Abstract
Background: The melanocortin-4 receptor (MC4R) plays a pivotal role in the regulation of appetite and eating behavior. Variants in the MC4R gene have been related to appetite and obesity.
Objective: We aimed to examine whether weight-loss diets modified the effect of the “obesity-predisposing” MC4R genotype on appetite-related measures in a randomized controlled trial.
Methods: A total of 811 overweight and obese subjects [25 ≤ body mass index (BMI; kg/m2) ≤ 40] aged 30–70 y were included in the 2-y POUNDS Lost (Preventing Overweight Using Novel Dietary Strategies) trial. We genotyped MC4R rs7227255 in 735 overweight adults and assessed appetite-related characteristics, including craving, fullness, hunger, and prospective consumption, as well as a composite appetite score. We examined the effects of the genotype-by-weight-loss diet intervention interaction on appetite variables by using general linear models in both the whole population and in white participants only.
Results: We found that dietary protein intake (low compared with high: 15% of energy compared with 25% of energy, respectively) significantly modified MC4R genetic effects on changes in appetite score and craving (P-interaction = 0.03 and 0.02, respectively) at 2 y, after adjustment for age, sex, ethnicity, baseline BMI, weight change, and baseline perspective phenotype. The obesity-predisposing A allele was associated with a greater increase in overall appetite score (β = 0.10, P = 0.05) and craving (β = 0.13, P = 0.008) compared with the non-A allele among participants who consumed a high-protein diet. MC4R genotype did not modify the effects of fat or carbohydrate intakes on appetite measures. Similar interaction patterns were observed in whites.
Conclusion: Our data suggest that individuals with the MC4R rs7227255 A allele rather than the non-A allele might experience greater increases in appetite and food craving when consuming a high-protein weight-loss diet. This trial was registered at clinicaltrials.gov as NCT00072995.
Keywords: MC4R genotype, appetite, gene-diet interaction, weight-loss trial, protein diet, food craving
Introduction
The melanocortin-4 receptor (MC4R)12 gene, which is primarily expressed in the brain (1), participates in complex neurohormonal pathways with reciprocal effects on energy intake and expenditure (2–5), primarily through its regulatory effect on appetite and satiety (6). MC4R is also a key factor in regulating body adiposity (7).
A common variant in MC4R, rs7227255, was found to be associated with BMI in recent genomewide association studies (8), probably through affecting regulation of the central nervous system on appetite. In our previous analysis, we found that MC4R genotype was related to dietary macronutrient intakes (3). In addition, an animal study showed that a high-fat diet upregulated MC4R gene expression in obese Berlin mice (9). Moreover, in response to a high-fat diet, MC4R−/− mice exhibited hyperphagia and accelerated weight gain compared with wild-type mice (10). Interestingly, a recent animal study examined the effects of high-protein or high-fat diets on appetite regulation and found that high-protein diets reduced food intake and hypothalamic gene expression of appetite-related factors such as MC4R (11). Therefore, we hypothesized that MC4R genotype might interact with dietary macronutrient intakes in affecting appetite measures and adiposity.
In the present study, we aimed to examine the effect of the obesity-predisposing MC4R genotype (8) on appetite measures in a 2-y dietary intervention study, the Preventing Overweight Using Novel Dietary Strategies (POUNDS Lost) trial. In particular, we assessed interactions between MC4R genotype and weight-loss diets varying in macronutrient intake on changes in appetite during an intervention.
Methods
The POUNDS Lost trial.
The POUNDS Lost trial (www.clinicaltrials.gov; NCT00072995), a 2-y randomized clinical trial for weight loss, was conducted in Boston, Massachusetts and Baton Rouge, Louisiana in 2004–2007. All of the participants provided informed consent, and the trial was approved and monitored by the Human Subjects Committee at the Harvard School of Public Health, Brigham and Women’s Hospital, Pennington Biomedical Research Center, and the National Heart, Lung, and Blood Institute. Detailed information on the study design and methods was provided previously (12). A total of 811 overweight and obese subjects [25 ≤ BMI (kg/m2) ≤ 40] aged 30–70 y were randomly assigned to receive 1 of 4 diets in which the target percentages of energy intake derived from fat, protein, and carbohydrate in the 4 diets were 20%, 15%, and 65%; 20%, 25%, and 55%; 40%, 15%, and 45%; and 40%, 25%, and 35%, respectively. After 24 mo, 79.5% of the participants (n = 645) completed the trial. Among those participants who had genotyping data (n = 735), 594 (80.9%) completed the trial. In the trial, the low-fat diets (20% of energy) were considered as high-carbohydrate diets (≥55% of energy), whereas the high-fat diets (40% of energy) can be thought of as low-carbohydrate diets (≤45% of energy). Participants from the 2 low-protein groups and the 2 high-protein groups were combined in order to compare the low-protein diets (15% of energy) with the high-protein diets (25% of energy).
