measurements of these metabolites to longitudinally assess whether 1) baseline and 1-y changes in tryptophan, kynurenine, kynurenic acid, 3-HAA, and quinolinic acid were associated with future risk of CVD; 2) these associations differed by the type of CVD events (stroke compared with nonstroke events [i.e., coronary artery disease (CAD) and nonstroke-related cardiac death]); 3) potentially harmful associations could be counteracted by a Mediterranean diet (MedDiet); and 4) the cardioprotective effect of a MedDiet is attenuated after adjusting for changes in these metabolites. In light of previous research on cognitive diseases, we hypothesized that a favorable tryptophan profile would be more beneficial in preventing stroke, because there may be common risk factors for both stroke and cognitive disorders, such as inflammation or preclinical cerebrovascular disease.
Methods
Study population and design.
The PREDIMED trial is a multicenter, randomized trial of dietary interventions with a MedDiet supplemented with either nuts or extra-virgin olive oil (EVOO) for the primary prevention of CVD compared with a low-fat control group (17). Protocol, design, and primary results are detailed elsewhere (18, 19). Briefly, 7447 eligible men and women at high risk of CVD were randomly assigned to a MedDiet supplemented with a free provision for the family of 1 L EVOO/wk (MedDiet+EVOO), a MedDiet supplemented with 30 g mixed nuts/d (15 g walnuts, 7.5 g hazelnuts, and 7.5 g almonds) (MedDiet+nuts), or a control diet consisting of advice to reduce the intake of all types of fat (control group). The primary endpoint was a composite CVD, defined as stroke, myocardial infarction, or death from cardiovascular causes. Information on primary endpoints was collected by physicians and from other sources of information, such as the National Death Index. Only endpoints that were confirmed by the adjudication committee and that occurred between October 2003 and December 2010 were included in the analyses.
For the present study, we sampled ∼10% of all PREDIMED participants in a case-cohort design. A random selection of 791 participants was chosen at baseline as the subcohort. From the full cohort (including the subcohort), there were 231 CVD cases during follow-up that were included in the analysis. Of the CVD cases, 118 were strokes (113 ischemic and 5 hemorrhagic), and 113 were nonstroke events. There were 37 individuals in the subcohort who later became cases. We excluded individuals from specific analyses if they were missing metabolite values for that analysis. At baseline and at 1-y of follow-up, 896 and 806 participants had valid measurements for all 5 metabolites, respectively.
Quantification of metabolites.
Fasting plasma EDTA-coated tubes were collected for all participants, and aliquots were coded and kept refrigerated until they were stored at −80°C. Pairs of samples (baseline and first-year visits from each participant) were randomly ordered and shipped to the Broad Institute of Harvard and MIT for metabolomics analyses. Liquid chromatography tandem mass spectrometry was used to quantitatively profile metabolites in fasting plasma collected at baseline and year 1 of the intervention. Reference standards were used to confirm metabolite identities. The technical details of this procedure are described elsewhere (20, 21).
Statistical analyses.
Rank-based inverse normal transformations were used to transform the nonnormal distributions of the 5 metabolites under study (22). We calculated a kynurenine risk score (KRS) using as weights the adjusted coefficients obtained in the assessment of the association between the respective metabolite and the risk of CVD. We first regressed composite CVD on each individual metabolite in a fully adjusted Cox model, then multiplied the resulting β by the normalized values for the corresponding metabolite. These 5 products were then summed to produce the KRS. If any of the individuals were missing any metabolite values, we did not calculate a KRS for that individual. Each normalized metabolite was also analyzed according to its quartile distribution. Quartile cutoffs were generated based on the distributions of metabolites and score among noncases. Scores for acylcarnitines (20), BCAAs (21), and ceramides were also calculated in this fashion. We conducted tests of linear trend by examining an ordinal score based on the median value in each quartile of metabolites and score in the multivariable models.
Baseline data by quartiles of the KRS were presented as means ± SDs for continuous variables and numbers and percentages for categorical variables. Baseline characteristics were compared across quartiles of the KRS by using chi-square tests for categorical variables and 1-factor ANOVA for continuous variables.
We used weighted Cox regression models with noncases upweighted by their sampling fraction (22) and used robust variance to account for correlation between observations (23). We calculated HRs and their 95% CIs for the composite CVD endpoint and separately for stroke and nonstroke cases (myocardial infarction and nonstroke CVD mortality). Follow-up time was calculated from the date of enrollment to the date of diagnosis of CVD for cases and to the date of the last visit or the end of the follow-up period (1 December 2010) for noncases. In model 1, we adjusted for age, sex, family history of CAD, smoking status, and BMI (in kg/m2) and stratified by intervention group. In model 2, we additionally adjusted for model 1 plus baseline hypertension, dyslipidemia, diabetes, and scores for BCAAs, acylcarnitines, and ceramides (previously reported as associated with CVD in our cohort). For models including 1-y change variables, we also adjusted for continuous baseline levels of that metabolite. We also excluded individuals with follow-up times of <1 y in these analyses. We additionally conducted subgroup analyses by restricting the analysis to the group of interest. To test for effect modification, we used a likelihood ratio test to compare the model without interaction terms with the model with interaction terms (indicator variable for subgroup × KRS) among all participants.
For the mediation analysis of the MedDiet+EVOO diet, we reported the coefficient for the MedDiet+EVOO group compared with the control group (excluding individuals allocated to the MedDiet+nuts group) with and without adjustment for both baseline and change in tryptophan. We repeated this analyses for the MedDiet+nuts diet excluding the MedDiet+EVOO participants.
To calculate 1-y changes in metabolites, we performed an ANOVA for each metabolite and calculated means of metabolite values at baseline and at 1 y, stratified by intervention group, adjusted for categories of age (<65 or ≥65 y), BMI (<30 or ≥30), and sex (male or female). We used paired t tests to test if changes in metabolites were significant within each arm of the trial.
For correlation between metabolites and scores, we used Spearman correlation coefficients and P values.
All statistical analyses were performed with SAS (v9.4; SAS Institute) and R (v2.13.0; R Foundation).
Results
Descriptive results.
Demographic and behavioral characteristics of the participants in the subcohort with valid measurements of all 5 metabolites at baseline (n = 724) were classified according to quartiles of KRS in Table 1. Increasing quartiles of KRS were significantly associated with age, percentage of female sex, percentage of hypertension, and percentage of current smokers. The median follow-up time was 4.7 y.
TABLE 1.
