Abstract
Background: Above-average dietary protein, as a single nutrient, improves musculoskeletal health. Evaluating the link between dietary protein and musculoskeletal health from a whole-diet perspective is important, as dietary guidelines focus on dietary patterns.
Objective: We examined the prospective association of novel dietary protein food clusters (derived from established dietary pattern techniques) with appendicular lean mass (ALM), quadriceps strength (QS), and bone mineral density (BMD) in 2986 men and women, aged 19–72 y, from the Framingham Third Generation Study.
Design: Total protein intake was estimated by food-frequency questionnaire in 2002–2005. A cluster analysis was used to classify participants into mutually exclusive groups, which were determined by using the percentage of contribution of food intake to overall protein intake. General linear modeling was used to 1) estimate the association between protein intake (grams per day) and BMD, ALM, appendicular lean mass normalized for height (ALM/ht2), and QS (2008–2011) and to 2) calculate adjusted least-squares mean outcomes across quartiles of protein (grams per day) and protein food clusters.
Results: The mean ± SD age of subjects was 40 ± 9 y; 82% of participants met the Recommended Daily Allowance (0.8 g · kg body weight–1 · d–1). The following 6 dietary protein food clusters were identified: fast food and full-fat dairy, fish, red meat, chicken, low-fat milk, and legumes. BMD was not different across quartiles of protein intake (P-trend range = 0.32–0.82); but significant positive trends were observed for ALM, ALM/ht2 (P < 0.001), and QS (P = 0.0028). Individuals in the lowest quartile of total protein intake (quartile 1) had significantly lower ALM, ALM/ht2, and QS than did those in the higher quartiles of intake (quartiles 2–4; (P ranges = 0.0001–0.003, 0.0007–0.003, and 0.009–0.05, respectively). However, there were no associations between protein clusters and any musculoskeletal outcome in adjusted models.
Conclusions: In a protein-replete cohort of adults, dietary protein is associated with ALM and QS but not with BMD. In this study, dietary protein food patterns do not provide further insight into beneficial protein effects on muscle outcomes.
Keywords: bone mineral density, dietary patterns, dietary protein, muscle mass, muscle strength
INTRODUCTION
Age-related musculoskeletal losses are a major public health burden because they can cause physical disability and increased mortality. Osteoporosis accounts for ∼50% of all hip fractures (1). Consequences of hip fracture include a substantially increased risk of mortality (≤30%) (2) and a decline in physical function (3, 4). Osteoporosis is commonly accompanied by sarcopenia, a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength with risk of adverse outcomes such as physical disability, poor quality of life, and death (5). The prevention of age-related losses of bone and muscle strength during adulthood via low-risk approaches such as nutrition and dietary alterations is of increasing research interest.
Greater dietary protein has been shown to reduce age-related loss of bone density (6–8), muscle mass (9–11), and muscle strength (12–15). Although the benefits of attaining adequate protein in the diet to optimize musculoskeletal health have been well established, it remains unknown whether these benefits occur as a result of absolute protein intake (i.e., attaining a specific dietary allowance in grams per day) or whether maximizing protein intake from specific food sources is of importance. Individual protein food sources may be beneficial to the musculoskeletal system because a specific food source may differ in its amino acid composition, digestibility, and nonprotein nutrient composition (16). Studies that have separated protein foods by animal protein compared with plant protein have had conflicting results regarding their associations with bone (17) and muscle (13, 18). This controversy is due in part to the complexity of what seems to be a simple research question. The influence of dietary protein on the musculoskeletal system is dependent on many other dietary factors such as other nonprotein nutrients (i.e., calcium, magnesium, and vitamin D) and the acidity of the diet (17). Although it is recommended that adults attain adequate protein intake for musculoskeletal health, it remains unclear if specific protein food sources or dietary patterns possess greater benefit to the musculoskeletal system. The assessment of whether dietary protein food patterns are differentially associated with bone and muscle health will have important public health implications, because precise recommendations on the type of protein-containing foods are lacking. The objective of our study was to examine the prospective association of novel dietary protein food clusters [derived from established dietary pattern techniques (19) and used by our group previously (20)] with appendicular lean mass (ALM),6 quadriceps strength (QS), and bone mineral density (BMD) in 2986 men and women from the Framingham Third Generation Study. We hypothesized that not all protein food clusters would be equally beneficial to bone and muscle health.
METHODS
Study population
The Framingham Third Generation Study is a longitudinal cohort study that began in 2002 by enrolling children of the Framingham Offspring Cohort (offspring of the Original Framingham Heart Study). By 2005, a total of 4095 Third Generation participants were recruited. The purpose of the Framingham Heart Study was to identify risk factors for coronary artery disease including familial factors. Visits occur every 4–8 y, at which participants take part in physical examinations, blood chemistries, assessment of risk factors, and questionnaires. The Framingham Third Generation exam 1 occurred between the years 2002 and 2005, and exam 2 occurred between the years 2008 and 2011. Of 4095 Third Generation participants, 3800 men and women had dietary data collected at exam 1. Of this sample, food-frequency questionnaire (FFQ) data from 188 individuals were removed because of an invalid FFQ on the basis of the following criteria: >12 items left blank or energy intakes <600 or >4000 kcal/d. Of 3612 participants with valid FFQ data, 8 individuals were removed after an outlier analysis (described in Statistical Analysis). The protein cluster procedure that was used to create protein clusters included 3604 men and women. Of this sample, 2986 subjects had one or more of the following outcome measures: BMD, ALM, or QS collected at exam 2 (2008–2011). In fully adjusted models, 15 participants were lost because of missing data on physical activity. All participants provided informed consent for their participation. This study was approved by the Institutional Review Board at Hebrew SeniorLife.
BMD
BMD of the hip (at the femoral neck, trochanter, and total hip) and spine (mean BMD from L2 to L4) were scanned with the use of a GE Lunar Prodigy fan-beam densitometer (GE Healthcare Inc.) between the years 2008 and 2011. BMD was measured in grams per centimeter squared. The right hip was routinely scanned; in cases where there was a history of a previous fracture or hip replacement, the left hip was scanned. The precision of the Prodigy machine was 1.8% for the femoral neck, 2.3% for the trochanter, 1.2% for the total hip, and 1.1% for the lumbar spine (21).
ALM
Whole-body and regional measures of lean mass were obtained with the GE Lunar Prodigy fan beam densitometer between the years 2008 and 2011. Arm and leg lean body mass were measured in grams. For participants who were too large to fit within the dimensions of the scanning field, a hemiscan was performed (46% of participants had a hemiscan). For the majority of participants with a hemiscan, the right side was scanned, and the machine imputed left-side measures to create whole-body measures. The CV for the Prodigy device was 0.9% for total body lean mass. ALM was calculated as the sum of leg and arm lean masses (grams) and was also adjusted for height [appendicular lean mass normalized for height (ALM/ht2) was calculated as ALM (grams) divided by the square of height (meters squared)].
