Abstract
Background: The assessment of polyphenol intake in free-living subjects is challenging, mostly because of the difficulty in accurately measuring phenolic content and the wide presence of phenolics in foods.
Objective: The aims of this study were to evaluate the validity of polyphenol intake estimated from food-frequency questionnaires (FFQs) by using the mean of 6 measurements of a 24-h dietary recall (24-HR) as a reference and to apply a unique method-of-triads approach to assess validity coefficients (VCs) between latent “true” dietary estimates, total urinary polyphenol (TUP) excretion, and a surrogate biomarker (plasma carotenoids).
Design: Dietary intake data from 899 adults of the Adventist Health Study 2 (AHS-2; 43% blacks and 67% women) were obtained. Pearson correlation coefficients (r), corrected for attenuation from within-person variation in the recalls, were calculated between 24-HRs and FFQs and between 24-HRs and TUPs. VCs and 95% CIs between true intake and polyphenol intakes from FFQs, 24-HRs, and the biomarkers TUPs and plasma carotenoids were calculated.
Results: Mean ± SD polyphenol intakes were 717 ± 646 mg/d from FFQs and 402 ± 345 mg/d from 24-HRs. The total polyphenol intake from 24-HRs was correlated with FFQs in crude (r = 0.51, P < 0.001) and deattenuated (r = 0.63; 95% CI: 0.61, 0.69) models. In the triad model, the VC between the FFQs and theoretical true intake was 0.46 (95% CI: 0.20, 0.93) and between 24-HRs and true intake was 0.61 (95% CI: 0.38, 1.00).
Conclusions: The AHS-2 FFQ is a reasonable indicator of total polyphenol intake in the AHS-2 cohort. Urinary polyphenol excretion is limited by genetic variance, metabolism, and bioavailability and should be used in addition to rather than as a replacement for dietary intake assessment.
Keywords: Adventist Health Study 2, method of triads, phytochemicals, polyphenols, urinary polyphenols
INTRODUCTION
Polyphenols are a large group of phytochemicals abundant in plants (1) that have shown various health functions in humans, including anti-inflammatory, antiobesity, and anticarcinogenic effects (2). Epidemiologic evidence indicates that polyphenol intake is associated with a reduced risk of chronic diseases, including type 2 diabetes and cardiovascular diseases (3–5). Quantifying polyphenol intake is challenging and susceptible to systematic and random errors related to the variability in the polyphenol content of foods, error related to dietary self-report, and differences in absorption and metabolism between individuals (6). Because of the inherent limitations of dietary self-report, which include the likely possibility that errors of 2 dietary assessment tools are correlated, the use of ≥1 biomarker is particularly advantageous for validation studies (7).
The Folin-Ciocalteu assay of polyphenols in urine samples is considered a specific biomarker for total polyphenol intake and may also serve as a marker of dietary fruit and vegetable intake (8, 9). The use of total urinary polyphenol (TUP)4 concentration as a biomarker is comparable to plasma measurements of polyphenols (10) and is currently the most well-studied and promising biomarker of total polyphenol intake (11). Polyphenols undergo microbial conversion through oxidation, hydroxylation, and conjugation, so that the diverse original compounds share common metabolites (12–14). Not all polyphenol compounds are excreted in urine (15); however, a variety of phenolic compounds reach maximum concentrations in urine ∼2.5 h after ingestion (16). Although total polyphenols measured by 24-h urine volume is the preferred urinary biomarker of polyphenol intake, the 4- or 12-h volume corrected by urinary creatinine is also a suitable biomarker of total polyphenols and may serve as a reliable tool to study relations between polyphenol intake and health (17).
Similar to polyphenols, the major dietary carotenoids (i.e., β-carotene, α-carotene, lycopene, lutein and zeaxanthin, and β-cryptoxanthin) are concentrated in fruit and vegetables (18). Plasma carotenoids and urinary flavonoids have been used together as biomarkers of fruit, vegetable, and juice intakes in previous validation studies (19). Other studies have shown correlations between intakes of polyphenol-rich food groups, such as citrus, coffee, wine, and vegetables, and plasma β-cryptoxanthin concentrations (20) or dietary carotenoid intake (21). Plasma concentrations of carotenoids can be quantified by using HPLC (22), and this biomarker is considered a valid and reliable measure of ∼1–2 wk of carotenoid intake (23–25).
The method of triads (26) is an application of factor analysis in which the correlation of the estimate by using the dietary assessment tool and a person’s theoretical true usual intake is estimated from 3 pairwise correlations between the food-frequency questionnaire (FFQ), the reference method, and a biomarker. This technique assumes that a positive correlation occurs between intake estimates and the latent true intake and that random errors in dietary assessments are not correlated (26, 27). In the present secondary analysis of the Adventist Health Study 2 (AHS-2) calibration study, a modified approach to the method of triads with the use of 2 concentration biomarkers (28) is applied to validate polyphenol intake estimated from the AHS-2 FFQ in a sample population with a wide range of dietary intakes.