Anthropometric measures and dietary adherence.
Body weight was measured by using calibrated hospital scales in the morning before breakfast and after voiding, with participants wearing a hospital gown, on 2 nonconsecutive days at baseline and at 6 and 24 mo and on a single day at 12 and 18 mo. The mean number of days between measurements was 5 for baseline, 10 for 6 mo, and 9 for 24 mo. Waist circumference was also measured at these visits by using a nonstretchable tape measure, 4 cm above the iliac crest. Height was measured at baseline. BMI was calculated as weight divided by height squared (kg/m2).
To assess dietary adherence across the intervention, dietary intake was assessed in a random sample of 50% of the participants by a review of the 5-d diet record at baseline and by 24-h recall during a telephone interview on 3 nonconsecutive days at 6 and 24 mo. Measurements of oxygen consumption and carbon dioxide production began after a 30-min rest and continued for 30 min. The respiratory quotient (RQ) and urinary nitrogen (grams) were measured at 6 and 24 mo of the intervention. RQ was computed as the quantity of carbon dioxide produced divided by the amount of oxygen consumed. The within-individual CV was 3.2%. The nonprotein RQ is a biomarker of carbohydrate intake and was computed from the RQ and urinary nitrogen measured in a 24-h sample collected contemporaneously. Urine nitrogen was a biomarker for the validation of dietary protein intake. A 24-h urine sample was collected at baseline and at 6 mo and 2 y for urea assessment (as a biomarker of protein intake) and was measured at the Core Laboratory at Pennington. Differences in macronutrient intake and these biomarkers among the intervention groups suggested that participants modified their intakes of macronutrients in the direction of the goals, although the targets were not fully achieved (12). Therefore, RQ and urinary nitrogen were used to assess dietary compliance.
Appetite measures.
Appetites of participants at baseline and at 6 and 24 mo were assessed by using a motivation-to-eat questionnaire (13, 14). The reliability and validity of visual analog scales (VASs) have been previously discussed (15). Participants completed the questionnaires in a fasting state before breakfast, between 0700 and 0900, on a weekly basis during the center-based feeding portion of this study. Participants remained seated throughout the experimental sessions. The questionnaire included 4 questions or scales: 1) How strong is your desire to eat? (“very weak” to “very strong” to assess craving); 2) How hungry do you feel? (“not hungry at all” to “as hungry as I’ve ever felt” to assess hunger); 3) How full do you feel? (“not full at all” to “very full” to assess fullness); and 4) How much food do you think you could eat? (“nothing at all” to “a large amount” to assess prospective consumption).
Each VAS consisted of a 100-mm line anchored at the beginning and end by opposing statements. When completing the questionnaire, participants were instructed to rate the intensity of their current state (i.e., “at that moment”) for a number of appetite markers by placing a cursor and clicking the mouse on a computerized 100-increment line representing the continuum from “not at all” to “extremely.” Scores in each domain (craving, hunger, fullness, and prospective consumption) were determined by measuring the distance (in millimeters) from the left starting point of the line to the intersection of the “X.” By using established appetite calculation methods previously published (13, 14), an average appetite score including responses to the 4 questions on motivation to eat was calculated at each time of measurement as appetite score = [craving + (100 − fullness) + prospective consumption + hunger]/4 (13, 14).
Genotyping.
DNA was extracted from the buffy coat fraction of centrifuged blood by using the QIAmp Blood Kit (Qiagen). The single nucleotide polymorphism (SNP) rs7227255 was selected because it was identified as the top variant of the MC4R locus for BMI (8) and because MC4R variants play a functional role in food intake and appetite (2). The SNP was genotyped successfully in 735 of 811 total participants by using the OpenArray SNP Genotyping System (BioTrove). The genotype success rate was 99% in available DNA samples. Replicated quality-control samples (10%) were included in every genotyping plate, with >99% concordance (16). The allele frequency in 2 major ethnic groups (white and black) was compatible with Hardy-Weinberg equilibrium (P > 0.05).
Statistical analysis.
The primary outcomes in the present analysis were changes in appetite-related measures such as appetite score, craving, fullness, prospective consumption, and hunger. The change in each measure was calculated as the difference between the measure at 24 mo of intervention and baseline (final – baseline). We compared baseline participant characteristics across genotypes by using general linear models (PROC GLM; SAS Institute) for continuous variables and chi-square tests (PROC FREQ; SAS Institute) for categorical variables in 735 participants with genetic information. To evaluate the effect of genotype, we compared the changes in the primary outcomes, biomarkers of adherence to intervention and reported nutrient intakes across genotype at 6 and 24 mo, by using general linear models.