Baseline characteristics of the full subcohort according to Qs of kynurenine risk score1
| Kynurenine risk score |
|||||
| Q1 | Q2 | Q3 | Q4 | P | |
| n | 180 | 181 | 179 | 188 | |
| Median score | −0.43 | −0.14 | 0.08 | 0.41 | |
| Range | −1.06 to −0.24 | −0.24 to −0.03 | −0.03 to 0.18 | 0.18–1.08 | |
| Age, y | 66.2 ± 5.8 | 66.8 ± 6.0 | 66.8 ± 5.8 | 69.0 ± 5.7 | <0.0001 |
| Sex (women) | 46.1 | 57.5 | 60.3 | 62.2 | 0.01 |
| BMI, kg/m2 | 29.5 ± 3.6 | 29.5 ± 3.4 | 29.8 ± 3.7 | 30.3 ± 3.5 | 0.09 |
| Intervention group | 0.21 | ||||
| MedDiet+EVOO | 33.3 | 32.6 | 40.2 | 44.2 | |
| MedDiet+nuts | 34.4 | 37.0 | 29.1 | 30.3 | |
| Control | 32.2 | 30.4 | 30.7 | 25.5 | |
| Family history of CAD | 21.7 | 27.6 | 22.9 | 27.7 | 0.41 |
| Hypertension | 78.3 | 84.5 | 82.1 | 87.8 | 0.10 |
| Dyslipidemia | 71.1 | 74.6 | 73.2 | 73.4 | 0.90 |
| Diabetes | 47.2 | 40.3 | 53.6 | 47.9 | 0.09 |
| Obesity (BMI ≥30) | 43.3 | 40.3 | 46.9 | 53.2 | 0.08 |
| Smoking | <0.0001 | ||||
| Never | 52.2 | 65.8 | 62.6 | 69.7 | |
| Former | 22.2 | 23.2 | 26.8 | 26.6 | |
| Current | 25.6 | 11.1 | 10.6 | 3.7 | |
Values are means ± SDs or percentages unless otherwise indicated. The P values are comparisons between cases and control groups across Qs (Pearson chi-square test for categorical variables or 1-factor ANOVA for continuous variables) as appropriate. CAD, coronary artery disease; EVOO, extra-virgin olive oil; MedDiet, Mediterranean diet; Q, quartile.
One-year changes in tryptophan metabolites according to intervention group are shown in Supplemental Figure 1. Metabolites of those randomly assigned to the MedDiet+EVOO group uniformly decreased after 1 y. Using paired t tests for the nontransformed metabolite quantifications at baseline and 1 y, we observed significant changes in tryptophan and 3-HAA in the MedDiet+EVOO group and in kynurenic acid and quinolinic acid in the MedDiet+nuts group.
Spearman correlation coefficients of tryptophan metabolites, BCAAs, ceramides, and acylcarnitine score are depicted in Supplemental Table 1. In general, tryptophan metabolites had low-to-moderate correlations with each other (ρ = 0.00–0.48), low-to-moderate correlation with BCAA scores (ρ = 0.03–0.49), and modest correlations with ceramide and acylcarnitine scores (ρ = −0.29 to 0.14).
Individual metabolites, risk score, and composite CVD.
Table 2 details the associations of baseline and 1-y changes of kynurenine pathway metabolites with composite CVD incidence. Only baseline kynurenic acid was associated with composite CVD risk in the fully adjusted model (HR per SD: 1.23; 95% CI: 1.02, 1.48; P < 0.05). By using 1-y changes, an increase in tryptophan was associated with a significantly reduced risk in the categorical analysis (HR for quartile 4 compared with quartile 1: 0.49; 95% CI: 0.26, 0.95; P-trend < 0.05) models, as well as in the continuous analysis (HR per SD: 0.79; 95% CI: 0.63, 0.98; P < 0.05)
TABLE 2.
HRs (95% CIs) for composite CVD (stroke, nonstroke, death from vascular causes) by baseline and 1-y changes in plasma metabolites (tryptophan, kynurenine, kynurenic acid, 3-HAA, and quinolinic acid) as continuous and categorical variables1
| Tryptophan | Kynurenine | Kynurenic acid | 3-HAA | Quinolinic acid | |
| Baseline2 | |||||
| Model 1, metabolite as continuous variable, per SD | |||||
| HR (95% CI) | 0.88 (0.74, 1.04) | 1.00 (0.86, 1.16) | 1.14 (0.99, 1.31) | 0.82 (0.69, 0.98) | 1.03 (0.86, 1.22) |
| P | 0.13 | 0.99 | 0.08 | 0.03 | 0.77 |
| Metabolite in Q categories as compared with Q1 (reference) | |||||
| Q2 | 0.91 (0.59, 1.40) | 0.86 (0.55, 1.35) | 1.49 (0.91, 2.42) | 0.83 (0.53, 1.30) | 0.93 (0.59, 1.47) |
| Q3 | 0.67 (0.42, 1.05) | 0.93 (0.61, 1.44) | 1.39 (0.86, 2.24) | 0.62 (0.38, 1.03) | 0.99 (0.64, 1.54) |
| Q4 | 0.75 (0.48, 1.19) | 0.91 (0.59, 1.40) | 1.49 (0.92, 2.40) | 0.69 (0.43, 1.11) | 1.17 (0.75, 1.83) |
| P-trend | 0.12 | 0.76 | 0.14 | 0.07 | 0.46 |
| Model 2, metabolite as continuous variable, per SD | |||||
| HR (95% CI) | 0.87 (0.67, 1.11) | 1.06 (0.89, 1.26) | 1.23 (1.02, 1.48) | 0.82 (0.67, 1.01) | 1.15 (0.94, 1.41) |
| P | 0.28 | 0.52 | 0.03 | 0.06 | 0.19 |
| Metabolite in Q categories as compared with Q1 (reference) | |||||
| Q2 | 0.95 (0.58, 1.57) | 1.27 (0.75, 2.13) | 1.58 (0.91, 2.76) | 0.72 (0.42, 1.23) | 1.00 (0.60, 1.67) |
| Q3 | 0.58 (0.33, 1.01) | 1.17 (0.72, 1.92) | 1.62 (0.92, 2.85) | 0.84 (0.46, 1.50) | 1.08 (0.65, 1.82) |
| Q4 | 0.75 (0.40, 1.44) | 1.21 (0.73, 1.99) | 1.73 (0.97, 3.10) | 0.64 (0.36, 1.16) | 1.53 (0.91, 2.57) |
| P-trend | 0.21 | 0.55 | 0.08 | 0.22 | 0.11 |
| 1-y changes3 | |||||
| Model 1, metabolite as continuous variable, per SD | |||||
| HR (95% CI) | 0.77 (0.64, 0.94) | 0.86 (0.69, 1.06) | 0.87 (0.73, 1.04) | 1.15 (0.91, 1.45) | 0.96 (0.80, 1.15) |
| P | 0.01 | 0.15 | 0.13 | 0.26 | 0.64 |
| Metabolite in Q categories as compared with Q1 (reference) | |||||
| Q2 | 1.18 (0.72, 1.94) | 0.79 (0.48, 1.30) | 0.95 (0.56, 1.61) | 1.59 (0.79, 3.19) | 0.84 (0.48, 1.47) |
| Q3 | 0.75 (0.44, 1.27) | 0.37 (0.20, 0.70) | 0.88 (0.50, 1.53) | 1.91 (0.91, 3.99) | 0.95 (0.54, 1.66) |
| Q4 | 0.49 (0.27, 0.87) | 0.68 (0.41, 1.13) | 0.69 (0.40, 1.20) | 1.46 (0.70, 3.05) | 0.89 (0.52, 1.51) |
| P-trend | 0.005 | 0.02 | 0.18 | 0.27 | 0.78 |
| Model 2, metabolite as continuous variable, per SD | |||||
| HR (95% CI) | 0.79 (0.63, 0.98) | 0.85 (0.67, 1.06) | 0.93 (0.75, 1.15) | 1.15 (0.90, 1.49) | 0.92 (0.75, 1.13) |
| P | 0.03 | 0.15 | 0.52 | 0.27 | 0.42 |
| Metabolite in Q categories as compared with Q1 (reference) | |||||
| Q2 | 1.19 (0.70, 2.02) | 0.78 (0.45, 1.34) | 1.00 (0.53, 1.87) | 1.26 (0.56, 2.84) | 0.82 (0.43, 1.57) |
| Q3 | 0.72 (0.40, 1.28) | 0.39 (0.20, 0.77) | 1.00 (0.52, 1.93) | 1.37 (0.55, 3.43) | 0.97 (0.51, 1.85) |
| Q4 | 0.49 (0.26, 0.95) | 0.60 (0.34, 1.07) | 0.84 (0.44, 1.58) | 1.32 (0.57, 3.03) | 0.78 (0.43, 1.42) |
| P-trend | 0.01 | 0.02 | 0.60 | 0.50 | 0.55 |
Model 1 was adjusted for age, sex, family history of coronary artery disease, smoking status, and BMI and was stratified by intervention group. Model 2 was adjusted as for covariates in model 1 plus for baseline hypertension, dyslipidemia, diabetes, and scores for BCAAs, acylcarnitines, and ceramides. For change analyses, metabolites were adjusted for baseline values. CVD, cardiovascular disease; Q, quartile; 3-HAA, 3-hydroxyanthranilic acid.