METHODS
Study population
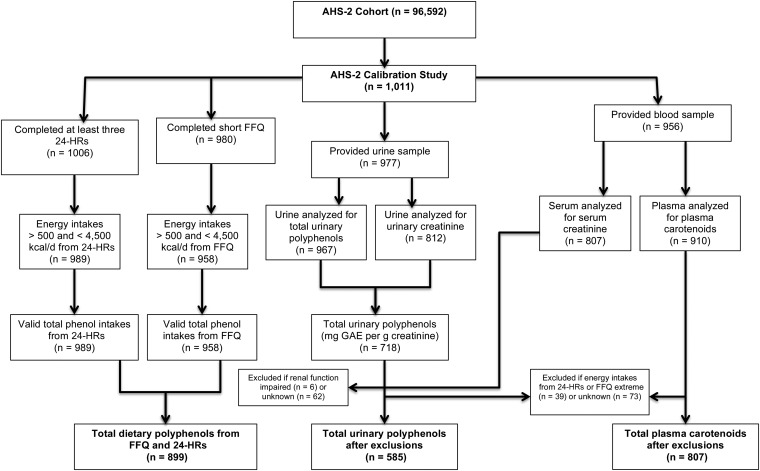
The AHS-2 is a prospective cohort of >96,000 Seventh-Day Adventist men and women recruited from the United States and Canada between 2002 and 2007. The study is designed primarily to investigate relations between diet and health outcomes. The profile and recruitment methods of the cohort have been previously described (29). In brief, each participant completed a 50-page, self-administered AHS-2 questionnaire at baseline that aimed to assess diet, physical activity, supplement use, and medical history. Participants were asked to report weight and height in the baseline questionnaire, and these values were used to calculate BMI. The level of education was determined by categories of the highest level of education completed. History of cigarette smoking and alcohol consumption was categorized on the basis of current or past use. Physical activity status was based on the average number of hours per week spent engaging in energetic physical exercise combined with physical activity level at work. To validate AHS-2 data on self-reported diet and physical activity, a calibration substudy was conducted between 2002 and 2007, which is described in full detail elsewhere (30). In addition to the baseline AHS-2 Diet and Lifestyle Questionnaire, participants of the AHS-2 calibration study (n = 1011) provided 2 sets of three 24-h dietary recalls (24-HRs) and an FFQ similar to that completed at baseline. In the middle of the 24-HR sets, subjects attended a clinic at their church where an overnight urine sample, blood sample, and body-composition measurements were collected. The calibration showed no significant differences in the distribution of dietary pattern, education, sex, or age between the substudy sample and the parent cohort (30). The AHS-2 is a health-oriented population with very low rates of smoking (1%) and alcohol intake (6.6%; usually at very low quantities) (31), as well as dietary patterns ranging from vegan to omnivorous. The diversity in diets introduces varying intakes of polyphenol-rich foods, including fruit and vegetables, grains, soy products, and other vegetarian protein sources. Participants included in this analysis are members of the AHS-2 calibration study (n = 906; see Figure 1). The calibration study was approved by the Loma Linda University institutional review board, and all of the participants gave their written informed consent.
FIGURE 1.
Study design and flowchart. AHS-2, Adventist Health Study 2; FFQ, food-frequency questionnaire; GAE, gallic acid equivalents; 24-HR, 24-h dietary recalls.
Dietary assessment
The 24-HRs were collected from AHS-2 calibration study participants by unannounced telephone interview with the use of a multiple-pass approach to gather information about food, supplements, and beverages consumed during the past 24 h. During the first 2 mo, three 24-HRs were obtained (i.e., intakes for 1 Saturday, 1 Sunday, and 1 weekday); ∼6 mo later, this process was repeated. The aim was to obtain 2 sets of recalls, each representing a synthetic week (a total of six 24-HRs), from each subject (30). The 24-HRs were gathered in 2 blocks 5–6 mo apart, and the FFQs and biomarkers were collected at clinics timed between the 2 recall blocks. The Nutrition Data System for Research version 4.06 or 5.0 (The Nutrition Coordinating Center) was used for 24-HR data entry.
During a 6-mo period between 24-HR sets, participants completed a self-administered FFQ. Responses were reviewed, and incomplete information was clarified by telephone follow-up. The FFQ is a 22-page instrument that contains a food list of 204 hard-coded items, write-ins for 46 open-ended items, and 54 questions about food preparation methods. The survey provides information on food item, frequency of consumption, and portion size, which are necessary to estimate mean daily food and nutrient intakes. Questionnaires were scanned by using the Nilson Candle Scanner 5000i Image Scanner with ScanTools Plus software (Pearson NCS) to archive an electronic copy of data.
Polyphenol food-composition database
The polyphenol content of foods in the AHS-2 cohort was produced by using a combination of all available data on polyphenol concentrations in foods derived from chromatography analysis. The concentrations of polyphenols in various foods were gathered from the Phenol-Explorer 3.6 database (32) and the USDA flavonoid version 3.1 (33) and isoflavones version 2.0 (34) databases, and the individual research literature was consulted for polyphenol concentrations of a select few foods [e.g., okra (35), rice flour (36), and oat bran and millet (12)]. Of the complete data, ∼78% were from Phenol-Explorer, 21% were from USDA databases, and 1% were from individual studies. The USDA isoflavones database provided data for a variety of soy foods, which allowed more comprehensive estimates of polyphenol intake in the AHS-2. The data obtained refer to intact polyphenols (i.e., glycosides and esters) for most compounds, except for values that were obtained from analysis by using chromatography after hydrolysis or from USDA databases. For foods that contained lignans and phenolic acids that are only detectable after hydrolysis (i.e., beans, olives, and cereals), data obtained by using chromatography after hydrolysis were also gathered. Foods with unknown polyphenol concentrations (e.g., cottonseed oil, wheat gluten, coconut milk, and cola) were considered to have no polyphenols. Polyphenol concentrations for foods in our database were standardized to milligrams per 100 g, and the data acquired for individual foods were expanded to incorporate the calculation of recipes as well as estimations of missing values.
Estimating polyphenol intake
Total dietary polyphenol (TDP) intake is the sum of all flavonoids, phenolic acids, stilbenes, lignans, and other phenolics in foods consumed (Supplemental Table 1). The TDP intake for each participant was estimated by using the following: TDP from FFQ = Σ Pn × Fn × Gn × Sn, where P = milligrams of phenolic compounds per 100 g foodn, F = the reported frequency of intake of foodn, G = the standard serving size of foodn in grams, and S = the reported servings of foodn. Foods recorded in 24-HRs and our polyphenol food-composition data were matched, and TDP intake was calculated on the basis of the portions reported, so that TDPs from 24-HRs = Σ Pn × Gn, where P = milligrams of phenolic compounds per 100 g foodn and G = the reported portion sizes of foodn in grams.
Urinary polyphenols
The Folin-Ciocalteu method was used to determine total polyphenol concentration in 12-h overnight urine samples of AHS-2 calibration study participants (n = 967) by using mixed-mode anion-exchange and reversed-phase solvent (Oasis MAX) cartridges (Waters Corporation) for solid-phase extraction (10). The Folin-Ciocalteu method was used because it provides the best recovery for total polyphenols and is a relatively simple and efficient technique that allows for the analysis of many samples at one time. Gallic acid was the chosen standard because it is most often used and is both a stable and pure substance. In addition, the response to gallic acid has been shown to be equivalent to most other phenolics when measuring polyphenols in food on a mass basis (17, 37, 38). The solid-phase cleanup before the Folin-Ciocalteu assay prevents interference of other substances such as ascorbic acid, aromatic amines, iron, organic acids, sugar, and sulfur dioxide present in urine (9).