To test for effect modification of the intervention by genotype, we tested the interactions between MC4R rs7227255 genotype and the intervention diets (2-factorial comparisons: low- compared with high-protein and high- compared with low-fat diets) on changes in appetite measures over 24 mo after adjustments for age, sex, ethnicity, baseline BMI, weight change (in appetite-related measures analyses), and the baseline value for the respective outcome, in general linear models in both the whole population and in white participants only. We also tested the gene × diet interaction in whites alone. Linear mixed models were used to test genetic associations with the trajectory of changes in appetite-related measures according to diet groups. Time was treated as a repeated measurement factor, and genotype × time interaction terms were included in the mixed models.
All reported P values are 2-sided, with P ≤ 0.05 considered to be significant. We used Quanto 1.2.4 (University of Southern California) to estimate the detectable effect sizes of genotype × intervention interactions. The study had 80% power to detect gene × intervention interaction effect sizes of 2.6 kg for weight loss, 2.5 mm for changes in appetite score, 2.3 mm for changes in craving, 4.1 mm for changes in fullness, 5.5 mm for changes in prospective consumption, and 6.7 mm for changes in hunger at 6 mo under an additive genetic model. Statistical analyses were performed with SAS version 9.1 (SAS Institute).
Results
Baseline characteristics of participants by MC4R rs7227255 genotype.
Baseline characteristics of participants according to MC4R rs7227255 genotype are presented in Table 1. The minor (effect) allele (A) frequency was 0.11 in the genotyped population. The genotype frequency differed significantly between sexes and ethnicities, with more men than women and more blacks than other race/ethnicities having a copy of the minor A allele. There were no significant differences between MC4R genotypes in other baseline characteristics or in changes in adiposity measures during the 2-y diet intervention.
TABLE 1.
Baseline characteristics of the 735 overweight and obese adults according to MC4R rs7227255 genotype1
|
MC4R rs7227255 genotype |
|||
| Characteristics | GG (n = 663) | GA+AA (n = 72) | P2 |
| Age, y | 51.3 ± 9.2 | 49.1 ± 9.0 | 0.66 |
| Race, n (%) | <0.0001 | ||
| White | 561 (84.6) | 29 (40.3) | |
| Black | 70 (10.6) | 40 (55.6) | |
| Hispanic | 23 (3.5) | 2 (2.8) | |
| Asian or other | 9 (1.4) | 1 (1.4) | |
| Female, n (%) | 398 (60.0) | 52 (72.0) | 0.04 |
| Weight, kg | 93.4 ± 15.6 | 91.7 ± 15.1 | 0.26 |
| BMI, kg/m2 | 32.7 ± 3.8 | 32.7 ± 4.1 | 0.28 |
| Dietary intake/d | |||
| Total energy, kcal | 1960 ± 545 | 1979 ± 693 | 0.26 |
| Carbohydrate, % of energy | 44 ± 8 | 47 ± 6 | 0.31 |
| Fat, % of energy | 37 ± 6 | 36 ± 6 | 0.91 |
| Protein, % of energy | 18 ± 3 | 17 ± 3 | 0.14 |
| Biomarkers of adherence | |||
| Respiratory quotient | 0.84 ± 0.04 | 0.84 ± 0.05 | 0.24 |
| Urinary nitrogen, g | 12.3 ± 4.4 | 11.4 ± 4.3 | 0.96 |
Values are means ± SDs unless otherwise indicated. MC4R, melanocortin-4 receptor.
P values were calculated by using the chi-square test for categorical variables and F tests after adjustment for sex (except for sex), age (except for age), and race (except for race).
Dietary adherence by MC4R rs7227255 genotype.
Table 2 shows dietary intake and adherence markers (urinary nitrogen and RQ) of the participants by genotype at 6 and 24 mo of the intervention. There were no significant differences between genotypes in mean values of nutrient intakes or biomarkers of adherence at either time point, except for total energy intake at 6 mo, which was slightly lower in carriers of the A allele than in noncarriers (P = 0.04). Differences in macronutrient intake between the intervention groups suggested that participants modified their intakes of macronutrients in the direction of the goals, although the targets were not fully achieved (12).
TABLE 2.
Nutrient intakes and biomarkers of weight-loss diet adherence in overweight and obese adults according to MC4R rs7227255 genotype at 6 and 24 mo1
| 6 mo |
24 mo |
|||||
| GG | GA+AA | P | GG | GA+AA | P | |
| Dietary intake/d2 | ||||||
| Energy, kcal | 1607 ± 479 | 1528 ± 478 | 0.04 | 1738 ± 810 | 1511 ± 583 | 0.60 |
| Carbohydrate, % of energy | 50 ± 11 | 49 ± 10 | 0.33 | 54 ± 9 | 56 ± 10 | 0.06 |
| Fat, % of energy | 30 ± 8 | 31 ± 9 | 0.95 | 29 ± 7 | 29 ± 6 | 0.50 |
| Protein, % of energy | 20 ± 4 | 20 ± 5 | 0.20 | 18 ± 5 | 18 ± 5 | 0.23 |
| Biomarkers of adherence | ||||||
| Respiratory quotient3 | 0.84 ± 0.04 | 0.83 ± 0.04 | 0.65 | 0.84 ± 0.04 | 0.83 ± 0.05 | 0.18 |
| Urinary nitrogen,4 g | 11.7 ± 4.5 | 12.0 ± 4.5 | 0.10 | 10.4 ± 4.8 | 11.8 ± 5.0 | 0.74 |
Values are means ± SEs. Generalized linear models were used to compare biomarkers of adherence and nutrient intakes across rs7227255 groups at 6 and 24 mo. MC4R, melanocortin-4 receptor.