Baseline analysis consisted of 986 participants and 231 cases for tryptophan (n = 63, 58, 50, and 60 cases for Q1–Q4, respectively), kynurenine (n = 61, 50, 57, and 63 cases for Q1–Q4, respectively), kynurenic acid (n = 42, 56, 62, and 71 cases for Q1–Q4, respectively), and quinolinic acid (n = 65, 46, 51, and 69 cases for Q1–Q4, respectively) and 904 participants and 203 cases for 3-HAA (n = 57, 58, 44, and 44 cases for Q1–Q4, respectively).
1-y change analysis consisted of 908 participants and 162 cases for tryptophan (n = 44, 54, 39, and 25 cases for Q1–Q4, respectively), 160 cases for kynurenine (n = 51, 43, 22, and 44 cases for Q1–Q4, respectively), kynurenic acid (n = 49, 43, 36, and 32 cases for Q1–Q4, respectively), and quinolinic acid (n = 41, 35, 41, and 43 cases for Q1–Q4, respectively) and 750 participants and 122 cases for 3-HAA (n = 22, 31, 40, and 29 cases for Q1–Q4, respectively).
Because the kynurenine pathway has been examined most often in relation to brain disorders, we hypothesized that associations of these metabolites would be stronger for stroke than for nonstroke events. Using stroke as the outcome in Table 3, we found no significant associations of any baseline metabolite concentration with stroke risk. Furthermore, the inverse association observed for 1-y changes in tryptophan was not present (HR per SD: 0.91; 95% CI: 0.70, 1.19; P = 0.49) when stroke alone was used as the outcome. In Table 3 when only nonstroke events were used as the outcome, kynurenic acid (HR per SD: 1.46; 95% CI: 1.12, 1.92; P < 0.05) was associated with higher subsequent incidence of outcomes different from stroke (myocardial infarction or any other cause of vascular death). Furthermore, the relation between 1-y increase in tryptophan and nonstroke outcomes was significantly associated with a lower risk of events in both the continuous models (HR per SD: 0.65; 95% CI: 0.46, 0.91; P < 0.05) and categorical models (HR for quartile 4 compared with quartile 1: 0.19; 95% CI: 0.06, 0.61; P-trend < 0.05). One-year changes in kynurenine were also inversely associated with nonstroke event risk in the categorical analysis (HR for quartile 4 compared with quartile 1: 0.62; 95% CI: 0.28, 1.36; P-trend < 0.05).
TABLE 3.
HRs (95% CIs) for stroke and nonstroke cases by baseline and 1-y changes in plasma metabolites (tryptophan, kynurenine, kynurenic acid, 3-HAA, and quinolinic acid) as continuous and categorical variables1
| Tryptophan | Kynurenine | Kynurenic acid | 3-HAA | Quinolinic acid | |
| Stroke | |||||
| Baseline2 | |||||
| Model 1, metabolite as continuous variable, per SD | |||||
| HR (95% CI) | 0.97 (0.76, 1.24) | 0.97 (0.79, 1.20) | 1.05 (0.87, 1.26) | 0.78 (0.61, 0.98) | 0.91 (0.72, 1.15) |
| P | 0.81 | 0.79 | 0.65 | 0.04 | 0.42 |
| Metabolite in Q categories as compared with Q1 (reference) | |||||
| Q2 | 0.68 (0.38, 1.22) | 0.87 (0.49, 1.54) | 1.43 (0.79, 2.61) | 0.67 (0.37, 1.21) | 0.81 (0.45, 1.46) |
| Q3 | 0.61 (0.34, 1.10) | 0.80 (0.46, 1.40) | 1.00 (0.54, 1.86) | 0.69 (0.37, 1.30) | 0.95 (0.55, 1.65) |
| Q4 | 0.95 (0.54, 1.66) | 0.89 (0.50, 1.57) | 1.16 (0.61, 2.18) | 0.63 (0.34, 1.17) | 0.96 (0.54, 1.72) |
| P-trend | 0.77 | 0.62 | 0.94 | 0.18 | 0.97 |
| Model 2, metabolite as continuous variable, per SD | |||||
| HR (95% CI) | 0.98 (0.71, 1.35) | 1.02 (0.82, 1.26) | 1.06 (0.83, 1.37) | 0.76 (0.57, 1.01) | 1.05 (0.81, 1.37) |
| P | 0.89 | 0.89 | 0.63 | 0.06 | 0.72 |
| Metabolite in Q categories as compared with Q1 (reference) | |||||
| Q2 | 0.73 (0.38, 1.41) | 1.22 (0.65, 2.30) | 1.43 (0.72, 2.82) | 0.56 (0.28, 1.12) | 0.93 (0.49, 1.75) |
| Q3 | 0.52 (0.25, 1.09) | 0.90 (0.48, 1.67) | 1.07 (0.52, 2.18) | 0.87 (0.42, 1.80) | 1.09 (0.58, 2.05) |
| Q4 | 0.96 (0.43, 2.16) | 1.13 (0.60, 2.12) | 1.19 (0.55, 2.61) | 0.52 (0.24, 1.12) | 1.26 (0.65, 2.47) |
| P-trend | 0.74 | 0.96 | 0.85 | 0.23 | 0.43 |
| 1-y changes3 | |||||
| Model 1, metabolite as continuous variable, per SD | |||||
| HR (95% CI) | 0.89 (0.70, 1.12) | 0.88 (0.66, 1.17) | 0.90 (0.71, 1.15) | 1.27 (0.93, 1.72) | 0.87 (0.69, 1.10) |
| P | 0.32 | 0.37 | 0.41 | 0.14 | 0.24 |
| Metabolite in Q categories as compared with Q1 (reference) | |||||