The 12-h overnight urine samples were collected by participants between 2002 and 2007 and processed by AHS-2 clinic staff. Urine volume was recorded, and then two 90-mL specimen cups were each filled to obtain ≤70-mL aliquots; 1 mL of 35% hydrochloric acid was added to 1 aliquot before freezing to prevent oxidation of labile compounds (39). In 2015, urine samples were thawed in a refrigerator at ∼1.6°C, mixed on a vortex machine for 10 s each, and then centrifuged for 10 min at 4°C at 2350 × g. All of the samples and standards were handled with minimal exposure to light. Synthetic urine was prepared by using 25 g pure urea in 500 mL distilled water. Standard gallic acid dilutions at concentrations of 50, 25, and 12.5 mg/L in synthetic urine were created for the calibration of each assay. The 50-mg/L concentration was made from 100 μL stock gallic acid plus 1900 μL synthetic urine, which was diluted 1:1 with synthetic urine to make the 25 and 12.5 mg/L dilutions. To equilibrate the cartridges, 1 mL 98% methanol and 1 mL sodium acetate (50 mmol/L, pH 7) were loaded. Urine samples were diluted 1:1 with distilled water, and then 1 mL of each diluted sample was loaded and rinsed with sodium acetate (50 mmol/L, pH 7) in 5% methanol. Phenolic compounds were eluted with 1900 μL 2% formic acid in methanol.
Standard concentrations of 50, 25, and 12.5 mg gallic acid/L were analyzed on each plate. After purification, 15 μL eluted fractions was added to 4 wells of the Thermo microtiter 96-well plate (Waters Corporation) so that 20 samples were run on each plate. Each well was mixed with 170 μL distilled water, and then 12 μL Folin-Ciocalteu reagent was added to each well. The multichannel pipette reduced differences in the times of the Folin-Ciocalteu reaction between the 8 lines of the 96-well plate so that the reaction time was similar for all samples analyzed on the same plate. Sodium carbonate was added to each well to initiate the reaction, and the plate was held in the dark for 60 min. After the reaction delay, 73 μL distilled water was deposited into each well and absorbance was measured at 765 nM in ultraviolet-visible spectroscopy in the Bio-Tek Synergy HT spectrometer (BioTek Instruments Inc.). Concentrations of total polyphenol equivalents were adjusted for dilutions, and mean TUP values were calculated by using creatinine correction. Repeatability was evaluated for quality assurance, and the CVs for intra- and interassays were 2.75% and 2.97%, respectively.
Urinary creatinine concentrations were determined previously in 2008 by the Cancer Research Center of Hawaii with a clinical auto-analyzer (Roche-Cobas MiraPlus; Roche Diagnostics) by using a test kit based on the Jaffé reaction (Randox Laboratories). Limits of detection for all analytes were 10 nmol creatinine/L or 2–50 pg creatinine/mg. Urinary polyphenol concentrations were adjusted for creatinine concentrations, and TUPs were expressed as milligrams of gallic acid equivalents per gram of creatinine. Renal function was estimated by using the Cockcroft-Fault equation (40): estimated glomerular filtration rate = (140 − age) weight × 0.85 (if female)/(serum creatinine × 72). Participants were categorized as having normal renal function if ≥60 mL/min or as having impaired renal function if <60 mL/min.
Plasma carotenoids
After fasting blood samples were drawn from AHS-2 calibration study participants, plasma was centrifuged immediately and transported to Loma Linda University on frozen gel packs within 30 h of collection, and samples were stored on liquid nitrogen. In 2008, plasma β- carotene, α-carotene, lycopene, lutein, and zeaxanthin were quantified at the UCLA Center for Human Nutrition from these blood samples by using HPLC. The Agilent 1050 HPLC (Agilent Technology) with multiple wavelength detectors was used for this analysis. A YMC carotenoids column (Waters Corporation), 250 × 4.6 mm, with a Vydac C18 guard column (Alltech) was used, and carotenoids were detected at 445 nm. The CVs for the intra-assay pooled plasma sample were 7.4% for lutein, 10.3% for β-carotene, 11.5% for β-cryptoxanthin, 12.2% for lycopene, and 14.2% for α-carotene (41).
Statistical analysis
The variables TUPs and plasma carotenoids were transformed by using log(x + 1) for statistical tests to approximate normality and to avoid zero values. Because renal function varies with creatinine excretion, participants with impaired renal function (i.e., estimated glomerular filtration rate <60 mL/min according to the Cockcroft-Fault equation) were excluded from analyses (n = 6) involving the TUP biomarker. Polyphenol intakes from food groups estimated by 24-HR and the FFQ were energy-adjusted by using a partitioned energy-adjustment method, in which data that are initially zero remain zero and energy adjustment is performed on nonzero data only. A similar approach has been described elsewhere (42). Briefly, a log(x + 1) transformation was applied to nonzero data before energy adjustment. Then, let y = energy-adjusted residual + mean of log(x + 1), which we used as the energy-adjusted values on the logarithmic scale for nonzero data. Original zero values remained as zero. For the 24-HR, the transformed values from each recall were weighted to produce a synthetic week [(Saturday intake + Sunday intake + 5 × weekday intake)/7]. Finally, synthetic weeks 1 and 2 were averaged to produce the energy-adjusted mean daily polyphenol intake on the logarithmic scale.