Data were included for 294 participants with the GG genotype and 33 participants with the GA+AA genotype at 6 mo and for 154 participants with the GG genotype and 13 participants with the GA+AA genotype at 24 mo.
Data were included for 537 participants with the GG genotype and 55 participants with the GA+AA genotype at 6 mo and for 424 participants with the GG genotype and 35 participants with the GA+AA genotype at 24 mo.
Data were included for 487 participants with the GG genotype and 49 participants with the GA+AA genotype at 6 mo and for 344 participants with the GG genotype and 25 participants with the GA+AA genotype at 24 mo.
Effects of the MC4R rs7227255 genotype on changes in appetite measures and body size.
There were no significant differences between MC4R genotype at baseline or between 6- and 24-mo changes in appetite-related measures during the intervention, except for the 24-mo change in craving, which was significantly greater in A allele carriers than in noncarriers (P = 0.01). We did not observe significant associations of the MC4R genotype with changes in body weight or waist circumference at 6 or 24 mo. However, we observed a more prolonged effect on loss in waist circumference than on weight loss (Supplemental Table 1).
Gene × diet interactions on changes in appetite measures.
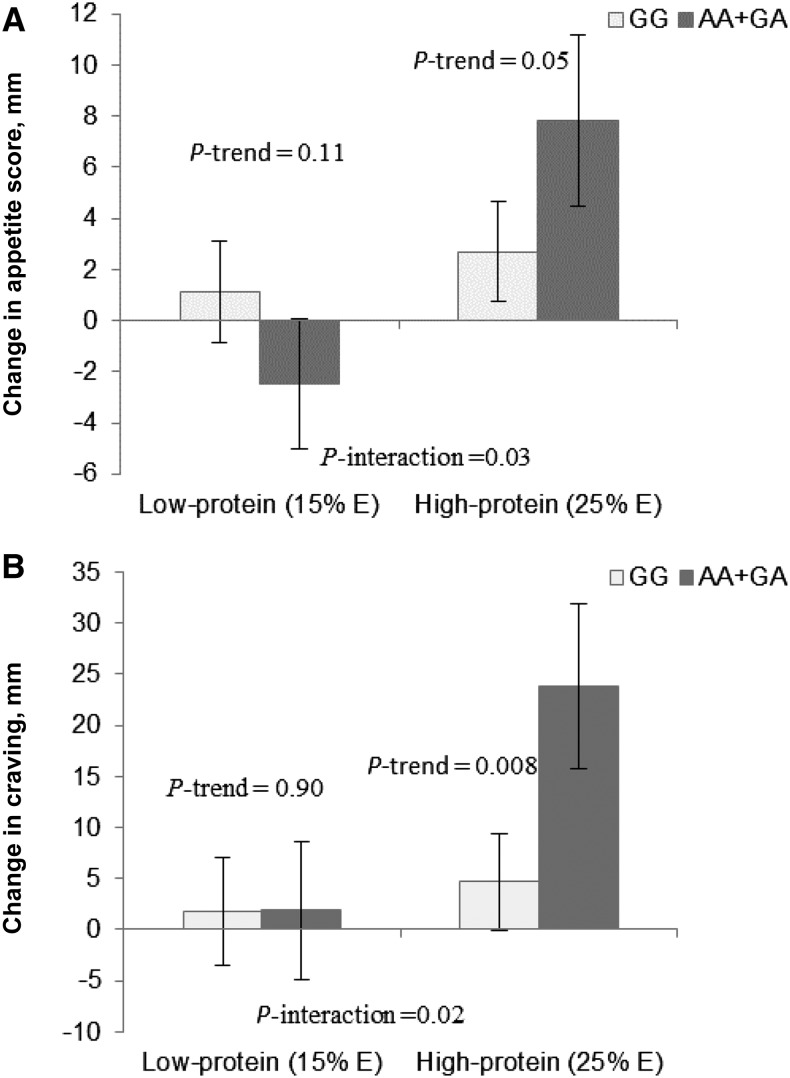
Dietary protein intake (low compared with high: 15% of energy compared with 25% of energy) significantly modified the effect of MC4R genotype on changes in appetite score and craving at 24 mo, after adjustment for age, sex, ethnicity, baseline BMI, weight loss, and baseline values for the respective outcome (P-interaction = 0.03 and 0.02, respectively). A marginal interaction for hunger was also observed (P-interaction = 0.06). The A allele was associated with a greater increase in appetite score (β = 0.10, P = 0.05) (Figure 1A) and craving (β = 0.13, P = 0.008) (Figure 1B) among the participants in the high-protein diet intervention groups, whereas opposite genotype effects were observed in the low-protein diet intervention groups. The MC4R rs7227255 SNP did not modify the associations of fat or carbohydrate intakes on the appetite measures. Further adjustment for dietary fat intake in the model yielded similar results. We did not observe interactions between diets and MC4R genotype on fullness or prospective consumption.