| Q2 | 1.51 (0.79, 2.90) | 0.64 (0.33, 1.26) | 0.46 (0.22, 0.99) | 1.65 (0.63, 4.31) | 1.11 (0.54, 2.27) |
| Q3 | 0.88 (0.43, 1.80) | 0.44 (0.20, 0.98) | 0.83 (0.41, 1.68) | 1.90 (0.68, 5.31) | 1.10 (0.54, 2.25) |
| Q4 | 0.84 (0.41, 1.70) | 0.67 (0.34, 1.31) | 0.59 (0.28, 1.23) | 1.79 (0.65, 4.92) | 0.85 (0.43, 1.70) |
| P-trend | 0.35 | 0.16 | 0.40 | 0.25 | 0.66 |
| Model 2, metabolite as continuous variable, per SD | |||||
| HR (95% CI) | 0.91 (0.70, 1.19) | 0.85 (0.63, 1.16) | 0.97 (0.72, 1.29) | 1.24 (0.90, 1.72) | 0.85 (0.66, 1.09) |
| P | 0.49 | 0.31 | 0.81 | 0.19 | 0.20 |
| Metabolite in Q categories as compared with Q1 (reference) | |||||
| Q2 | 1.60 (0.82, 3.16) | 0.59 (0.28, 1.25) | 0.44 (0.18, 1.04) | 1.30 (0.46, 3.74) | 1.03 (0.46, 2.29) |
| Q3 | 0.90 (0.42, 1.92) | 0.51 (0.23, 1.14) | 0.86 (0.38, 1.92) | 1.42 (0.41, 4.85) | 1.08 (0.49, 2.36) |
| Q4 | 0.89 (0.42, 1.92) | 0.56 (0.26, 1.18) | 0.71 (0.31, 1.58) | 1.55 (0.53, 4.56) | 0.78 (0.37, 1.63) |
| P-trend | 0.46 | 0.12 | 0.78 | 0.43 | 0.54 |
| Nonstroke | |||||
| Baseline4 | |||||
| Model 1, metabolite as continuous variable, per SD | |||||
| HR (95% CI) | 0.78 (0.63, 0.97) | 1.03 (0.85, 1.26) | 1.24 (1.02, 1.51) | 0.85 (0.69, 1.06) | 1.17 (0.92, 1.47) |
| P | 0.03 | 0.74 | 0.03 | 0.14 | 0.20 |
| Metabolite in Q categories as compared with Q1 (reference) | |||||
| Q2 | 1.20 (0.67, 2.16) | 0.80 (0.43, 1.50) | 1.50 (0.73, 3.09) | 1.04 (0.56, 1.91) | 1.05 (0.57, 1.95) |
| Q3 | 0.78 (0.42, 1.44) | 1.03 (0.58, 1.85) | 1.90 (0.96, 3.76) | 0.53 (0.26, 1.10) | 1.00 (0.54, 1.86) |
| Q4 | 0.58 (0.30, 1.11) | 0.94 (0.52, 1.71) | 2.02 (1.02, 3.97) | 0.74 (0.40, 1.40) | 1.48 (0.80, 2.73) |
| P-trend | 0.05 | 0.94 | 0.03 | 0.13 | 0.26 |
| Model 2, metabolite as continuous variable, per SD | |||||
| HR (95% CI) | 0.76 (0.54, 1.05) | 1.10 (0.87, 1.39) | 1.46 (1.12, 1.92) | 0.85 (0.66, 1.10) | 1.30 (0.97, 1.73) |
| P | 0.09 | 0.42 | 0.01 | 0.22 | 0.08 |
| Metabolite in Q categories as compared with Q1 (reference) | |||||
| Q2 | 1.16 (0.60, 2.26) | 1.26 (0.60, 2.65) | 1.77 (0.77, 4.08) | 0.99 (0.49, 2.00) | 1.12 (0.54, 2.35) |
| Q3 | 0.69 (0.33, 1.43) | 1.49 (0.75, 2.94) | 2.49 (1.10, 5.66) | 0.81 (0.36, 1.82) | 1.10 (0.53, 2.30) |
| Q4 | 0.52 (0.21, 1.29) | 1.33 (0.65, 2.74) | 2.89 (1.26, 6.65) | 0.74 (0.35, 1.60) | 2.05 (1.00, 4.21) |
| P-trend | 0.10 | 0.39 | 0.01 | 0.39 | 0.07 |
| 1-y changes5 | |||||
| Model 1, metabolite as continuous variable, per SD | |||||
| HR (95% CI) | 0.64 (0.48, 0.86) | 0.82 (0.62, 1.07) | 0.82 (0.65, 1.05) | 1.04 (0.76, 1.42) | 1.03 (0.79, 1.33) |
| P | 0.003 | 0.15 | 0.11 | 0.82 | 0.84 |
| Metabolite in Q categories as compared with Q1 (reference) | |||||
| Q2 | 0.93 (0.47, 1.84) | 0.94 (0.48, 1.81) | 1.69 (0.85, 3.36) | 1.59 (0.62, 4.11) | 0.59 (0.26, 1.37) |
| Q3 | 0.63 (0.31, 1.30) | 0.27 (0.11, 0.68) | 0.79 (0.34, 1.86) | 2.03 (0.76, 5.45) | 0.81 (0.36, 1.83) |
| Q4 | 0.22 (0.08, 0.56) | 0.64 (0.32, 1.28) | 0.79 (0.37, 1.70) | 1.19 (0.44, 3.21) | 0.90 (0.43, 1.88) |
| P-trend | 0.001 | 0.03 | 0.24 | 0.64 | 0.99 |
| Model 2, metabolite as continuous variable, per SD | |||||
| HR (95% CI) | 0.65 (0.46, 0.90) | 0.81 (0.60, 1.09) | 0.85 (0.64, 1.14) | 1.09 (0.78, 1.53) | 0.96 (0.71, 1.30) |
| P | 0.01 | 0.17 | 0.29 | 0.61 | 0.81 |
| Metabolite in Q categories as compared with Q1 (reference) | |||||
| Q2 | 0.84 (0.39, 1.84) | 0.98 (0.48, 2.00) | 2.14 (0.91, 5.04) | 1.30 (0.39, 4.36) | 0.59 (0.22, 1.57) |
| Q3 | 0.55 (0.24, 1.27) | 0.22 (0.07, 0.64) | 1.08 (0.39, 2.97) | 1.45 (0.40, 5.28) | 0.84 (0.30, 2.35) |
| Q4 | 0.19 (0.06, 0.61) | 0.62 (0.28, 1.36) | 0.98 (0.38, 2.51) | 1.20 (0.37, 3.88) | 0.74 (0.31, 1.77) |
| P-trend | 0.003 | 0.02 | 0.62 | 0.73 | 0.70 |
Model 1 was adjusted for age, sex, family history of coronary artery disease, smoking status, and BMI and was stratified by intervention group. Model 2 was adjusted as for model 1 plus for baseline hypertension, dyslipidemia, diabetes, and scores for BCAAs, acylcarnitines, and ceramides. For change analyses, metabolites were adjusted for baseline values. Q, quartile; 3-HAA, 3-hydroxyanthranilic acid.