QS
The isometric QS of the right leg was measured with the use of Lafayette Manual Muscle Test System (model 01163; Lafayette Instrument) between the years 2008 and 2011. In cases where the right leg could not be assessed (e.g., because of pain or wearing a leg brace), the left leg was measured. In a seated position with hands on the lap and the back supported against the chair back, the participant’s right knee was positioned at 60 degrees of flexion with the use of a fixed goniometer with the right foot placed flat on the floor. The test system was held perpendicular to the leg on the anterior surface of the tibia 6 cm above the lateral malleolus, and the participant was instructed to kick their leg against the test system as hard as they could for 3 s. The procedure was repeated, and the force (kilograms) for the maximum of both trials was used in the analyses.
Dietary assessment
Typical dietary intakes of foods and nutrients were assessed with the use of the Harvard 126-item semiquantitative and validated general population 88 FFQ (22, 23) during the years 2002–2005. The Harvard FFQ has been validated extensively, and nutrient intakes have been well correlated with those obtained with the use of a multiple food records and biochemical measures of several nutrients (23, 24) including dietary protein. Protein intake from each food consumed was calculated for all participants in grams per day. The percentage contribution of each food to total protein intake for all individuals was calculated as
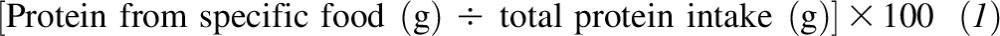 |
for use in the cluster analysis (described in Statistical analysis).
Covariates
Variables that are known to affect the outcome measures (BMD, ALM, and QS) were used in the adjusted models to assess the relation between dietary protein food clusters and musculoskeletal health. These covariates (collected between 2002 and 2005) included age (years), sex, estrogen use, menopausal status, height (meters), BMI (in kg/m2; calculated as weight divided by the square of height), physical activity (continuous score), smoking status (current smoker or noncurrent smoker), total energy intake (kilocalories per day), alcohol intake (grams per day), calcium intake (milligrams per day), vitamin D intake (International Units per day), calcium-supplement use (3 categories, described in this section), and vitamin D–supplement use (3 categories).
Height was measured with the subject not wearing shoes to the nearest 0.25 in (0.64 cm) with the use of a stadiometer. Weight was measured in pounds with the use of a standard balance-beam scale (Detecto; Worcester Scale Co. Inc.). Physical activity was assessed with the use of a structured questionnaire to indicate the number of hours spent performing 5 levels of activity (i.e., being asleep, being sedentary, and performing light, moderate, and heavy activity). The responses contributed to a weighted sum (i.e., the physical activity index) with a score of 120 representing 24 h of constant strenuous activity and a score of 24 representing 24 h of sleeping (25). The physical activity index was calculated from the number of hours that a subjects participated in an activity, which were multiplied by a metabolic factor, as follows:
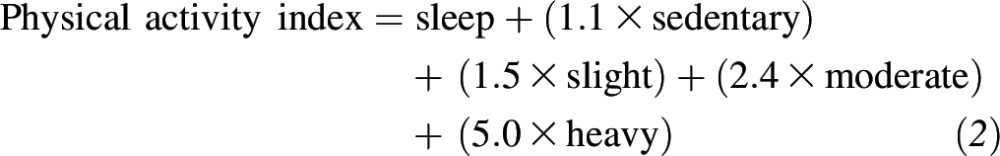 |
The hormone status of women and the sex of participants were combined into one variable to reduce the df in regression models. Therefore, this variable had 3 levels: 1) men, 2) estrogenic women (premenopausal or currently taking postmenopausal estrogen), and 3) nonestrogenic women [postmenopausal (periods stopped for >1 y) and nonestrogen user]. Menopausal status and estrogen use were self-reported. A medical chart review was conducted for all participants who reported being menopausal or who used estrogen to verify the self-report. Typical intakes of total energy, alcohol, calcium, and vitamin D were assessed with the use of an FFQ. Smoking status was classified as current smoker (defined as smoking cigarettes within the past month) or noncurrent smoker (all other participants who were not currently smoking).
Supplement use was captured with the same FFQ that was used to capture dietary data (22). Supplement categories for calcium and vitamin D were chosen to differentiate between participants who received these nutrients from a multivitamin (a marker of a healthy lifestyle) and subjects who were taking specific supplements that were likely to benefit their bone health. Therefore, calcium-supplement use was categorized as follows: non–supplement user (0 mg/d), supplement use from a multivitamin (supplemental calcium intake >0 to <200 mg/d), or additional supplement use (supplemental calcium intake ≥200 mg/d). Vitamin D–supplement use was categorized similarly as follows: non–supplement user, supplement use from a multivitamin (>0 to ≤400 IU vitamin D/d), or additional supplement use (>400 IU vitamin D/d).
Statistical analysis
The generation of protein food clusters was accomplished in several steps. First, the percentage of total daily protein that was contributed from each food was calculated for each individual. Foods containing protein were grouped into 20 predefined food groups on the basis of nutrient-composition similarities, protein type, or source (Supplemental Table 1). Foods not containing protein were not included in the formation food groups (i.e., condiments, alcohol, and diet drinks). Food groups that contributed <0.5% of total daily dietary protein were removed before the cluster analysis. Dietary protein food clusters were generated a posteriori with the use of the FASTCLUS procedure in SAS software (version 9.4; SAS Institute Inc.). This statistical method applied the K-means method of cluster analysis to classify individuals into mutually exclusive groups by comparing Euclidean distances between each person and each cluster center in an iterative process (26). The cluster analysis was sensitive to outliers; therefore, the data were checked to ensure that no participants with protein contributions from food groups that were >5 SDs away from the mean protein contribution for that group were included. In addition, we ran the clustering procedure by forcing a predefined number of 20 clusters and removed individuals who fell into clusters of <10 subjects (8 participants were removed at this step). Thus, the final sample of 3604 adults was used to create the study protein food clusters. In this sample, the FASTCLUS procedure was run with predetermined numbers of clusters (2–8 times) to determine which number of clusters best interpreted the current sample’s dietary protein food patterns of intake. The 6-cluster set was chosen to best represent individual protein food intake patterns because it presented the most-meaningfully separated clusters, including a high F ratio, and it distributed participants well between all clusters (each cluster contained >100 participants). Clusters that were derived in this study were similar to those derived in the older Framingham Offspring Cohort (20). Further background and discussion of these methods have been described elsewhere (26).