FIGURE 1.
Effect of the MC4R rs72272552 genotype on 24-mo changes in appetite measures in response to protein intake in overweight and obese adults. Values are means ± SEs. P values were adjusted for age, sex, ethnicity, baseline BMI, weight loss, and baseline values for respective outcomes. Data included 333 (GG) and 40 (AA+GA) overweight and obese adults in the low-protein (15% of energy) diet group and 330 (GG) and 32 (AA+GA) overweight and obese adults in the high-protein (25% of energy) diet group at 24 mo (n = 735). Appetite score (A) (“0 = very weak” to “100 = very strong”) = [craving + (100 − fullness) + prospective consumption + hunger]/4. The question: “How strong is your desire to eat? (‘0 = very weak’ to ‘100 = very strong’)” was used to assess craving (B). MC4R, melanocortin-4 receptor; % E, percentage of energy.
In subgroup analyses in white participants, we observed similar interactions between MC4R rs7227255 and dietary protein on changes in appetite score (P-interaction = 0.04) and craving (P-interaction = 0.02), whereas the interaction for changes in hunger (P = 0.06) did not reach significance (Supplemental Table 2). We did not observe significant interactions between MC4R rs7227255 and fat or carbohydrate intakes on weight loss or changes in appetite measures in the total sample or in white participants only (all P-interaction > 0.10).
Trajectory of changes in appetite measures by MC4R rs7227255 genotype in response to weight-loss diets.
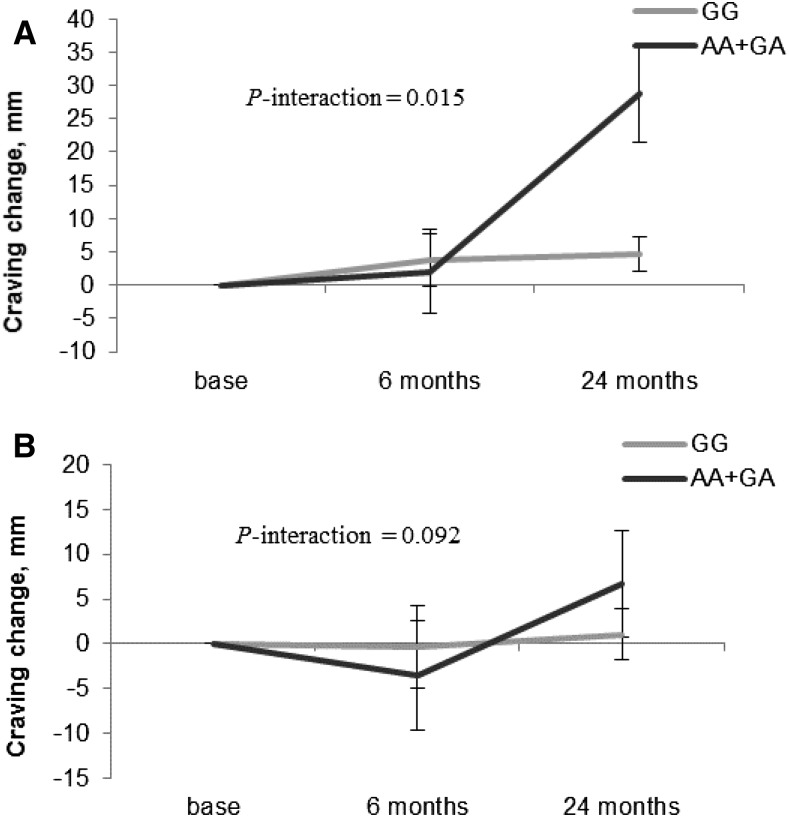
We observed significant interactions between MC4R rs7227255 genotype and intervention time on 24-mo changes in craving in the high-protein diet group (P-interaction < 0.05; Figure 2A). Participants with the MC4R rs7227255 A allele showed greater increases in craving than those without the A allele across the 24-mo intervention in the high-protein diet group. However, we did not find any significant differences in appetite measures between genotype in the low-protein diet group (Figure 2B). No significant gene × time interaction was observed for changes in other appetite measures (all P-interaction > 0.05).
FIGURE 2.