Baseline analysis for stroke consisted of 872 participants and 118 cases for tryptophan (n = 36, 23, 24, and 35 cases for Q1–Q4, respectively), kynurenine (n = 33, 28, 26, and 31 cases for Q1–Q4, respectively), kynurenic acid (n = 25, 35, 27, and 31 cases for Q1–Q4, respectively) and quinolinic acid (n = 35, 23, 28, and 32 cases for Q1–Q4, respectively) and 804 participants and 103 cases for 3-HAA (n = 32, 25, 26, and 20 cases for Q1–Q4, respectively). Nonstroke cases are excluded.
One-year change analysis for stroke consisted of 835 participants and 86 cases for tryptophan (n = 19, 29, 20, and 18 cases for Q1–Q4, respectively), kynurenine (n = 28, 19, 14, and 25 cases for Q1–Q4, respectively), kynurenic acid (n = 29, 15, 24, and 18 cases for Q1–Q4, respectively), and quinolinic acid (n = 19, 23, 23, and 21 cases for Q1–Q4, respectively) and 692 participants and 63 cases for 3-HAA (n = 10, 16, 20, and 17 cases for Q1–Q4, respectively). Nonstroke cases are excluded.
Baseline analysis for nonstroke consisted of 868 participants and 113 cases for tryptophan (n = 27, 35, 26, and 25 cases for Q1–Q4, respectively), kynurenine (n = 28, 22, 31, and 32 cases for Q1–Q4, respectively), kynurenic acid (n = 17, 21, 35, and 40 cases for Q1–Q4, respectively) and quinolinic acid (n = 30, 23, 23, and 37 cases for Q1–Q4, respectively) and 800 participants and 100 cases for 3-HAA (n = 25, 33, 18, and 24 cases for Q1–Q4, respectively). Stroke cases are excluded.
One-year change analysis for nonstroke consisted of 825 participants and 76 cases for tryptophan (n = 25, 25, 19, and 7 cases for Q1–Q4, respectively), cases for kynurenine (n = 23, 24, 8, and 19 cases for Q1–Q4, respectively), 75 cases for kynurenic acid (n = 20, 28, 12, and 15 cases for Q1–Q4, respectively), and 74 cases for quinolinic acid (n = 22, 12, 18, and 22 cases for Q1–Q4, respectively) and 687 participants and 59 cases for 3-HAA (n = 12, 15, 20, and 12 cases for Q1–Q4, respectively). Stroke cases are excluded.
In Table 4, using the KRS as exposure, we observed a similar pattern of elevated incidence of the composite CVD outcome (HR per SD: 1.41; 95% CI: 1.14, 1.75; P < 0.05), and a strengthened association with nonstroke events (HR per SD: 1.70; 95% CI: 1.27, 2.29; P < 0.001). We also considered the ratio of kynurenine to tryptophan in Supplemental Table 2 but found no significant associations of this ratio with any CVD outcome. Thus, we proceeded with the KRS to test effect modification by a MedDiet.
TABLE 4.
HRs (95% CIs) for composite CVD, stroke only, and nonstroke only by baseline kynurenine risk score among participants with available data for all 5 metabolites under study1
| Outcome |
|||
| Composite CVD2 | Stroke only3 | Nonstroke only4 | |
| n | 896 | 797 | 793 |
| Cases | 202 | 103 | 99 |
| Model 1, metabolite as continuous variable, per SD | |||
| HR (95% CI) | 1.33 (1.11, 1.60) | 1.17 (0.91, 1.50) | 1.56 (1.22, 2.00) |
| P | 0.002 | 0.22 | <0.001 |
| Metabolite in Q categories as compared with Q1 (reference) | |||
| Q2 | 1.49 (0.88, 2.50) | 1.17 (0.62, 2.20) | 1.97 (0.92, 4.22) |
| Q3 | 1.03 (0.60, 1.78) | 0.75 (0.38, 1.47) | 1.48 (0.68, 3.23) |
| Q4 | 2.11 (1.26, 3.52) | 1.37 (0.70, 2.65) | 3.72 (1.80, 7.71) |
| P-trend | 0.02 | 0.64 | 0.002 |
| Model 2, metabolite as continuous variable, per SD | |||
| HR (95% CI) | 1.41 (1.14, 1.75) | 1.27 (0.94, 1.71) | 1.70 (1.27, 2.29) |
| P | 0.002 | 0.12 | <0.001 |
| Metabolite in Q categories as compared with Q1 (reference) | |||
| Q2 | 1.60 (0.89, 2.86) | 1.34 (0.66, 2.76) | 2.07 (0.86, 5.01) |
| Q3 | 1.00 (0.55, 1.82) | 0.71 (0.33, 1.55) | 1.59 (0.67, 3.78) |
| Q4 | 2.41 (1.36, 4.27) | 1.76 (0.80, 3.88) | 4.11 (1.84, 9.19) |
| P-trend | 0.02 | 0.40 | 0.002 |
Model 1 was adjusted for age, sex, family history of coronary artery disease, smoking status, and BMI and was stratified by intervention group. Model 2 was adjusted as for covariates in model 1 plus for baseline hypertension, dyslipidemia, diabetes, and scores for BCAAs, acylcarnitines, and ceramides. Kynurenine risk score was built by multiplying normalized individual metabolites (tryptophan, kynurenine, kynurenic acid, 3-hydroxyanthranilic acid, and quinolinic acid) by their β coefficient in a fully adjusted model with that metabolite alone and then summing up the products for each metabolite × coefficient value. The weights were −0.13474 for tryptophan, 0.0558 for kynurenine, 0.20359 for kynurenic acid, −0.20066 for 3-hydroxyanthranilic acid, and 0.13657 for quinolinic acid. CVD, cardiovascular disease; Q, quartile.
Baseline analysis for composite CVD consisted of 40, 49, 39, and 74 cases for Q1–Q4, respectively.
Baseline analysis for stroke consisted of 24, 26, 19, and 34 cases for Q1–Q4, respectively.
Baseline analysis for nonstroke consisted of 16, 23, 20, and 40 cases for Q1–Q4, respectively
Effect modification by a MedDiet.
In Supplemental Table 3 we sought to address whether several baseline variables potentially modified the positive association of the KRS with CVD. We only found significant interaction according to intervention group (P-interaction = 0.003), with those in the control group experiencing a greater elevation in risk per SD increase (HR per SD: 2.02; 95% CI: 1.31, 3.13) than those in the MedDiet+EVOO group (HR per SD: 1.27; 95% CI: 0.83, 1.94) and the MedDiet+nuts group (HR per SD: 1.23; 95% CI: 0.79, 1.92).