Nutrient intakes were adjusted for total energy with the use of the residual method (27). Means ± SDs for continuous variables and proportions of participants for categorical variables were calculated for both the total sample and within each protein food cluster. The mean ± SE percentages of protein intake from individual food groups were calculated across the protein food clusters, and general linear modeling was used to make statistical comparisons of intakes across the clusters. P values were adjusted for multiple comparisons with the use of the Tukey-Kramer test. Clusters were named on the basis of the relative comparison of protein source foods across clusters. For example, if the percentage of protein from fish was higher within cluster 2 than those in clusters 1 and 3–6, cluster 2 was named fish although individuals in this cluster also consumed dietary protein from other sources (such as chicken).
Separate analyses were conducted for each musculoskeletal measure including each BMD measure (femoral neck, trochanter, total femur, and lumbar spine), ALM, ALM/ht2, and QS. General linear modeling was used to assess the relation between dietary protein (grams per day; adjusted for energy intake) and each musculoskeletal outcome. Dietary protein was ranked in quartiles, and general linear modeling was used to compare least-squares mean BMD, ALM, ALM/ht2, and QS across protein intake quartiles. If the protein-quartile multilevel variable was significant (P < 0.05), pairwise comparisons across protein quartiles were made. Crude models were adjusted for energy intake only; fully adjusted models were controlled for age, sex, estrogen status (men, estrogenic women, and nonestrogenic women), BMI, height, total energy intake, smoking status, energy-adjusted alcohol intake, physical activity, and dietary protein as well as for calcium-supplement use and vitamin D–supplement use (in BMD models only). Both height and BMI were included in the models to adequately capture the joint relation of body composition (BMI) and body size (height) to the health outcomes as recommended previously (28).
General linear modeling was used to compare adjusted least-squares mean BMD, ALM, ALM/ht2, or QS between protein food clusters. Initial BMD analyses were adjusted for age, sex, estrogen status, BMI, height, and total energy intake. Final BMD models were further adjusted for current smoking status, energy-adjusted alcohol intake, calcium-supplement use, vitamin D–supplement use, physical activity, and dietary protein. In sensitivity analyses in which BMI was replaced with total fat mass, there were nearly identical results; therefore, BMI was used in all models. The resulting least-squares means for each BMD site, ALM, ALM/ht2, and QS were compared across all pairwise combinations of protein food-cluster groups if the multilevel variable for protein food clusters was significant (P < 0.05). The Tukey-Kramer test was used to adjust for multiple comparisons.
The sex-by-cluster interaction term was not significantly associated with any of the musculoskeletal outcomes in the general linear models (P range = 0.41–0.89). Therefore, results are presented for men and women combined. Additional models also included an interaction term for age because losses of bone and muscle accelerate with age, and the cohort included a wide age range (19–72 y). No significant interactions with age were detected with any musculoskeletal outcome (P range = 0.23–0.99). All analyses were performed with the use of SAS software (version 9.4).
RESULTS
The mean ± SD age of subjects was 40.6 ± 8.7 y with a range of 19–72 y (Table 1). Median amounts of dietary protein within quartiles of intake were as follows: quartile 1, 59 g/d (0.8 g/kg body weight); quartile 2, 80 g/d (1.1 g/kg body weight); quartile 3, 99 g/d (1.3 g/kg body weight); and quartile 4, 129 g/d (1.8 g/kg body weight). Overall, 82% of the total sample met the Recommended Daily Allowance (RDA) for dietary protein of 0.8 g intake · kg body weight–1 · d–1.
TABLE 1.
Characteristics of participants in the Framingham Third Generation Study (n = 2986)1
| Characteristic | Value |
| Men, % | 46 |
| Age, y | 40.6 ± 8.7 (19–72)2 |
| Physical activity index | 37.3 ± 7.53 |
| Total energy intake, kcal/d | 2048 ± 662 |
| Dietary protein, g/d | 93 ± 32 |
| Dietary calcium, mg/d | 870 ± 419 |
| Total calcium, mg/d | 1023 ± 516 |
| Dietary vitamin D, IU/d | 221 ± 162 |
| Total vitamin D, IU/d | 378 ± 284 |
| Alcohol intake, g/d | 10.7 ± 14.3 |
| Smoking status, current, % | 13 |
| Body composition and muscle strength | |
| BMI, kg/m2 | 26.8 ± 5.5 |
| Appendicular lean mass, kg | 21.7 ± 7.0 |
| Appendicular lean mass ÷ height squared, kg/m2 | 7.3 ± 1.9 |
| Quadriceps strength, kg | 27.5 ± 9.2 |
| BMD, g/cm2 | |
| Femoral neck | 1.003 ± 0.135 |
| Trochanter | 0.826 ± 0.140 |
| Total femur | 1.036 ± 0.140 |
| Lumbar spine | 1.255 ± 0.171 |
| Estrogen status in women,4 % | |
| Estrogenic | 90 |
| Nonestrogenic | 10 |
| Calcium-supplement use, % | |
| None | 64 |
| MVI, >0 to <200 mg/d | 12 |
| Additional, ≥200 mg/d | 24 |
| Vitamin D–supplement use, % | |
| None | 54 |
| MVI, >0 to ≤400 IU/d | 39 |
| Additional, >400 IU/d | 7 |
BMD, bone mineral density; MVI, multivitamin intake.
Mean ± SD; range in parentheses.
Mean ± SD (all such values).
Defined as estrogenic if premenopausal or postmenopausal (periods stopped for >1 y) and taking hormone replacement therapy and as nonestrogenic if postmenopausal (periods stopped for >1 y) and not taking hormone replacement therapy.
Cluster patterns were named on the basis of the highest percentage of protein intake from one or more food groups (Table 2, footnote 2). Compared with all other protein food clusters, the fast-food and full fat–dairy group (n = 458) presented with greater protein intake from whole milk, cream, cheese, grains, and fast food (i.e., pizza and French fries). The fish cluster (n = 605) presented with relatively greater protein intake from fish, yogurt, and fruit and vegetables. The red-meat cluster (n = 640) presented with relatively greater protein intake from red meat. The chicken cluster (n = 735) presented with relatively greater protein intake from chicken. The low fat–milk cluster (n = 434) presented with relatively greater protein intake from low-fat milk. Last, compared with most of the other protein food clusters, the legume protein food cluster (n = 114) presented with greater protein intake from legumes, nuts and seeds, fruit and vegetables, cereals, and grains.
TABLE 2.