Genotype effect of the MC4R rs7227255 on trajectory of changes in craving in the high-protein (A) and the low-protein (B) diet groups over 24 mo in overweight and obese adults. Linear mixed models were used to test genetic associations with the trajectory of changes in appetite-related measures according to diet groups. Time was treated as a repeated measurement factor, and genotype × time interaction terms were included in the mixed models. Values are means ± SEs. P values were adjusted for age, sex, ethnicity, baseline BMI, and baseline values for craving. Data included 408, 324, and 235 overweight and obese adults in the low-protein (15% of energy) diet group and 403, 332, and 259 overweight and obese adults in the high-protein (25% of energy) diet group for craving at baseline and at 6 and 24 mo, respectively. The question “How strong is your desire to eat? (‘0 = very weak’ to ‘100 = very strong’)” was used to assess craving. MC4R, melanocortin-4 receptor.
Discussion
In a 2-y randomized weight-loss intervention trial, we found that the MC4R genotype was significantly associated with 24-mo change in food craving during the intervention. The change in craving at 24 mo was significantly greater in A allele carriers than in noncarriers. We further found significant interactions between MC4R rs7227255 and protein intake in relation to changes in appetite and craving. Carriers of the A allele exhibited greater increases in appetite and craving when assigned to a high-protein weight-loss diet.
Compelling evidence suggests that MC4R is a key regulator of energy balance through functionally divergent central melanocortin neuronal pathways (17), as well as a regulator of appetite and satiety (2, 18, 19). MC4R coding mutations cause monogenic early-onset obesity through appetite deregulation (20). A report by Qi et al. (3) observed that this MC4R variant was associated with higher intakes of total energy and dietary fat. Genomewide association studies found that the MC4R variant rs7227255 was associated with BMI (8), and rs7227255 located in the intergenic region, a noncoding region, is in perfect linkage disequilibrium (r2 = 1) with a relatively rare MC4R missense variant (rs2229616, p.Val103Ile; minor allele frequency = 1.7%) that has been associated with BMI (21). In the present study, we found that MC4R rs7227255 genotype was associated with long-term changes in food craving. Our observations are in line with previous reports showing that the MC4R variant was associated with emotional eating and food cravings (5). Other studies have observed that MC4R interacts with the dopamine system, which is highly expressed in the brain’s reward pathways and plays a key role in affecting eating behavior such as overeating (22, 23).
Intriguingly, we found that protein intake modified the effect of MC4R rs7227255 on appetite measures, suggesting that the A allele was related to greater increases in appetite and food craving in response to a hypocaloric and high-protein diet. Our results are supported by a recent study that found that obese people with MC4R mutant genotypes showed different brain responses to food images than obese individuals with normal MC4R genotypes (24). In addition, central dopaminergic circuitry also plays an important role in feeding and satiety (25). There are extensive interactions between dopamine and the melanocortin system. The MC4R antagonist stimulated and inhibited, respectively, dopamine metabolite concentrations (26). These findings may also help us understand the underlying mechanisms through which the MC4R variant alters food intake (27). Furthermore, other studies have suggested that MC4R regulates metabolic and behavioral responses to high-protein and low-fat intake (10). Protein intake is known to increase secretion of gastrointestinal hormones, which are regulated by the MC4R variant (28), and to induce satiety (29), and thereby to sustain reductions in appetite and ad libitum caloric intake (30) and to increase weight loss and prevent weight regain (31). In addition, dietary protein regulates MC4R gene expression (32). Taken together, we assume that high-protein diets may influence appetite, food cravings, and body weight through differentially affecting gene expression according to various MC4R genotypes. Furthermore, we acknowledge that participants had difficulty achieving the goals of the macronutrient intake of their assigned group, and the differences in protein intakes and in urinary nitrogen between the low- and high-protein groups at 2 y were marginally significant. However, the difference in protein intake was significant during the majority of the intervention course, which was the driving force of the observed interaction. Therefore, the interaction might be stronger if such differences in intake remained as substantial at 2 y as they had been during the intervention.
To the best of our knowledge, this was the first study to investigate interactions between this MC4R genetic variant and weight-loss diets on appetite and food craving in the context of a large, long-term randomized trial. Our findings provide new insights into the role of MC4R genotype in determining appetite and food intake. However, several limitations need to be considered. Because the adherence to various diets declined after 6 mo, the power to detect a long-term genotype effect in response to the real difference in macronutrient intake among the diet groups was reduced. In addition, it is difficult to distinguish which macronutrient plays the key role behind the observed interactions because increased carbohydrate intake reflects decreased fat intake. In addition, we used a previously validated VAS to assess appetite, and we acknowledge that measurement errors might have affected our results; the overweight and obese participants might have underreported their appetite. This might lead to an artifactual positive association between MC4R rs7227255 and appetite and food craving indexes. Therefore, analyses that use more accurate measurement of appetite-related outcomes are needed to validate our findings. Furthermore, we only examined the common variant rs7227255 in the MC4R gene for BMI and acknowledge that a comprehensive analysis of variants capturing the overall variance of the gene was needed. Finally, most of the participants (80%) in our study were white and the minor allele frequency of MC4R rs7227255 varied across ethnicities. Further studies are needed to determine whether our findings are generalizable to other ethnic groups, although our results in whites were similar to those in the total study sample. Even though randomized clinical trials are thought to be the best model to test gene × environment interactions, we cannot exclude the possibility of false-positive findings, and replication in diverse populations is needed to verify our findings.