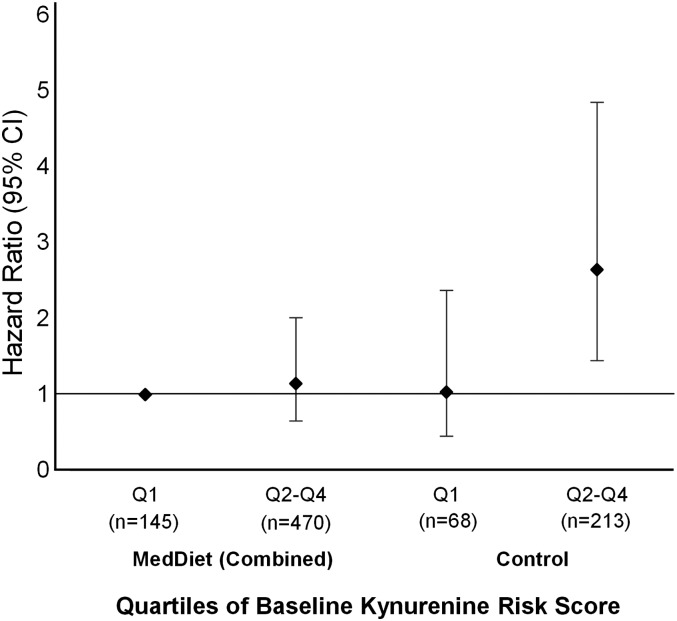
Figure 1 depicts the fully adjusted HRs stratified by intervention (combined MedDiet compared with control group) and quartiles of the baseline KRS. Compared with those randomly assigned to a MedDiet and in the lowest quartile of baseline KRS, we found a significant positive association for individuals randomly assigned to the control group and quartiles 2–4 of the KRS but not for those randomly assigned to the MedDiet and quartiles 2–4 of the KRS.
FIGURE 1.
Multivariate adjusted HRs (95% CIs) of composite CVD by Qs of baseline kynurenine risk score stratified by intervention group (Mediterranean interventions combined compared with the control group) among participants with available data for all 5 metabolites under study (n = 896). MedDiet, Mediterranean diet; Q, quartile.
Attenuation of the benefits of a MedDiet by tryptophan changes.
In Supplemental Tables 4 and 5 we explore the strength of the protective association of a MedDiet before and after adjustment of tryptophan. When comparing the MedDiet+EVOO group with the control group, adjustment for baseline and 1-y changes in tryptophan attenuated the inverse association from an HR per SD of 0.62 (95% CI: 0.38, 1.01) to an HR per SD of 0.95 (95% CI: 0.66, 1.36). This attenuation appeared to be weaker for stroke and greater for nonstroke outcomes. However, when comparing the MedDiet+nuts group with the control group, we did not observe these patterns of attenuation. After controlling for baseline and 1-y changes in tryptophan, the inverse association for the MedDiet+nuts diet only slightly changed from an HR per SD of 0.61 (95% CI: 0.37, 1.00) to an HR per SD of 0.60 (95% CI: 0.35, 1.02). Similarly, effect size was lower for stroke events and higher for nonstroke events.
Discussion
In the present case-cohort study, we report that lower baseline kynurenic acid and higher baseline tryptophan concentrations were associated with a lower risk of nonstroke events, and a 1-y increase in tryptophan concentrations was strongly associated with a lower risk of CVD and nonstroke events. A combined score of 5 plasma metabolites in this pathway (tryptophan, kynurenine, kynurenic acid, 3-HAA, and quinolinic acid), designed to summarize the pathway in a single variable by maximizing predictiveness of outcome within the present study, was significantly associated with the risk of hard clinical events of CVD. Furthermore, consuming a MedDiet counteracted the harmful effect of an unfavorable metabolite profile in the tryptophan-kynurenine pathway. Lastly, changes in tryptophan may be involved in the cardioprotective benefits of the MedDiet.
The finding that tryptophan is inversely associated with CVD incidence is in line with previous publications (24–26). However, we found no significant associations of the kynurenine:tryptophan ratio with any CVD outcome. Clinical studies have reported that tryptophan degradation, as operationalized by low blood tryptophan concentrations or a high kynurenine:tryptophan ratio, was predictive of CAD status (15), elevated oxidative stress in patients with kidney dysfunction (13), and greater mean carotid artery intima-media thickness (27). Large-scale epidemiologic studies have reported positive associations of tryptophan catabolism (i.e., lower concentrations of tryptophan and higher concentrations of downstream metabolites in the tryptophan-kynurenine pathway) with primary acute coronary events (28), worse prognosis after diagnosis of coronary artery disease (14), and greater risk of mortality from CVD (16).
By using repeated measurements of metabolites, our observation that baseline and 1-y increases in tryptophan are associated with a lower risk of CVD events (especially when stroke was excluded) needs to be compared with the existing literature, which has suggested that tryptophan levels are decreased in stroke patients compared with in control participants (12), and that lower blood tryptophan concentrations are related to greater infarct volume in stroke patients (11) and worse prognosis (29) and faster cognitive decline (30) after stroke. Our results are consistent with an inverse association between tryptophan and CVD, but they may differ from those of previous studies according to the subtype of CVD event, given that we have not found a stronger effect for stroke than for other events. A potential explanation might be that existing publications largely used cross-sectional designs examining stroke patients at baseline, whereas we used a longitudinal design for both the exposure and the outcome and followed disease-free individuals for repeated measurements of metabolites after an intervention and for incident endpoints. Mangge et al. (26) suggested that decreases in tryptophan concentrations may be a consequence of chronic low-grade inflammation rather than a cause of disease. Our findings of an inverse association between changes in tryptophan and nonstroke endpoints would support the inflammation hypothesis for nonstroke events, given the anti-inflammatory properties of the MedDiet. However, further studies are needed to elucidate the differing effects of tryptophan on stroke compared with nonstroke CVD events.
The causal role of tryptophan-kynurenine pathway metabolites in CVD remains poorly understood. It is thought that IFN-γ plays a central role in the activation of IDO and subsequent degradation of tryptophan (4); however, activation of the kynurenine pathway has also been shown to have anti-inflammatory effects (3). Treatment of human peripheral blood mononuclear cells and monocyte-derived macrophages with IFN-γ attenuated the extent of LDL oxidation, and tryptophan degradation in concert with 3-HAA formation was instrumental in this inhibitory effect (31). 3-HAA has also been independently identified as having antiatherogenic properties by regulating lipid metabolism and inflammation (32, 33). Other experimental studies suggest a beneficial effect of IDO on the vasculature. IDO-deficient mice fed high-fat diets showed marked increases in F4/80 and TNF mRNA concentrations, as well as greater hepatic inflammation compared with control mice (34). IDO inhibition also blunted the protective effects of eicosapentaenoic acid in LDL receptor–deficient mice (35). In light of the prevailing theme in experimental studies that IDO-mediated degradation of tryptophan is beneficial, we speculate that activation of the tryptophan-kynurenine pathway may be a compensatory mechanism to, rather than a cause of, inflammation and cardiovascular dysfunction. Furthermore, because most individuals consume adequate amounts of tryptophan (i.e., do not suffer from tryptophan deficiency), the protection conferred by the MedDiet is unlikely to be related to greater availability of tryptophan (36).