The mean ± SD polyphenol intake was estimated for each dietary assessment method. Unadjusted, deattenuated, and partial correlations (also deattenuated) with adjustments for age, race, sex, and BMI were determined between each dietary assessment, and the biomarkers TUPs and plasma carotenoids. In race-specific analysis, race was excluded from the model. Correlations between 24-HRs and FFQs, TUPs, plasma carotenoids, and polyphenol intakes from select food groups were corrected for attenuation due to within-person variation in the recalls (43). A partitioned deattenuation (42) was applied, so that where all six 24-HRs are zero values, the within-person variance is zero or nearly zero, so that no within-person adjustment for error is necessary. The deattenuated values were obtained by removing the deattenuating effects of random, within-person errors of the recall weeks when correlating recalls with questionnaire data, as described by Willett (44). The following formula was used for correlating FFQ (Q) and recall (R) data:
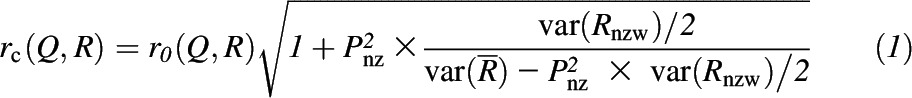 |
where r0 = the uncorrected correlation, rc = the corrected correlation, Pnz = the proportion of nonzeros, and Rnzw = the within-person variance of nonzeros from recalls.
If at least one 24-HR week contained nonzero data, the within-person variance was corrected and deattenuated correlations between total polyphenol intake and polyphenol intakes from select food groups from 24-HRs compared with FFQs were estimated. The 95% CIs for corrected validity coefficients (VCs) were calculated by using bootstrap 2000 resampling and the bias-corrected and accelerated bootstrap (BCa) method (45). The method of triads was applied to compute VCs between theoretical true intake and the biomarkers and polyphenol intakes from FFQs and 24-HRs (which were also deattenuated). Here we include either the 24-HRs (R) or the FFQs (Q) as dietary estimators and 2 independent biomarkers of intake, total urinary polyphenols (M) and plasma carotenoids (P), as biomarkers. Where r is an estimated correlation coefficient, the VCs are calculated as follows:
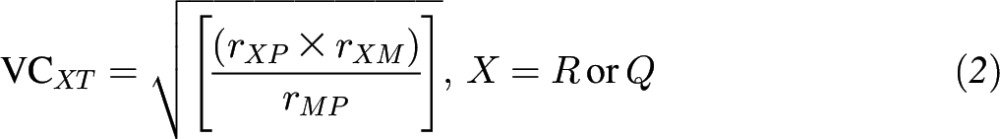 |
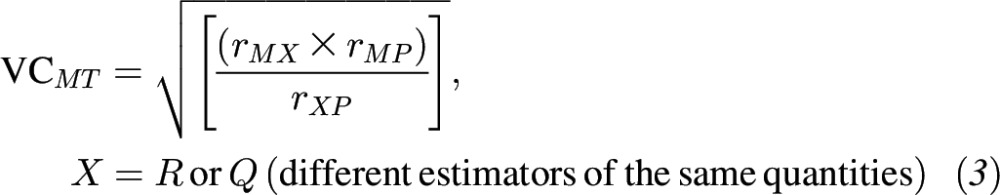 |
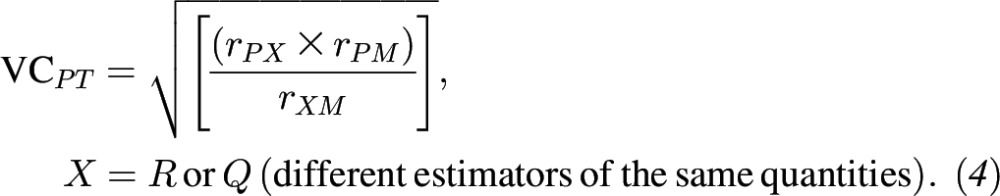 |
Analyses were completed by using IBM SPSS version 2.1 (IBM Corporation) and R version 3.2.2 (R Foundation for Statistical Computing).
RESULTS
The mean unadjusted polyphenol intake estimates were 717 ± 646 mg/d from FFQs and 402 ± 345 mg/d from 24-HRs; the mean TUP concentration was 108 ± 98.9 mg gallic acid equivalents/g creatinine (Table 1). Correlations between energy-adjusted polyphenol intakes from 24-HRs with FFQs, TUPs, and plasma carotenoids in crude models and after correction for attenuation due to within-person variation in 24-HRs, with further adjustment for differences in age, race, sex, and BMI, are shown in Table 2. In race-specific analysis, correlations between TDPs from 24-HRs and FFQs improved after deattenuation in the recalls in nonblack and black subjects by 18% and 27%, respectively. Correlations between TDPs from 24-HRs and biomarkers were higher among black subjects than in nonblacks.
TABLE 1.
General descriptive characteristics of the study subjects1
| Characteristics | Value |
| Age, y | 58.2 ± 13.32 |
| Sex, % female | 66.5 |
| Race, % | |
| Black | 43.1 |
| Nonblack | 56.9 |
| BMI, kg/m2, % | |
| Underweight or normal weight (18.5–24.9) | 40.4 |
| Overweight (25–29.9) | 34.4 |
| Obese (≥30) | 25.2 |
| Energy intake, kcal/d | |
| From FFQ | 1887 ± 755 |
| From 24-HR | 1555 ± 492 |
| Renal function,3 % | |
| Normal | 99 |
| Impaired | <1 |
| Urinary creatinine, g/L | 0.69 ± 0.58 |
| Total dietary polyphenol intake,4 mg/d | |
| From 24-HR | 402 ± 345 |
| From short FFQ | 717 ± 646 |
| Total urinary polyphenols, mg GAE/g creatinine | 108 ± 98.9 |
| Plasma total carotenoids, μg/L | 1.6 ± 1.0 |
n = 899. The proportions of missing data are as follows: age, 8%; sex, 7.4%; race, 8.4%; BMI, 7.8%; renal function, 24%; urinary creatinine, 24%; total urinary polyphenols, 29%; and plasma carotenoids, 9.8%. FFQ, food-frequency questionnaire; GAE, gallic acid equivalents; 24-HR, 24-h dietary recall.
Mean ± SD (all such values).
Renal function was defined by estimated glomerular filtration rate according to the Cockcroft-Fault equation: normal renal function if ≥60 mL/min or impaired renal function if <60 mL/min.
Unadjusted intake estimates are shown.
TABLE 2.