In conclusion, we found that MC4R rs7227255 was associated with 24-mo change in food craving during an intervention and that MC4R rs7227255 modified appetite and food craving in response to weight-loss diets in a 24-mo randomized trial. Individuals with the MC4R rs7227255 A allele might expect to experience greater increases in appetite and food craving than those without this allele when choosing a high-protein weight-loss diet. These findings provide supportive evidence for the notion of personalized nutrition in the prevention of obesity.
Acknowledgments
TH, YZ, and LQ contributed to the study concept and design, acquisition of data, analysis and interpretation of the data, drafting of the manuscript, and critical revision of the manuscript; YZ, AH, GAB, and FMS contributed to the study concept and design and to critical revision of the manuscript; TH, YZ, and YS contributed to statistical analysis; DAW, GAB, and FMS were involved in the collection of the data, analysis of the data, and funding of the initial project; FMS and LQ contributed to administration, material support, and study supervision; and LQ is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: MC4R, melanocortin-4 receptor; POUNDS Lost, Preventing Overweight Using Novel Dietary Strategies; RQ, respiratory quotient; SNP, single nucleotide polymorphism; VAS, visual analog scale.
References
- 1.Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci 2003;6:736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cole SA, Butte NF, Voruganti VS, Cai G, Haack K, Kent JW Jr, Blangero J, Comuzzie AG, McPherson JD, Gibbs RA. Evidence that multiple genetic variants of MC4R play a functional role in the regulation of energy expenditure and appetite in Hispanic children. Am J Clin Nutr 2010;91:191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qi L, Kraft P, Hunter DJ, Hu FB. The common obesity variant near MC4R gene is associated with higher intakes of total energy and dietary fat, weight change and diabetes risk in women. Hum Mol Genet 2008;17:3502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stutzmann F, Cauchi S, Durand E, Calvacanti-Proenca C, Pigeyre M, Hartikainen AL, Sovio U, Tichet J, Marre M, Weill J, et al. Common genetic variation near MC4R is associated with eating behaviour patterns in European populations. Int J Obes (Lond) 2009;33:373–8. [DOI] [PubMed] [Google Scholar]
- 5.Yilmaz Z, Davis C, Loxton NJ, Kaplan AS, Levitan RD, Carter JC, Kennedy JL. Association between MC4R rs17782313 polymorphism and overeating behaviors. Int J Obes (Lond) 2015;39:114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sartin JL, Marks DL, McMahon CD, Daniel JA, Levasseur P, Wagner CG, Whitlock BK, Steele BP. Central role of the melanocortin-4 receptors in appetite regulation after endotoxin. J Anim Sci 2008;86:2557–67. [DOI] [PubMed] [Google Scholar]
- 7.Lubrano-Berthelier C, Cavazos M, Le Stunff C, Haas K, Shapiro A, Zhang S, Bougneres P, Vaisse C. The human MC4R promoter: characterization and role in obesity. Diabetes 2003;52:2996–3000. [DOI] [PubMed] [Google Scholar]
- 8.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, Lango Allen H, Lindgren CM, Luan J, Magi R, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 2010;42:937–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Widiker S, Karst S, Wagener A, Brockmann GA. High-fat diet leads to a decreased methylation of the Mc4r gene in the obese BFMI and the lean B6 mouse lines. J Appl Genet 2010;51:193–7. [DOI] [PubMed] [Google Scholar]
- 10.Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci 2001;4:605–11. [DOI] [PubMed] [Google Scholar]
- 11.McConn BR, Matias J, Wang G, Cline MA, Gilbert ER. Dietary macronutrient composition affects hypothalamic appetite regulation in chicks. Nutr Neurosci 2016. Aug 16 (Epub ahead of print; DOI: 10.1080/1028415X.2016.1219103). [DOI] [PubMed] [Google Scholar]
- 12.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 2009;360:859–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson GH, Catherine NL, Woodend DM, Wolever TM. Inverse association between the effect of carbohydrates on blood glucose and subsequent short-term food intake in young men. Am J Clin Nutr 2002;76:1023–30. [DOI] [PubMed] [Google Scholar]