The novel finding that a MedDiet may offset the deleterious effects of a high-risk profile in metabolites of the tryptophan-kynurenine pathway also warrants discussion. To our knowledge, this is the first report of an association of tryptophan metabolites with CVD in the context of a nutritional intervention in a large randomized trial. Previous clinical trials have concluded that close adherence to a MedDiet has a beneficial effect on inflammatory markers (37, 38), and other PREDIMED reports have noted that participants randomly assigned to the MedDiet interventions had lower incidence of CVD events than those in the control group, even among those with comparable concentrations of plasma BCAAs (21) or acylcarnitines (20). These publications, in addition to supporting the favorable interaction of the MedDiet with various CVD biomarkers, also point to the need of future experimental studies to clarify the biological mechanisms underlying this effect.
The randomization of dietary interventions at baseline allowed us to study possible mediating effects of tryptophan in the association between MedDiet and the risk of CVD. We found that among the MedDiet+EVOO arm, adjustment for changes in tryptophan attenuated the HRs for CVD. These results suggest a possible role of tryptophan degradation (or preservation) as a mediator in the causal pathway of EVOO consumption and CVD prevention. We acknowledge that our statistical power for detecting interactions is limited. Consumption of both EVOO (39) and nuts (40, 41) has been associated with reduced circulating concentrations of inflammatory biomarkers, although the specific roles of IFN-γ and tryptophan degradation in diets enriched with these foods are not well characterized. Primary results from the PREDIMED trial also reported no striking differences between the 2 MedDiet groups in relation to composite CVD or secondary outcomes (18). However, dietary exposure to walnuts has been characterized by changes in intermediate metabolites of the tryptophan pathway (42). Future lines of inquiry should investigate the biological roles of IFN-γ, tryptophan, and related kynurenine metabolites in diets involving EVOO or nuts.
Strengths of the present study include blood draws at repeated intervals to assess changes in metabolites, the prospective design of the cohort, as well as adjustment for potential confounders related to CVD. Our study also has limitations. First, we did not measure all metabolites of interest in the tryptophan-kynurenine pathway, such as picolinic acid or 3-hydroxykynurenine. Second, we cannot rule out the possibility that concentrations of the metabolites were different between missing and nonmissing cases. Lastly, results from our study among a population of high-risk participants living in the Mediterranean region may not be generalizable to individuals of different demographics.
Our results indicate that 1-y increases in tryptophan are predictive of lower CVD incidence, especially nonstroke incidence, and that a score combining 5 metabolites in the tryptophan-kynurenine pathway is also prospectively associated with clinical cases of CVD. The harmful effects of an unfavorable tryptophan metabolite profile were in part mitigated by consuming a MedDiet. The cardioprotective effect of MedDiets supplemented with EVOO or nuts could be mediated in part by processes associated with changes in plasma tryptophan.
Acknowledgments
EY conducted the analysis and wrote the manuscript; MR-C, MG-F, YZ, and DDW provided code and guidance needed to complete the analysis; and ET, CBC, JS-S, LL, DC, MF, EG-G, JL, RE, ER, MC, FA, DR, LS-M, JVS, FBH, and MAM-G designed the research plan and oversaw the study. All authors read and approved the final version of the manuscript.
Footnotes
Abbreviations used: CAD, coronary artery disease; CVD, cardiovascular disease; EVOO, extra-virgin olive oil; IDO, indoleamine 2,3-dioxygenase; KRS, kynurenine risk score; MedDiet, Mediterranean diet; PREDIMED, Prevención con Dieta Mediterránea; 3-HAA, 3-hydroxyanthranilic acid.
References
- 1.Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation 2004;109(21 Suppl 1):II2–10. [DOI] [PubMed] [Google Scholar]
- 2.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-γ: an overview of signals, mechanisms and functions. J Leukoc Biol 2004;75:163–89. [DOI] [PubMed] [Google Scholar]
- 3.Mühl H, Pfeilschifter J. Anti-inflammatory properties of pro-inflammatory interferon-gamma. Int Immunopharmacol 2003;3:1247–55. [DOI] [PubMed] [Google Scholar]
- 4.Schroecksnadel K, Frick B, Winkler C, Fuchs D. Crucial role of interferon-gamma and stimulated macrophages in cardiovascular disease. Curr Vasc Pharmacol 2006;4:205–13. [DOI] [PubMed] [Google Scholar]
- 5.Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J 1991;5:2516–22. [PubMed] [Google Scholar]
- 6.Schwarcz R. The kynurenine pathway of tryptophan degradation as a drug target. Curr Opin Pharmacol 2004;4:12–7. [DOI] [PubMed] [Google Scholar]
- 7.Nishizuka Y, Hayaishi O. Studies on the biosynthesis of nicotinamide adenine dinucleotide. I. Enzymic synthesis of niacin ribonucleotides from 3-hydroxyanthranilic acid in mammalian tissues. J Biol Chem 1963;238:3369–77. [PubMed] [Google Scholar]
- 8.Chen Y, Guillemin GJ. Kynurenine pathway metabolites in humans: disease and healthy states. Int J Tryptophan Res 2009;2:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernstrom JD, Wurtman RJ. Brain serotonin content: physiological dependence on plasma tryptophan levels. Science 1971;173:149–52. [DOI] [PubMed] [Google Scholar]
- 10.Lovelace MD, Varney B, Sundaram G, Lennon MJ, Lim CK, Jacobs K, Guillemin GJ, Brew BJ. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology 2017;112:373–88. [DOI] [PubMed] [Google Scholar]
- 11.Darlington LG, Mackay GM, Forrest CM, Stoy N, George C, Stone TW. Altered kynurenine metabolism correlates with infarct volume in stroke. Eur J Neurosci 2007;26:2211–21. [DOI] [PubMed] [Google Scholar]
- 12.Ormstad H, Verkerk R, Aass HC, Amthor KF, Sandvik L. Inflammation-induced catabolism of tryptophan and tyrosine in acute ischemic stroke. J Mol Neurosci 2013;51:893–902. [DOI] [PubMed] [Google Scholar]
- 13.Pawlak K, Domaniewski T, Mysliwiec M, Pawlak D. The kynurenines are associated with oxidative stress, inflammation and the prevalence of cardiovascular disease in patients with end-stage renal disease. Atherosclerosis 2009;204:309–14. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen ER, Svingen GF, Schartum-Hansen H, Ueland PM, Ebbing M, Nordrehaug JE, Igland J, Seifert R, Nilsen RM, Nygard O. Urinary excretion of kynurenine and tryptophan, cardiovascular events, and mortality after elective coronary angiography. Eur Heart J 2013;34:2689–96. [DOI] [PubMed] [Google Scholar]
- 15.Wirleitner B, Rudzite V, Neurauter G, Murr C, Kalnins U, Erglis A, Trusinskis K, Fuchs D. Immune activation and degradation of tryptophan in coronary heart disease. Eur J Clin Invest 2003;33:550–4. [DOI] [PubMed] [Google Scholar]
- 16.Zuo H, Ueland PM, Ulvik A, Eussen SJPM, Vollset SE, Nygård O, Midttun Ø, Theofylaktopoulou D, Meyer K, Tell GS. Plasma biomarkers of inflammation, the kynurenine pathway, and risks of all-cause, cancer, and cardiovascular disease mortality: the Hordaland Health Study. Am J Epidemiol 2016;183:249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.PREDIMED. Mediterranean diet [Internet]. [cited 2016 Nov 24]. Available from: http://www.predimed.es.