Uncorrected, unadjusted deattenuated, and adjusted deattenuated correlations between energy-adjusted TDPs from 24-HRs and TDPs from FFQs, TUP biomarker, and total plasma carotenoids, stratified by race1
| TDPs (FFQ), mg/d |
TUPs,2 mg GAE/g urinary creatinine |
Plasma carotenoids,2 μg/L |
|||||||
| Uncorrected correlation | Deattenuated unadjusted correlation (95% CI) | Deattenuated adjusted correlation (95% CI)3 | Uncorrected correlation | Deattenuated unadjusted correlation (95% CI) | Deattenuated adjusted correlation (95% CI)3 | Uncorrected correlation | Deattenuated unadjusted correlation (95% CI) | Deattenuated adjusted correlation (95% CI)3 | |
| TDPs (24-HR),4 mg/d | |||||||||
| All (n = 823) | 0.51* | 0.64 (0.55, 0.71) | 0.63 (0.61, 0.69) | 0.15* | 0.19 (0.09, 0.29) | 0.20 (0.13, 0.32) | 0.14* | 0.18 (0.10, 0.27) | 0.18 (0.12, 0.27) |
| Nonblacks (n = 468) | 0.58* | 0.71 (0.54, 0.81) | 0.71 (0.64, 0.81) | 0.14* | 0.16 (0.04, 0.27) | 0.17 (0.07, 0.30) | 0.12* | 0.14 (0.03, 0.26) | 0.13 (0.03, 0.24) |
| Blacks (n = 355) | 0.38* | 0.52 (0.36, 0.66) | 0.52 (0.47, 0.66) | 0.17* | 0.23 (0.05, 0.45) | 0.23 (0.09, 0.42) | 0.21* | 0.28 (0.13, 0.42) | 0.29 (0.20, 0.44) |
TDPs from 24-HRs or FFQs for uncorrected, deattenuated unadjusted, and adjusted deattenuated correlations were energy adjusted. *P < 0.001 (2-tailed). FFQ, food-frequency questionnaire; GAE, gallic acid equivalents; TDP, total dietary polyphenol; TUP, total urinary polyphenol; 24-HR, 24-h dietary recall.
Transformed by using natural log + 1.
Adjusted for age, race, sex, and BMI. For race-specific analysis, race was removed from the model.
Corrected for attenuation due to within-person error in the recalls. Bias-corrected and accelerated bootstrap CIs were based on 2000 resampling.
Uncorrected and deattenuated partial correlations between FFQ and 24-HR measurements of polyphenol intakes from select food groups, with adjustments for age, race, sex, and BMI, were all significant (Table 3). Correlations between polyphenol intakes from select food groups as estimated by 24-HRs and FFQs ranged from 0.19 to 0.72 in all subjects after deattenuation and adjustment for covariates. The highest correlations were observed for polyphenols from coffee (0.72; 95% CI: 0.65, 0.81) and fruit juice (0.58; 95% CI: 0.57, 0.66). The mean of the deattenuated adjusted correlations between 24-HR and FFQ measurements for polyphenol intakes from various food groups was 0.46, which indicated reasonable validity of the FFQ. The mean deattenuated adjusted correlation was higher for nonblack (0.50) than for black (0.41) subjects (data not shown in Table 3).
TABLE 3.
Energy-adjusted polyphenol intakes from specific food groups: validity correlations between FFQs and 24-HRs1
| All (n = 823) |
Nonblacks (n = 468) |
Blacks (n = 355) |
|||||||
| Polyphenols from food group,2 mg/d | Uncorrected | Deattenuated (95% CI) | Deattenuated adjusted (95% CI) | Uncorrected | Deattenuated (95% CI) | Deattenuated adjusted (95% CI) | Uncorrected | Deattenuated (95% CI) | Deattenuated adjusted (95% CI) |
| Total fruit (n = 899) | 0.39** | 0.48 (0.40, 0.55) | 0.50 (0.47, 0.60) | 0.46** | 0.57 (0.45, 0.66) | 0.60 (0.55, 0.70) | 0.37** | 0.49 (0.37, 0.60) | 0.49 (0.42, 0.63) |
| Red and purple fruit (n = 884) | 0.29** | 0.35 (0.28, 0.42) | 0.35 (0.29, 0.44) | 0.33** | 0.41 (0.31, 0.51) | 0.41 (0.33, 0.52) | 0.27** | 0.31 (0.21, 0.41) | 0.34 (0.23, 0.45) |
| Berries (n = 810) | 0.35** | 0.42 (0.33, 0.50) | 0.45 (0.39, 0.54) | 0.41** | 0.50 (0.39, 0.60) | 0.53 (0.46, 0.65) | 0.16** | 0.20 (0.06, 0.35) | 0.19 (0.04, 0.37) |
| Citrus (n = 834) | 0.25** | 0.39 (0.28, 0.51) | 0.34 (0.24, 0.44) | 0.30** | 0.53 (0.35, 0.76) | 0.43 (0.29, 0.57) | 0.19** | 0.26 (0.12, 0.40) | 0.24 (0.11, 0.38) |
| Apples and pears (n = 877) | 0.32** | 0.41 (0.33, 0.48) | 0.44 (0.40, 0.54) | 0.30** | 0.41 (0.29, 0.52) | 0.42 (0.35, 0.62) | 0.37** | 0.45 (0.34, 0.54) | 0.49 (0.41, 0.60) |