Total protein intake from individual food groups across protein food clusters in 2986 men and women in the Framingham Third Generation Study1
| Food group | Fast food, full-fat dairy (n = 458) | Fish (n = 605) | Red meat (n = 640) | Chicken (n = 735) | Low-fat milk (n = 434) | Legumes (n = 114) |
| Beans and peas, % | 1.3 (0.5–2.3) | 1.8 (0.9–3.6) | 1.2 (0.4–2.1) | 1.3 (0.4–2.5) | 1.3 (0.6–2.3) | 18.1 (12.3–24.6)2 |
| Nuts and seeds, % | 1.4 (0.8–2.8) | 1.7 (0.8–3.5) | 1.2 (0.6–2.4) | 1.1 (0.6–2.3) | 1.3 (0.6–2.7) | 2.2 (1.2–6.1)2 |
| Low-fat milk, % | 2.7 (0–6.7) | 3.8 (0.8–7.2) | 1.2 (0–5.1) | 3.3 (0.5–7.0) | 19.5 (15.5–23.9)2 | 1.1 (0–7.0) |
| Whole milk, % | 0 (0–0.9) | 0 (0–0) | 0 (0–0.5) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Cream, % | 1.7 (0.9–2.4) | 1.2 (0.4–2.0) | 1.3 (0.6–1.9) | 1.1 (0.5–1.9) | 1.1 (0.5–1.8) | 0.8 (0.3–1.7) |
| Yogurt, % | 0.8 (0–2.5) | 1.2 (0–4.2) | 0 (0–1.5) | 0.8 (0–2.1) | 0.8 (0–3.0) | 1.5 (0–4.5) |
| Cheese products, % | 6.0 (3.5–9.6)2 | 4.3 (2.2–7.0) | 4.2 (2.6–6.4) | 3.9 (1.7–6.3) | 4.0 (2.2–6.5) | 4.2 (1.6–7.8) |
| Red meat, % | 14.8 (11.1–18.5) | 11.8 (8.0–16.0) | 27.4 (24.2–32.6)2 | 11.6 (7.7–15.3) | 13.3 (8.7–18.4) | 0 (0–6.1) |
| Processed meat, % | 2.0 (1.1–3.7) | 1.1 (0.4–2.0) | 1.8 (0.9–3.5) | 1.1 (0.4–2.1) | 1.2 (0.5–2.1) | 0 (0–0.4) |
| Chicken, % | 16.3 (11.3–20.4) | 20.4 (15.6–24.1) | 18.7 (13.0–23.1) | 33.1 (29.5–38.6)2 | 15.5 (10.0–20.0) | 5.5 (0–12.2) |
| Fish, % | 5.6 (3.4–8.3) | 15.7 (11.8–20.5)2 | 6.6 (3.8–9.0) | 6.6 (3.4–9.7) | 6.0 (3.4–9.2) | 7.8 (3.0–13.2) |
| Eggs, % | 1.2 (0.8–2.9) | 1.2 (0.7–2.8) | 1.1 (0.7–2.7) | 1.0 (0.6–2.6) | 0.9 (0.5–2.0) | 1.1 (0.6–2.9) |
| Fruit and vegetables, % | 6.0 (4.5–7.9) | 7.5 (5.6–9.5) | 5.8 (4.4–7.8) | 6.2 (4.6–8.2) | 6.3 (4.8–7.9) | 10.1 (7.3–13.1)2 |
| Cereal, % | 1.1 (0.3–2.6) | 1.7 (0.7–3.4) | 0.9 (0.2–2.4) | 1.1 (0.3–2.6) | 2.2 (0.7–4.1) | 2.9 (1.0–5.3)2 |
| Sweet baked products, % | 2.5 (1.5–4.1) | 1.8 (0.9–3.1) | 2.0 (1.1–3.0) | 1.5 (0.7–2.8) | 1.9 (1.0–2.8) | 1.4 (0.9–2.8) |
| White grains, % | 6.7 (4.6–9.5)2 | 4.7 (2.9–7.0) | 5.3 (3.3–7.4) | 4.7 (2.7–7.4) | 4.7 (3.1–7.0) | 6.0 (2.9–9.7) |
| Whole grains, % | 1.6 (0.5–3.4) | 1.9 (1.0–3.6) | 1.3 (0.5–2.6) | 1.5 (0.5–2.7) | 1.8 (0.6–3.3) | 5.3 (2.6–10.4)2 |
| Snacks, % | 2.7 (1.6–4.1) | 1.8 (1.0–3.0) | 1.9 (1.1–2.9) | 1.8 (1.1–3.0) | 1.8 (1.1–3.1) | 1.6 (0.7–3.0) |
| Fast food, % | 7.7 (4.8–14.4)2 | 3.7 (2.6–5.1) | 4.2 (3.0–5.8) | 4.1 (2.9–5.5) | 3.8 (2.6–5.3) | 3.9 (2.2–5.7) |
| Protein supplements, % | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
All values are medians (IQRs). Values are protein intake from individual food groups within each cluster. Some food groups presented with zero medians and IQRs because of a small percentage of individuals with high intake (>75th percentile). Naming of clusters was determined by comparing the percentage of protein intake of a food group (from a reference cluster) with that of all other clusters. The percentages of protein intake from individual food groups cumulatively add up to ∼100% and may be <100% because of the omission of food groups that provided <0.5% of total protein intake in the diet.
Value represents the highest consumption of a food group compared with that of all other clusters and is a distinct characteristic by which the cluster was named.
Participant characteristics are described across protein food clusters in Table 3. Dietary protein was not associated with BMD at any site when protein intake was modeled as a continuous variable (data not shown; P range = 0.35–0.63) or as a categorical variable (Table 4; P-trend range = 0.32–0.82). No differences at any BMD site were observed across the 6 protein food clusters in either crude models (data not shown) or adjusted models (Table 5). There was no significant interaction between the protein food cluster and age (P range = 0.09–0.95).
TABLE 3.