- 18.Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, et al. Primary prevention of cardiovascular disease with a mediterranean diet. N Engl J Med 2013;368:1279–90. [DOI] [PubMed] [Google Scholar]
- 19.Martínez-Gonzalez MÁ, Corella D, Salas-Salvadó J, Ros E, Covas MI, Fiol M, Wärnberg J, Arós F, Ruiz-Gutiérrez V, Lamuela-Raventós RM, et al. Cohort profile: design and methods of the PREDIMED study. Int J Epidemiol 2012;41:377–85. [DOI] [PubMed] [Google Scholar]
- 20.Guasch-Ferré M, Zheng Y, Ruiz-Canela M, Hruby A, Martínez-González MA, Clish CB, Corella D, Estruch R, Ros E, Fitó M, et al. Plasma acylcarnitines and risk of cardiovascular disease: effect of Mediterranean diet interventions. Am J Clin Nutr 2016;103:1408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruiz-Canela M, Toledo E, Clish CB, Hruby A, Liang L, Salas-Salvadó J, Razquin C, Corella D, Estruch R, Ros E, et al. Plasma branched-chain amino acids and incident cardiovascular disease in the PREDIMED trial. Clin Chem 2016;62:582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol 1999;52:1165–72. [DOI] [PubMed] [Google Scholar]
- 23.Lee EW, Wei LJ, Amato DA, Leurgans S. Cox-type regression analysis for large numbers of small groups of correlated failure time observations. In: Klein JP, Goel PK, editors. Survival analysis: state of the art. Dordrecht (Netherland): Springer Netherlands; 1992. p. 237–47. [Google Scholar]
- 24.Polyzos KA, Ketelhuth DF. The role of the kynurenine pathway of tryptophan metabolism in cardiovascular disease. An emerging field. Hamostaseologie 2015;35:128–36. [DOI] [PubMed] [Google Scholar]
- 25.Murr C, Grammer TB, Kleber ME, Meinitzer A, Marz W, Fuchs D. Low serum tryptophan predicts higher mortality in cardiovascular disease. Eur J Clin Invest 2015;45:247–54. [DOI] [PubMed] [Google Scholar]
- 26.Mangge H, Reininghaus E, Fuchs D. Role of kynurenine pathway in cardiovascular diseases. In: Mittal S, editor. Targeting the broadly pathogenic kynurenine pathway. Cham (Switzerland): Springer International Publishing; 2015. p. 133–43. [Google Scholar]
- 27.Kato A, Suzuki Y, Suda T, Suzuki M, Fujie M, Takita T, Furuhashi M, Maruyama Y, Chida K, Hishida A. Relationship between an increased serum kynurenine/tryptophan ratio and atherosclerotic parameters in hemodialysis patients. Hemodial Int 2010;14:418–24. [DOI] [PubMed] [Google Scholar]
- 28.Sulo G, Vollset SE, Nygard O, Midttun O, Ueland PM, Eussen SJ, Pedersen ER, Tell GS. Neopterin and kynurenine-tryptophan ratio as predictors of coronary events in older adults, the Hordaland Health Study. Int J Cardiol 2013;168:1435–40. [DOI] [PubMed] [Google Scholar]
- 29.Brouns R, Verkerk R, Aerts T, De Surgeloose D, Wauters A, Scharpe S, De Deyn PP. The role of tryptophan catabolism along the kynurenine pathway in acute ischemic stroke. Neurochem Res 2010;35:1315–22. [DOI] [PubMed] [Google Scholar]
- 30.Stone TW, Forrest CM, Stoy N, Darlington LG. Involvement of kynurenines in Huntington’s disease and stroke-induced brain damage. J Neural Transm 2012;119:261–74. [DOI] [PubMed] [Google Scholar]
- 31.Christen S, Thomas SR, Garner B, Stocker R. Inhibition by interferon-gamma of human mononuclear cell-mediated low density lipoprotein oxidation. Participation of tryptophan metabolism along the kynurenine pathway. J Clin Invest 1994;93:2149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Ovchinnikova O, Jonsson A, Lundberg AM, Berg M, Hansson GK, Ketelhuth DF. The tryptophan metabolite 3-hydroxyanthranilic acid lowers plasma lipids and decreases atherosclerosis in hypercholesterolaemic mice. Eur Heart J 2012;33:2025–34. [DOI] [PubMed] [Google Scholar]
- 33.Krause D, Suh H-S, Tarassishin L, Cui QL, Durafourt BA, Choi N, Bauman A, Cosenza-Nashat M, Antel JP, Zhao M-L, et al. The tryptophan metabolite 3-hydroxyanthranilic acid plays anti-inflammatory and neuroprotective roles during inflammation: role of hemeoxygenase-1. Am J Pathol 2011;179:1360–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagano J, Shimizu M, Hara T, Shirakami Y, Kochi T, Nakamura N, Ohtaki H, Ito H, Tanaka T, Tsurumi H, et al. Effects of indoleamine 2,3-dioxygenase deficiency on high-fat diet-induced hepatic inflammation. PloS One 2013;8:e73404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakajima K, Yamashita T, Kita T, Takeda M, Sasaki N, Kasahara K, Shinohara M, Rikitake Y, Ishida T, Yokoyama M, et al. Orally administered eicosapentaenoic acid induces rapid regression of atherosclerosis via modulating the phenotype of dendritic cells in LDL receptor-deficient mice. Arterioscler Thromb Vas Biol 2011;31:1963–72. [DOI] [PubMed] [Google Scholar]
- 36.Moehn S, Pencharz PB, Ball RO. Lessons learned regarding symptoms of tryptophan deficiency and excess from animal requirement studies. J Nutr 2012;142:2231S–5S. [DOI] [PubMed] [Google Scholar]
- 37.Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: the Attica study. J Am Coll Cardiol 2004;44:152–8. [DOI] [PubMed] [Google Scholar]
- 38.Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, D’Armiento M, D’Andrea F, Giugliano D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA 2004;292:1440–6. [DOI] [PubMed] [Google Scholar]
- 39.Cicerale S, Lucas LJ, Keast RSJ. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr Opin Biotechnol 2012;23:129–35. [DOI] [PubMed] [Google Scholar]
- 40.Salas-Salvadó J, Casas-Agustench P, Murphy MM, López-Uriarte P, Bulló M. The effect of nuts on inflammation. Asia Pac J Clin Nutr 2008;17 Suppl 1:333–6. [PubMed] [Google Scholar]
- 41.Yu Z, Malik VS, Keum N, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS, Bao Y. Associations between nut consumption and inflammatory biomarkers. Am J Clin Nutr 2016;104:722–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Aloy M, Llorach R, Urpi-Sarda M, Tulipani S, Estruch R, Martinez-Gonzalez MA, Corella D, Fito M, Ros E, Salas-Salvado J, et al. Novel multimetabolite prediction of walnut consumption by a urinary biomarker model in a free-living population: the PREDIMED study. J Proteome Res 2014;13:3476–83. [DOI] [PubMed] [Google Scholar]