| Orange and yellow fruit (n = 857) | 0.33** | 0.45 (0.34, 0.56) | 0.43 (0.35, 0.57) | 0.40** | 0.58 (0.44, 0.74) | 0.52 (0.41, 0.67) | 0.24** | 0.33 (0.18, 0.51) | 0.27 (0.12, 0.45) |
| Dried fruit (n = 884) | 0.34** | 0.39 (0.25, 0.53) | 0.42 (0.29, 0.58) | 0.42** | 0.49 (0.31, 0.67) | 0.48 (0.31, 0.68) | 0.21** | 0.23 (0.08, 0.41) | 0.33 (0.18, 0.52) |
| Total vegetables (n = 899) | 0.20** | 0.37 (0.22, 0.53) | 0.35 (0.31, 0.48) | 0.24** | 0.48 (0.26, 0.75) | 0.43 (0.36, 0.63) | 0.18** | 0.33 (0.11, 0.62) | 0.31 (0.18, 0.58) |
| Cruciferous vegetables (n = 883) | 0.28** | 0.49 (0.34, 0.62) | 0.52 (0.50, 0.62) | 0.30** | 0.59 (0.36, 0.91) | 0.61 (0.59, 0.74) | 0.26** | 0.40 (0.18, 0.59) | 0.42 (0.29, 0.59) |
| Orange and yellow vegetables (n = 879) | 0.21** | 0.47 (0.29, 0.80) | 0.40 (0.39, 0.55) | 0.21** | 0.53 (0.28, 1.00) | 0.42 (0.41, 0.59) | 0.21** | 0.46 (0.20, 0.95) | 0.38 (0.27, 0.61) |
| Leafy greens (n = 897) | 0.14** | 0.21 (0.10, 0.32) | 0.19 (0.12, 0.33) | 0.12* | 0.20 (0.04, 0.36) | 0.15 (0.04, 0.30) | 0.16** | 0.24 (0.07, 0.40) | 0.28 (0.14, 0.47) |
| Tomatoes (n = 895) | 0.30** | 0.52 (0.38, 0.68) | 0.50 (0.49, 0.64) | 0.26** | 0.50 (0.28, 0.81) | 0.48 (0.44, 0.70) | 0.30** | 0.56 (0.30, 0.86) | 0.44 (0.34, 0.67) |
| Legumes (n = 898) | 0.32** | 0.52 (0.42, 0.64) | 0.49 (0.48, 0.56) | 0.30** | 0.48 (0.34, 0.62) | 0.48 (0.45, 0.60) | 0.32** | 0.55 (0.35, 0.81) | 0.49 (0.41, 0.75) |
| Soy (n = 858) | 0.39** | 0.46 (0.37, 0.53) | 0.47 (0.41, 0.57) | 0.42** | 0.49 (0.38, 0.58) | 0.50 (0.40, 0.59) | 0.34** | 0.41 (0.28, 0.52) | 0.41 (0.30, 0.56) |
| Nuts and seeds (n = 893) | 0.37** | 0.45 (0.36, 0.53) | 0.46 (0.40, 0.56) | 0.40** | 0.48 (0.36, 0.59) | 0.50 (0.41, 0.63) | 0.24** | 0.30 (0.16, 0.42) | 0.32 (0.19, 0.47) |
| Grains (n = 899) | 0.33** | 0.55 (0.43, 0.69) | 0.54 (0.55, 0.63) | 0.30** | 0.52 (0.35, 0.69) | 0.52 (0.48, 0.67) | 0.31** | 0.55 (0.34, 0.82) | 0.56 (0.50, 0.75) |
| Added fats (n = 898) | 0.19** | 0.53 (0.32, 1.00) | 0.38 (0.37, 0.50) | 0.23** | 0.57 (0.29, 1.00) | 0.50 (0.49, 0.68) | 0.15** | 0.43 (0.16, 1.00) | 0.37 (0.33, 0.77) |
| Beverages (n = 890) | 0.57** | 0.70 (0.63, 0.75) | 0.71 (0.69, 0.76) | 0.64** | 0.73 (0.65, 0.78) | 0.73 (0.69, 0.82) | 0.44** | 0.65 (0.52, 0.79) | 0.67 (0.63, 0.80) |
| Fruit juices (n = 853) | 0.40** | 0.55 (0.46, 0.63) | 0.58 (0.57, 0.66) | 0.39** | 0.49 (0.38, 0.59) | 0.51 (0.44, 0.64) | 0.41** | 0.64 (0.50, 0.82) | 0.72 (0.70, 0.86) |
| Vegetable juices (n = 507) | 0.32** | 0.34 (0.16, 0.51) | 0.40 (0.18, 0.57) | 0.34** | 0.37 (0.17, 0.57) | 0.40 (0.19, 0.63) | 0.33** | 0.36 (0.10, 0.62) | 0.39 (0.05, 0.65) |
| Coffee (n = 235) | 0.69** | 0.74 (0.65, 0.80) | 0.72 (0.65, 0.81) | 0.71** | 0.77 (0.66, 0.84) | 0.75 (0.67, 0.84) | 0.55** | 0.58 (0.39, 0.73) | 0.57 (0.31, 0.76) |
| Tea (n = 539) | 0.43** | 0.48 (0.38, 0.57) | 0.49 (0.41, 0.60) | 0.46** | 0.50 (0.33, 0.63) | 0.56 (0.49, 0.68) | 0.39** | 0.45 (0.33, 0.57) | 0.42 (0.31, 0.54) |
Pearson’s partial correlations were adjusted for age, race, sex, and BMI. For deattenuated correlations, a mixed model was used to estimate the within- and between-subject variances. The mixed model included subjects as random effects and age, sex, race (black or nonblack), and BMI as fixed effects. For race-specific analysis, race was removed from the model. *P < 0.05, **P < 0.01 (2-tailed). FFQ, food-frequency questionnaire; 24-HR, 24-h dietary recall.
For unadjusted and corrected correlations, the polyphenol intake from food groups was energy adjusted.
The modified approach that used the method of triads (Figure 2) showed that the VC between the FFQ and the latent true value of polyphenol intake (T) was lower than that of the corresponding coefficient between the 24-HR and T but greater than the coefficient between TUP and T. The deattenuated VCs and 95% CIs for each dietary assessment method and both biomarkers are presented in Table 4. When included together with the FFQ estimate of polyphenol intake, the plasma total carotenoid concentration was more strongly correlated to true polyphenol intake than the TUP concentration.
FIGURE 2.