Characteristics of 2986 study participants from the Third Generation Framingham Cohort across protein food clusters1
| Characteristic | Fast food, full-fat dairy (n = 458) | Fish (n = 605) | Red meat (n = 640) | Chicken (n = 735) | Low-fat milk (n = 434) | Legumes (n = 114) |
| Age, y | 39.3 ± 8.52 | 42.2 ± 9.0 | 41.5 ± 8.3 | 39.3 ± 8.3 | 40.9 ± 8.6 | 38.6 ± 9.4 |
| Men, % | 56 | 42 | 52 | 42 | 42 | 21 |
| Smoking status, current, % | 18 | 9 | 18 | 12 | 12 | 8 |
| BMI, kg/m2 | 26.5 ± 5.0 | 26.8 ± 5.3 | 27.4 ± 5.6 | 26.7 ± 5.3 | 26.8 ± 5.0 | 23.9 ± 4.6 |
| Physical activity index | 37.2 ± 7.4 | 37.4 ± 7.6 | 37.5 ± 8.3 | 37.0 ± 7.2 | 37.8 ± 7.3 | 36.1 ± 5.8 |
| Nonestrogenic women, % | 6 | 14 | 13 | 7 | 11 | 7 |
| Calcium-supplement user, % | ||||||
| None | 71 | 57 | 70 | 64 | 60 | 53 |
| MVI, <200 mg/d | 13 | 12 | 11 | 11 | 14 | 8 |
| Additional, ≥200 mg/d | 16 | 31 | 19 | 25 | 26 | 39 |
| Vitamin D–supplement user, % | ||||||
| None | 60 | 47 | 61 | 54 | 50 | 44 |
| MVI, ≤400 IU/d | 35 | 43 | 34 | 38 | 40 | 48 |
| Additional, >400 IU/d | 5 | 10 | 5 | 8 | 10 | 8 |
| Nutrient intake | ||||||
| Total energy, kcal/d | 2221 ± 706 | 1922 ± 631 | 2164 ± 632 | 1891 ± 629 | 2157 ± 674 | 1957 ± 628 |
| Total protein, g/d | 88 ± 31 | 90 ± 31 | 97 ± 29 | 95 ± 35 | 98 ± 31 | 83 ± 34 |
| Dietary calcium, mg/d | 924 ± 422 | 789 ± 333 | 722 ± 262 | 745 ± 315 | 1372 ± 484 | 824 ± 392 |
| Total calcium, mg/d | 1021 ± 477 | 985 ± 490 | 846 ± 374 | 900 ± 439 | 1529 ± 563 | 1080 ± 521 |
| Dietary vitamin D, IU/d | 165 ± 140 | 276 ± 192 | 166 ± 91 | 171 ± 108 | 374 ± 167 | 205 ± 207 |
| Total vitamin D, IU/d | 291 ± 248 | 456 ± 317 | 294 ± 221 | 333 ± 251 | 558 ± 291 | 402 ± 343 |
| Alcohol, g/d | 12.3 ± 15.7 | 10.9 ± 13.3 | 13.4 ± 17.9 | 9.2 ± 11.5 | 7.5 ± 11.2 | 8.8 ± 10.9 |
MVI, multivitamin intake.
Mean ± SD (all such values).
TABLE 4.
Bone mineral density of the hip and spine, ALM, and quadriceps strength across dietary protein quartiles1
| Dietary protein quartile, g/d |
||||||
| n | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-trend | |
| Bone mineral density,2 g/cm2 | ||||||
| Femoral neck | 2903 | 1.001 ± 0.0063 | 0.992 ± 0.006 | 0.998 ± 0.006 | 1.001 ± 0.006 | 0.82 |
| Total femur | 2903 | 1.016 ± 0.006 | 1.017 ± 0.006 | 1.023 ± 0.006 | 1.019 ± 0.006 | 0.47 |
| Trochanter | 2903 | 0.800 ± 0.006 | 0.806 ± 0.006 | 0.810 ± 0.006 | 0.805 ± 0.006 | 0.32 |
| Lumbar spine | 2831 | 1.230 ± 0.008 | 1.228 ± 0.008 | 1.239 ± 0.008 | 1.235 ± 0.008 | 0.37 |
| Muscle measures4 | ||||||
| ALM, kg | 2905 | 21.2 ± 0.1a | 21.6 ± 0.1b | 21.7 ± 0.1b | 21.7 ± 0.1b | 0.0001 |
| ALM/ht2, kg/m2 | 2905 | 7.2 ± 0.03a | 7.3 ± 0.03b | 7.3 ± 0.03b | 7.3 ± 0.03b | 0.0002 |
| Quadriceps strength, kg | 2885 | 25.9 ± 0.40a | 27.1 ± 0.40b | 27.2 ± 0.40b | 27.4 ± 0.40b | 0.0028 |
Adjusted for energy intake with the use of the residual method. Values with different superscript lowercase letters were statistically significant, P < 0.05. ALM, appendicular lean mass; ALM/ht2, appendicular lean mass normalized for height.
Adjusted for sex and estrogen status combined (men, estrogenic women, and nonestrogenic women), age, BMI, height, total energy, current smoking, energy-adjusted alcohol consumption, calcium-supplement use, vitamin D–supplement use, physical activity, dietary calcium, and dietary vitamin D.
Least-squares mean ± SE (all such values).
Adjusted for sex, estrogen status, age, BMI, height, total energy, current smoking, supplemental calcium, supplemental vitamin D, and physical activity.
TABLE 5.
Association of dietary protein food clusters with BMD in men and women in the Third Generation Framingham Cohort1
| BMD by protein food group, g/cm2 |
|||||||
| BMD | Fast food, full-fat dairy | Fish | Red meat | Chicken | Low-fat milk | Legumes | P2 |
| Femoral neck (n = 2903) | 1.004 ± 0.007 | 1.000 ± 0.006 | 0.989 ± 0.006 | 1.002 ± 0.006 | 0.993 ± 0.007 | 1.016 ± 0.012 | 0.12 |
| Trochanter (n = 2903 | 0.815 ± 0.006 | 0.805 ± 0.006 | 0.800 ± 0.006 | 0.806 ± 0.006 | 0.803 ± 0.007 | 0.806 ± 0.012 | 0.44 |
| Total femur (n = 2903) | 1.028 ± 0.007 | 1.016 ± 0.007 | 1.012 ± 0.006 | 1.022 ± 0.006 | 1.016 ± 0.007 | 1.025 ± 0.012 | 0.35 |
| Lumbar spine (n = 2831) | 1.240 ± 0.009 | 1.239 ± 0.009 | 1.227 ± 0.009 | 1.233 ± 0.008 | 1.228 ± 0.010 | 1.224 ± 0.017 | 0.67 |
All values are least-squares means ± SEs. General linear modeling was used to compare adjusted least-squares mean BMDs across protein food clusters. Analyses were adjusted for age, sex and estrogen status combined (men, estrogenic women, and nonestrogenic women), BMI, height, total energy intake, current smoking status, energy-adjusted alcohol intake, calcium-supplement use, vitamin D–supplement use, physical activity index, and energy-adjusted protein intake. Adjustment for multiple comparisons was performed with the use of the Tukey-Kramer test. There were no significant differences across groups for any BMD measure. BMD, bone mineral density.
Overall P value for protein food clusters.