- 14.Samra RA, Anderson GH. Insoluble cereal fiber reduces appetite and short-term food intake and glycemic response to food consumed 75 min later by healthy men. Am J Clin Nutr 2007;86:972–9. [DOI] [PubMed] [Google Scholar]
- 15.Stubbs RJ, Hughes DA, Johnstone AM, Rowley E, Reid C, Elia M, Stratton R, Delargy H, King N, Blundell JE. The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br J Nutr 2000;84:405–15. [DOI] [PubMed] [Google Scholar]
- 16.Qi Q, Bray GA, Smith SR, Hu FB, Sacks FM, Qi L. Insulin receptor substrate 1 gene variation modifies insulin resistance response to weight-loss diets in a 2-year randomized trial: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Circulation 2011;124:563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 2005;123:493–505. [DOI] [PubMed] [Google Scholar]
- 18.Yeo GS, Heisler LK. Unraveling the brain regulation of appetite: lessons from genetics. Nat Neurosci 2012;15:1343–9. [DOI] [PubMed] [Google Scholar]
- 19.Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, Elmquist JK. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab 2011;13:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med 2003;348:1085–95. [DOI] [PubMed] [Google Scholar]
- 21.Young EH, Wareham NJ, Farooqi S, Hinney A, Hebebrand J, Scherag A, O’Rahilly S, Barroso I, Sandhu MS. The V103I polymorphism of the MC4R gene and obesity: population based studies and meta-analysis of 29 563 individuals. Int J Obes (Lond) 2007;31:1437–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis C, Levitan RD, Kaplan AS, Carter J, Reid C, Curtis C, Patte K, Hwang R, Kennedy JL. Reward sensitivity and the D2 dopamine receptor gene: a case-control study of binge eating disorder. Prog Neuropsychopharmacol Biol Psychiatry 2008;32:620–8. [DOI] [PubMed] [Google Scholar]
- 23.Levitan RD, Masellis M, Basile VS, Lam RW, Kaplan AS, Davis C, Muglia P, Mackenzie B, Tharmalingam S, Kennedy SH, et al. The dopamine-4 receptor gene associated with binge eating and weight gain in women with seasonal affective disorder: an evolutionary perspective. Biol Psychiatry 2004;56:665–9. [DOI] [PubMed] [Google Scholar]
- 24.van der Klaauw AA, von dem Hagen EA, Keogh JM, Henning E, O’Rahilly S, Lawrence AD, Calder AJ, Farooqi IS. Obesity-associated melanocortin-4 receptor mutations are associated with changes in the brain response to food cues. J Clin Endocrinol Metab 2014;99:E2101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature 2006;443:289–95. [DOI] [PubMed] [Google Scholar]
- 26.Vucetic Z, Reyes TM. Central dopaminergic circuitry controlling food intake and reward: implications for the regulation of obesity. Wiley Interdiscip Rev Syst Biol Med 2010;2:577–93. [DOI] [PubMed] [Google Scholar]
- 27.Srisai D, Gillum MP, Panaro BL, Zhang XM, Kotchabhakdi N, Shulman GI, Ellacott KL, Cone RD. Characterization of the hyperphagic response to dietary fat in the MC4R knockout mouse. Endocrinology 2011;152:890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nonogaki K, Suzuki M, Sanuki M, Wakameda M, Tamari T. The contribution of serotonin 5-HT2C and melanocortin-4 receptors to the satiety signaling of glucagon-like peptide 1 and liraglutide, a glucagon-like peptide 1 receptor agonist, in mice. Biochem Biophys Res Commun 2011;411:445–8. [DOI] [PubMed] [Google Scholar]
- 29.Rebello CJ, Johnson WD, Martin CK, Xie W, O’Shea M, Kurilich A, Bordenave N, Andler S, Klinken BJ, Chu YF, et al. Acute effect of oatmeal on subjective measures of appetite and satiety compared to a ready-to-eat breakfast cereal: a randomized crossover trial. J Am Coll Nutr 2013;32:272–9. [DOI] [PubMed] [Google Scholar]
- 30.Weigle DS, Breen PA, Matthys CC, Callahan HS, Meeuws KE, Burden VR, Purnell JQ. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr 2005;82:41–8. [DOI] [PubMed] [Google Scholar]
- 31.Stocks T, Angquist L, Hager J, Charon C, Holst C, Martinez JA, Saris WH, Astrup A, Sorensen TI, Larsen LH. TFAP2B -dietary protein and glycemic index interactions and weight maintenance after weight loss in the DiOGenes trial. Hum Hered 2013;75:213–9. [DOI] [PubMed] [Google Scholar]
- 32.Cripps RL, Martin-Gronert MS, Archer ZA, Hales CN, Mercer JG, Ozanne SE. Programming of hypothalamic neuropeptide gene expression in rats by maternal dietary protein content during pregnancy and lactation. Clin Sci (Lond) 2009;117:85–93. [DOI] [PubMed] [Google Scholar]