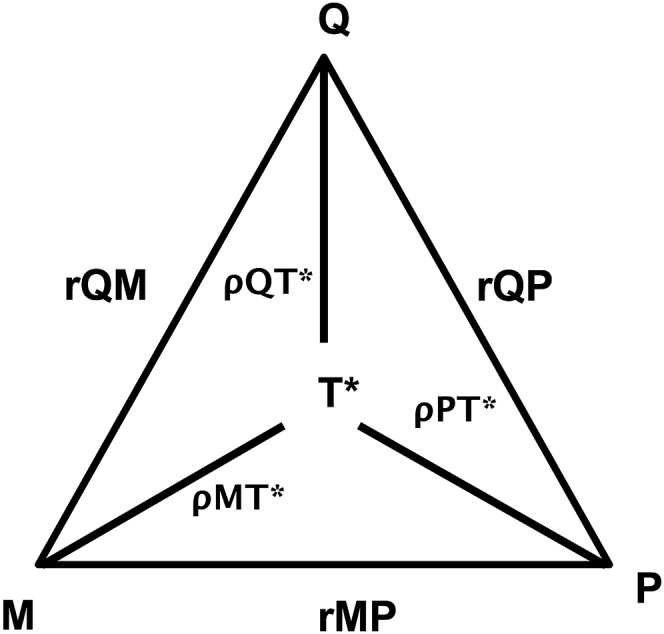
Triads method, comparison between food-frequency questionnaires, total urinary polyphenols, and plasma carotenoids. *T is latent (not directly observed). M, total urinary polyphenols; P, plasma carotenoids; Q, food-frequency questionnaire; rMP, correlation between total urinary polyphenols and plasma carotenoids; rQM, correlation between the food-frequency questionnaire and total urinary polyphenols; rQP, correlation between the food-frequency questionnaire and plasma carotenoids; T, theoretical true intake; ρMT, validity coefficient of total urinary polyphenols; ρPT, validity coefficient of plasma carotenoids as a measure of polyphenol intake; ρQT, validity coefficient of the food-frequency questionnaire.
TABLE 4.
VCs and 95% CIs between T and Q, R, and M and P, calculated by using the method of triads1
| VC | 95% CI | |
| FFQs (Q, T)2 | 0.46 | 0.20, 0.93 |
| TUPs (M, T)3 | 0.18 | 0.04, 0.32 |
| Plasma carotenoids (P, T)3 | 0.49 | 0.25, 1.004 |
| 24-HRs (R, T)2 | 0.61 | 0.38, 1.00 |
| TUPs (M, T)5 | 0.32 | 0.12, 0.58 |
| Plasma carotenoids (P, T)5 | 0.27 | 0.09, 0.46 |
n = 570. Correlations were adjusted for age, race, sex, and BMI. BCa CIs were based on 2000 resampling. Correlations including 24-HRs deattenuated by using a mixed model were used to estimate the within- and between-subject variances. BCa, bias-corrected and accelerated bootstrap; FFQ, food-frequency questionnaire; M, biomarker total urinary polyphenol variable; P, biomarker plasma carotenoids variable; Q, polyphenol intake estimates from FFQs variable; R, polyphenol intake estimates from 24-HRs variable; T, true polyphenol intake variable; TUP, total urinary polyphenol; 24-HR, 24-h dietary recall; VC, validity coefficient.
Polyphenol intakes from FFQs and 24-HRs were transformed by using log (x + 1) and each FFQ and recall were energy-adjusted by using a partitioned approach. The 24-HR variable represents polyphenol intake from the mean of synthetic week 1 and week 2 of recalls.
Uses Q as the dietary estimator.
The estimated upper limit exceeded 1 and was thus set to 1.00.
Uses R as the dietary estimator.
DISCUSSION
In the present study in a generally healthy US adult population, polyphenol intake was within the range of intakes observed in European populations (46, 47). The mean TUP excretion observed in our population sample was lower than that reported in other studies (8). The mean total polyphenol intake from 24-HRs was lower than the polyphenol intake from the FFQ. This is consistent with the results of Jaceldo-Siegl et al. (30), in which the authors validated the AHS-2 FFQ for intakes of various food groups and found that mean intakes of most foods and nutrients were notably higher with the FFQ than with the 24-HR. This often occurs when participants are asked to recall intake of numerous food items, which leads to overreporting the consumption of some foods. In the AHS-2 cohort, beverages and fruit are key contributors to total daily polyphenol intakes (48). It is possible that individuals overreported these items in the FFQ when asked to recall the intake of the several different types of fruit and fruit juice.
Although most FFQ validity studies use data from a single reference method, we used the 24-HR as a reference and in separate analyses applied the method of triads with 2 biomarkers (i.e., TUPs and plasma carotenoids) to find correlations with (latent) true polyphenol intake. The correlations between dietary polyphenols and urinary polyphenols determined from our analysis are higher than those published in other studies that used urinary polyphenols corrected with creatinine normalization (8). Because creatinine is associated with renal function, renal function was accounted for in the model by excluding participants with impaired renal status. In addition, age, sex, and race are covariates that usually affect creatinine clearance and excretion (49). The correlation between polyphenol intake measured by 24-HR and that measured by the FFQ was significant in unadjusted and adjusted models, and correction for attenuation due to within-person error in the 24-HR improved all correlations. Further adjustment for covariates did not greatly alter deattenuated unadjusted correlations between TDPs from 24-HRs and FFQs or the biomarkers. The VC from the method-of-triads analysis estimated for the FFQ was lower than that of the deattenuated 24-HR value, which suggested that the recalls may have superior validity when assessing polyphenol intake, but the wide CIs permit alternative conclusions. Our sample size in this analysis was smaller than that generally recommended when estimating VCs (28), which may have influenced the interval estimates. By using 2 biomarkers and 1 dietary assessment tool in each triad model, we overcame the unrealistic assumption that errors associated with FFQs and 24-HRs are unrelated (28), which is inherent in a naive interpretation of standard correlation coefficients.
The relatively low correlations between polyphenol intake and urinary polyphenols may be explained by individual differences in the metabolism of polyphenols and their excretion in urine. Large variations in polyphenol pharmacokinetics have been observed, ranging from virtually zero polyphenols showing up in urine to a 5-fold increase in polyphenol excretion in urine after the ingestion of identical amounts of a polyphenol-containing food (50). Therefore, it is reasonable to assume that polyphenol metabolism may vary substantially in the general population. Another limitation of measuring polyphenols in urine is that some phenolic compounds use alternative excretion routes (e.g., bile) and are not recovered in urine (37). Phenolic compounds from food and beverages have shown structural changes before excretion in urine (51), so the total phenolic compounds reflected by dietary intake measurements are likely to be different from what is measured in urine. In addition, the food composition and the chemical form of the polyphenol in the food may alter the dose-response relation between polyphenol intake and urine concentration (19, 20, 26). The bioavailability of individual phenolic compounds also varies: for example, citrus polyphenols are more accessible (52), whereas polyphenols bound to fiber fractions of foods are less available (15, 53).