Dietary protein was significantly positively associated with ALM in the adjusted continuous models (ALM: β = 0.008 ± 0.002, P < 0.001; ALM/ht2: β = 0.003 ± 0.001, P < 0.001) and trended toward significance with QS (β = 0.017 ± 0.009, P = 0.06). There was a significant positive trend that was observed across dietary protein quartiles with ALM, ALM/ht2, and QS after adjustment for potential confounders (Table 4). Individuals in the lowest quartile of dietary protein intake had significantly lower ALM, ALM/ht2, and QS compared with those of subjects in all other quartiles of intake (Table 4) after adjustment for potential confounders.
In crude models, ALM and ALM/ht2 were significantly associated with the protein food cluster whereby individuals in the legume protein food cluster presented with significantly lower ALM and ALM/ht2 compared with those of subjects in all other protein food clusters (Table 6). Individuals in the low fat–milk cluster presented with significantly lower ALM and ALM2 compared with those of participants in the fast-food and full fat–dairy cluster. However, fully adjusted models showed no differences in either ALM or ALM/ht2 across the 6 protein food clusters (Table 6). No differences in QS were observed across protein food clusters in either the crude or adjusted models.
TABLE 6.
Association of dietary protein food clusters with APL, ALM/ht2, and quadriceps strength in men and women in the Third Generation Framingham Cohort1
| Muscle by protein food group |
|||||||
| Variable | Fast food, full-fat dairy | Fish | Red meat | Chicken | Low-fat milk | Legumes | P2 |
| ALM, kg | |||||||
| Crude (n = 2922) | 23.2 ± 0.3a | 22.2 ± 0.3a,b | 22.7 ± 0.2a | 22.3 ± 0.2a,b | 21.7 ± 0.3b | 19.8 ± 0.6c | <0.001 |
| Adjusted (n = 2905) | 21.7 ± 0.2 | 21.6 ± 0.2 | 21.4 ± 0.2 | 21.5 ± 0.2 | 21.5 ± 0.2 | 21.8 ± 0.4 | 0.20 |
| ALM/ht2, kg/m2 | |||||||
| Crude (n = 2922) | 7.7 ± 0.07a | 7.5 ± 0.06a,b | 7.7 ± 0.06a | 7.6 ± 0.05a,b | 7.4 ± 0.07b | 6.9 ± 0.14c | <0.001 |
| Adjusted (n = 2905) | 7.4 ± 0.04 | 7.3 ± 0.03 | 7.3 ± 0.03 | 7.3 ± 0.03 | 7.3 ± 0.04 | 7.4 ± 0.07 | 0.11 |
| Quadriceps strength | |||||||
| Crude (n = 2899) | 28.1 ± 0.4 | 27.4 ± 0.4 | 27.5 ± 0.4 | 27.4 ± 0.3 | 27.4 ± 0.4 | 27.0 ± 0.9 | 0.78 |
| Adjusted (n = 2885) | 27.4 ± 0.5 | 26.7 ± 0.4 | 26.8 ± 0.4 | 26.7 ± 0.4 | 27.0 ± 0.5 | 27.6 ± 0.9 | 0.78 |
All values are least-squares means ± SEs. General linear modeling was used to compare adjusted least-squares mean ALM, ALM/ht2, or quadriceps strength across protein food clusters. The crude model was adjusted for total energy. The adjusted model was adjusted as for the crude model and for age, sex, menopause status, physical activity index, BMI, height, smoking status, energy-adjusted alcohol intake, and energy-adjusted protein intake. Means that do not share a common superscript letter were significantly different at P < 0.05 on the basis of the Tukey-Kramer test. ALM, appendicular lean mass; ALM/ht2, appendicular lean mass normalized for height.
Overall P value for protein food clusters.
DISCUSSION
When dietary protein was examined as grams of intake per day (continuous and ranked intake into quartiles), overall intake was not associated with any measure of hip or spine BMD in this age-diverse cohort of men and women from the Framingham Third Generation Study. Participants with the lowest quartile of total protein intake showed significantly lower ALM and QS compared with those of individuals in the upper quartiles of protein intake. Six protein food clusters were identified with the use of novel protein-centric food cluster modeling. There were no significant differences in BMD, ALM, or QS across protein food clusters after accounting for other known confounders.
Results of the current study from the Framingham Study Third Generation cohort showed no association between BMD and dietary protein when assessed as total daily intake. This result is contrary to previous research in large population-based cohorts in older adults (>60 y of age), which showed dietary protein to be positively associated with BMD cross-sectionally (29) with reduced bone loss over time in the Framingham Original Cohort (6) and with reduced risk of falls (30) and fracture in the Framingham Original Cohort (31, 32) longitudinally. Null results in the current study may be explained by the overall young age of the cohort (mean age: 40 y) of whom only 15% of subjects were aged >50 y, which is a time when the age-related loss of bone mass typically becomes evident. However, this cohort was specifically chosen because of their wide age range to examine more-diverse protein food intake (because older adults typically show comparable protein intakes from similar sources). In addition, the Framingham Third Generation Cohort was largely protein replete with 82% of the sample meeting the RDA for dietary protein (0.8 g · kg–1 · d–1). Dietary protein may maximally benefit bone health in older, more frail, or protein-insufficient populations.
Randomized controlled trials have shown inconsistent results on whether additional protein intake can improve bone health. Daily supplementation with whey protein showed no change in BMD, measured by dual-energy X-ray absorptiometry, or in volumetric BMD that was measured with the use of a quantitative computed tomography in either a 2-y (33) or 18-mo (34) follow-up period compared with placebo. In a weight-loss trial, a high-protein diet (24% of kilocalories from protein) attenuated the loss of BMD at the radius, spine, and total hip over 1 y compared with the effect in the normal-protein group (18% of kilocalories) (35). Women were counseled to increase their protein intake with lean meat, fish, legumes, and dairy with an optional whey-protein supplement if needed. Supplementation with protein from dietary sources compared with a whey-protein supplement may account for the differences in results between these studies.
Our study sought to determine whether individuals from a large community-based cohort would have different BMDs that were dependent on their protein-derived dietary pattern. A recently published study in the older Framingham Offspring Cohort (mean age: 60 y) showed that individuals in the protein food cluster who had high protein intakes from red meat and processed foods had lower BMD than that of individuals in the low fat–milk protein food cluster (20). In contrast, the current study showed no differences in BMD across protein food clusters in the younger generation of the Framingham Heart Study cohort. Age may have played a role in the different results. In addition, there was a stark difference in protein intake between the 2 cohorts, which may also partially explain the contrast in results (93 g/d in the younger cohort compared with 78 g/d in the older cohort).
Dietary protein is an important contributor to muscle status because it is an important building block for muscle-fiber synthesis, and the breakdown of muscle has been widely shown under conditions of inadequate protein intake. Consistent with the literature (9, 10), we showed that total protein intake was positively associated with ALM. Although studies that have examined the relation between total protein intake and strength in adults have shown nonsignificant results (11, 36, 37), the current study showed that individuals in the lowest quartile of dietary protein had significantly lower QS than did individuals in the higher quartiles of intake. Previous research in the older Framingham Offspring Cohort showed that greater plant protein intake was positively associated with QS, whereas animal protein intake showed no significant association (13). Therefore, the current study further examined protein intake by dietary patterns. With the use of this novel protein-centric dietary pattern methodology, no significant differences across protein food clusters were shown with either ALM or QS after other known risk factors were taken into account. These results suggest that, in a protein-replete population of largely middle-aged adults, higher intake of dietary protein is linked with lean mass and strength, but the dietary protein food pattern does not further clarify the associations with these measures of muscle health. The lack of differences in either muscle mass or strength across protein food clusters is an important finding because future public-health messages that encourage older adults to meet required protein intakes do not need to provide complicated recommendations about specific protein-containing foods. Increased dietary protein intake, regardless of the food source, will likely aid in the success of reaching required amounts in a population in whom adequate energy intake is already a problem.
Because of the single time point of the bone and muscle assessment, this study was unable to determine whether dietary protein food choices and patterns would alter bone and muscle health over time. In addition, there were some inherent limitations of the cluster methodology used such as its sensitivity to outliers (26). To overcome these limitations, individuals who fell >5 SDs from the mean of any one protein food group were removed in addition to participants who formed a cluster with <10 individuals. The naming of protein food clusters is subjective; therefore, the current study described methods of interpreting cluster formations in detail. Note that, although the clusters were named by their greatest contributions to overall protein intakes (such as the chicken cluster), the clusters all contained a mix of protein food sources. However, the cluster analysis offered advantages over alternative quantitative approaches because it classified participants into mutually exclusive, relatively homogenous clusters on the basis of a specified attribute (i.e., the percentage contribution of food groups to total protein intake). The use of the FFQ had inherent limitations because of the lack of detailed information on portion sizes and specific recipes with the potential for systematic errors that could have been due to the underreporting or overreporting of food intakes. Systematic errors can be partially mitigated through energy adjustment as was used in the current study. The use of the FFQ is best suited for ranking the typical nutrient intakes of individuals and for food-patterning techniques as were used in the current study.
To our knowledge, the use of the percentage contribution of protein intake from foods to total dietary protein intake is novel. The only other use of this methodology that was specific to protein intake was in the older Framingham Offspring Cohort (20). Each cohort produced a different number of clusters (5 clusters in the Offspring Cohort and 6 clusters in the Third Generation Cohort). Although some of these clusters showed similar patterns of protein intake (both cohorts identified a low fat–milk cluster, a red-meat cluster, and a chicken cluster) there were some differences whereby the younger generation classified some participants into a cluster of legumes, nuts, seeds, fruit, and vegetables. It is important to take these differences in intakes of dietary protein food patterns across generations into consideration when interpreting studies with varied results on this topic.
In conclusion, in this large cohort of non-Hispanic white men and women aged 19–72 y, total protein intake is positively associated with ALM and QS but not with BMD. The protein intake pattern, as described by a cluster analysis, is not associated with differences in BMD, muscle mass, or muscle strength in this population. Null results may be explained by the protein-replete population studied with intakes well in excess of the RDA for dietary protein (on average, 30–40 g above the RDA). In protein-replete adults, protein food pattern (source) may not contribute to musculoskeletal outcomes in a meaningful manner.
Acknowledgments
The authors’ responsibilities were as follows—KMM, SS, KLT, and MTH: designed the research; KMM, SS, DPK, KLT, and MTH: conducted the research; KMM and ABD: analyzed the data; KMM and MTH: had primary responsibility for the final content of the manuscript; and all authors: wrote the manuscript, read and approved the final manuscript, and approved the decision to submit the manuscript for publication. DPK reported being on the scientific advisory boards of Merck Sharp and Dohme, Amgen, and Ammonett Pharma and having received royalties from Springer for editorial work and author royalties from Wolter Kluwer for the UpToDate resource (Wolter Kluwer). Both DPK and MTH have received institutional grants from Merck Sharp and Dohme. None of the other authors reported any conflicts of interest related to the study.
Footnotes
Abbreviations used: ALM, appendicular lean mass; ALM/ht2, appendicular lean mass normalized for height; BMD, bone mineral density; FFQ, food-frequency questionnaire; QS, quadriceps strength; RDA, Recommended Daily Allowance.
REFERENCES
- 1.Odén A, McCloskey EV, Johansson H, Kanis JA. Assessing the impact of osteoporosis on the burden of hip fractures. Calcif Tissue Int 2013;92:42–9. [DOI] [PubMed] [Google Scholar]
- 2.Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA 2009;302:1573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall SE, Williams JA, Senior JA, Goldswain PR, Criddle RA. Hip fracture outcomes: quality of life and functional status in older adults living in the community. Aust N Z J Med 2000;30:327–32. [DOI] [PubMed] [Google Scholar]
- 4.Randell AG, Nguyen TV, Bhalerao N, Silverman SL, Sambrook PN, Eisman JA. Deterioration in quality of life following hip fracture: a prospective study. Osteoporos Int 2000;11:460–6. [DOI] [PubMed] [Google Scholar]
- 5.McLean RR, Shardell MD, Alley DE, Cawthon PM, Fragala MS, Harris TB, Kenny AM, Peters KW, Ferrucci L, Guralnik JM, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol A Biol Sci Med Sci 2014;69:576–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hannan MT, Tucker KL, Dawson-Hughes B, Cupples LA, Felson DT, Kiel DP. Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res 2000;15:2504–12. [DOI] [PubMed] [Google Scholar]
- 7.Rapuri PB, Gallagher JC, Haynatzka V. Protein intake: effects on bone mineral density and the rate of bone loss in elderly women. Am J Clin Nutr 2003;77:1517–25. [DOI] [PubMed] [Google Scholar]
- 8.Beasley JM, LaCroix AZ, Larson JC, Huang Y, Neuhouser ML, Tinker LF, Jackson R, Snetselaar L, Johnson KC, Eaton CB, et al. Biomarker-calibrated protein intake and bone health in the Women’s Health Initiative clinical trials and observational study. Am J Clin Nutr 2014;99:934–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, Lee JS, Sahyoun NR, Visser M, Kritchevsky SB, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the health, aging, and body composition (Health ABC) Study. Am J Clin Nutr 2008;87:150–5. [DOI] [PubMed] [Google Scholar]
- 10.Meng X, Zhu K, Devine A, Kerr DA, Binns CW, Prince RL. A 5-year cohort study of the effects of high protein intake on lean mass and BMC in elderly postmenopausal women. J Bone Miner Res 2009;24:1827–34. [DOI] [PubMed] [Google Scholar]
- 11.Scott D, Blizzard L, Fell J, Giles G, Jones G. Associations between dietary nutrient intake and muscle mass and strength in community-dwelling older adults: the Tasmanian Older Adult Cohort Study. J Am Geriatr Soc 2010;58:2129–34. [DOI] [PubMed] [Google Scholar]
- 12.Pasiakos SM, McLellan TM, Lieberman HR. The effects of protein supplements on muscle mass, strength, and aerobic and anaerobic power in healthy adults: a systematic review. Sports Med 2015;45:111–31. [DOI] [PubMed] [Google Scholar]
- 13.Sahni S, Mangano KM, Hannan MT, Kiel DP, McLean RR. Higher protein intake is associated with higher lean mass and quadriceps muscle strength in adult men and women. J Nutr 2015;145:1569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLean RR, Mangano KM, Hannan MT, Kiel DP, Sahni S. Dietary protein intake is protective against loss of grip strength among older adults in the Framingham Offspring Cohort. J Gerontol A Biol Sci Med Sci 2016;71:356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isanejad M, Mursu J, Sirola J, Kroger H, Rikkonen T, Tuppurainen M, Erkkila AT. Dietary protein intake is associated with better physical function and muscle strength among elderly women. Br J Nutr 2016;115:1281–91. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert JA, Bendsen NT, Tremblay A, Astrup A. Effect of proteins from different sources on body composition. Nutr Metab Cardiovasc Dis 2011;21(Suppl 2):B16–31. [DOI] [PubMed] [Google Scholar]
- 17.Massey LK. Dietary animal and plant protein and human bone health: a whole foods approach. J Nutr 2003;133:862S–5S. [DOI] [PubMed] [Google Scholar]
- 18.Genaro Pde S, Martini LA. Effect of protein intake on bone and muscle mass in the elderly. Nutr Rev 2010;68:616–23. [DOI] [PubMed] [Google Scholar]
- 19.Quatromoni PA, Copenhafer DL, Demissie S, D’Agostino RB, O’Horo CE, Nam BH, Millen BE. The internal validity of a dietary pattern analysis. The Framingham Nutrition Studies. J Epidemiol Community Health 2002;56:381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mangano KM, Sahni S, Kiel DP, Tucker KL, Dufour AB, Hannan MT. Bone mineral density and protein-derived food clusters from the Framingham Offspring Study. J Acad Nutr Diet 2015;115:1605–13.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gagnon DR, McLean RR, Hannan MT, Cupples LA, Hogan M, Kiel DP. Cross-calibration and comparison of variability in 2 bone densitometers in a research setting: the Framingham experience. J Clin Densitom 2010;13:210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26, discussion 27–36. [DOI] [PubMed] [Google Scholar]
- 23.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 24.Jacques PF, Sulsky SI, Sadowski JA, Phillips JC, Rush D, Willett WC. Comparison of micronutrient intake measured by a dietary questionnaire and biochemical indicators of micronutrient status. Am J Clin Nutr 1993;57:182–9. [DOI] [PubMed] [Google Scholar]
- 25.Kannel WB, Sorlie P. Some health benefits of physical activity. The Framingham Study. Arch Intern Med 1979;139:857–61. [PubMed] [Google Scholar]
- 26.Newby PK, Tucker KL. Empirically derived eating patterns using factor or cluster analysis: a review. Nutr Rev 2004;62:177–203. [DOI] [PubMed] [Google Scholar]
- 27.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65(4 Suppl):1220S–8S; discussion 1229S–31S. [DOI] [PubMed] [Google Scholar]
- 28.Michels KB, Greenland S, Rosner BA. Does body mass index adequately capture the relation of body composition and body size to health outcomes? Am J Epidemiol 1998;147:167–72. [DOI] [PubMed] [Google Scholar]
- 29.Kerstetter JE, Looker AC, Insogna KL. Low dietary protein and low bone density. Calcif Tissue Int 2000;66:313. [DOI] [PubMed] [Google Scholar]
- 30.Zoltick ES, Sahni S, McLean RR, Quach L, Casey VA, Hannan MT. Dietary protein intake and subsequent falls in older men and women: the Framingham Study. J Nutr Health Aging 2011;15:147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Misra D, Berry SD, Broe KE, McLean RR, Cupples LA, Tucker KL, Kiel DP, Hannan MT. Does dietary protein reduce hip fracture risk in elders? The Framingham Osteoporosis Study. Osteoporos Int 2011;22:345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahni S, Cupples LA, McLean RR, Tucker KL, Broe KE, Kiel DP, Hannan MT. Protective effect of high protein and calcium intake on the risk of hip fracture in the Framingham Offspring Cohort. J Bone Miner Res 2010;25:2770–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu K, Meng X, Kerr DA, Devine A, Solah V, Binns CW, Prince RL. The effects of a two-year randomized, controlled trial of whey protein supplementation on bone structure, IGF-1, and urinary calcium excretion in older postmenopausal women. J Bone Miner Res 2011;26:2298–306. [DOI] [PubMed] [Google Scholar]
- 34.Kerstetter JE, Bihuniak JD, Brindisi J, Sullivan RR, Mangano KM, Larocque S, Kotler BM, Simpson CA, Cusano AM, Gaffney-Stomberg E, et al. The effect of a whey protein supplement on bone mass in older Caucasian adults. J Clin Endocrinol Metab 2015;100:2214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sukumar D, Ambia-Sobhan H, Zurfluh R, Schlussel Y, Stahl TJ, Gordon CL, Shapses SA. Areal and volumetric bone mineral density and geometry at two levels of protein intake during caloric restriction: a randomized, controlled trial. J Bone Miner Res 2011;26:1339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartali B, Frongillo EA, Stipanuk MH, Bandinelli S, Salvini S, Palli D, Morais JA, Volpato S, Guralnik JM, Ferrucci L. Protein intake and muscle strength in older persons: does inflammation matter? J Am Geriatr Soc 2012;60:480–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulla UZ, Cooper R, Mishra GD, Kuh D, Stephen AM. Adult macronutrient intake and physical capability in the MRC National Survey of Health and Development. Age Ageing 2013;42:81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