Of the major food groups examined, beverage polyphenols produced the highest correlation for both nonblack and black participants. Coffee and fruit juices represent the greatest contributors to polyphenol intake in this cohort (48), and the phenolic compounds from low-fiber beverages (e.g., coffee, juice) tend to be more accessible than those linked to fiber fractions of a food (15). The food groups with the lowest performance included leafy greens and added fats, perhaps because these foods are often eaten as mixed dishes or because they are consumed less frequently than beverages (54).
Strengths and limitations
The primary strengths of the present validation study include the relatively large sample of participants and the use of a modified method-of-triads approach that used 2 independent biomarkers to assess the validity of the FFQ for assessing polyphenol intake. Furthermore, we used up to six 24-HRs as a reference to validate dietary intake. The concentration of TUPs estimated by using creatinine-corrected 12-h urine samples analyzed by Folin-Ciocalteu assay is considered to be an acceptable biomarker of total polyphenol intake (8). Plasma carotenoid concentration is also a reasonable proxy indicator of the intake of polyphenol-rich foods, because both carotenoids and polyphenols are concentrated in fruit, fruit juices, and vegetables. Although concentration biomarkers cannot be translated into absolute amounts of intake, they do correlate to dietary polyphenol intake. In this study, TUPs and plasma carotenoids are from different tissue types (urine and plasma) and vary in their half-life: plasma carotenoids represent the preceding 1–2 wk of dietary exposure (55) and urinary polyphenols indicate a period of 1–2 d of intake (38). Thus, associated measurement errors are less likely to be correlated. In addition, because recalls were gathered in 2 blocks 5 to 6 mo apart and the biomarkers were collected between the 2 recall blocks, measurements were likely not close enough to be affected by short-term correlations. It is important to note that concentration biomarkers have inherent limitations, including low reproducibility, alternative excretion routes, and susceptibility to interindividual variation in physiology and environment that may affect the detection of metabolites (37). Due to the size and geographic spread of our calibration cohort (n ∼ 1011), repeated sampling of TUPs and plasma carotenoids was not practical, although deattenuation of the biomarkers was not relevant to the method that we used. One additional assumption of the method of triads is that different assessment methods have linear relations with underlying true intake. Finally, the reference period differs between the FFQ and the 24-HR.
Conclusions
To our knowledge, our findings provide a novel objective assessment of polyphenol intake in a North American population of persons with diverse dietary intakes. The positive correlation observed between the FFQ estimates and the 24-HR shows that the FFQ is a reasonable indicator of total polyphenol intake in the AHS-2 cohort. In addition, the AHS-2 FFQ has relatively good validity for polyphenol intake from many food groups, particularly for beverages and other foods that represent good dietary sources of polyphenols in the cohort. The application of a modified method-of-triads model that used 2 biomarkers provided an additional means of validation. The VCs suggest that the total urinary polyphenol biomarker does not perform as well as the FFQ, and therefore should be used to supplement rather than substitute for intake data when assessing polyphenol consumption. Exploring associations between polyphenol intake and health outcomes in this cohort and identifying alternative biomarkers of polyphenol intake are reasonable goals of future research.
Acknowledgments
We thank Rita Amen for her assistance in laboratory analysis in measuring total polyphenols in urine samples. We also acknowledge Susanna Henning and her laboratory at the University of California–Los Angeles for assessing total plasma carotenoids.
The authors’ responsibilities were as follows—NMB-C, SSR, EHH, GEF, and KJ-S: designed the research; NMB-C and EHH: conducted laboratory analyses; NMB-C and KO: analyzed the data; SSR, EHH, GEF, and KJ-S: assisted with data interpretation; NMB-C: wrote the manuscript; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: AHS-2, Adventist Health Study 2; FFQ, food-frequency questionnaire; TDP, total dietary polyphenol; TUP, total urinary polyphenol; VC, validity coefficient; 24-HR, 24-h dietary recall.
REFERENCES
- 1.Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr 2004;79:727–47. [DOI] [PubMed] [Google Scholar]
- 2.Vauzour D, Rodriguez-Mateos A, Corona G, Oruna-Concha MJ, Spencer J. Polyphenols and human health: prevention of disease and mechanisms of action. Nutrients 2010;2:1106–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arts IC, Hollman P. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr 2005;81(Suppl):317S–25S. [DOI] [PubMed] [Google Scholar]
- 4.Tomas-Barberan FA, Andres-Lacueva C. Polyphenols and health: current state and progress. J Agric Food Chem 2012;60:8773–5. [DOI] [PubMed] [Google Scholar]
- 5.Tresserra-Rimbau A, Medina-Remón A, Pérez-Jiménez J, Martínez-González M, Covas M, Corella D, Salas-Salvadó J, Gómez-Gracia E, Lapetra J, Arós F. Dietary intake and major food sources of polyphenols in a Spanish population at high cardiovascular risk: the PREDIMED study. Nutr Metab Cardiovas 2013;23:953–9. [DOI] [PubMed] [Google Scholar]
- 6.Voutilainen S, Nurmi T, Mursu J, Rissanen T. Carotenoids and cardiovascular health. Am J Clin Nutr 2006;83:1265–71. [DOI] [PubMed] [Google Scholar]
- 7.Zamora-Ros R, Forouhi NG, Sharp SJ, González CA, Buijsse B, Guevara M, van der Schouw YT, Amiano P, Boeing H, Bredsdorff L, et al. Dietary intakes of individual flavanols and flavonols are inversely associated with incident type 2 diabetes in European populations. J Nutr 2014;144:335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zamora-Ros R, Rabassa M, Cherubini A, Urpi-Sarda M, Llorach R, Bandinelli S, Ferrucci L, Andres-Lacueva C. Comparison of 24-h volume and creatinine-corrected total urinary polyphenol as a biomarker of total dietary polyphenols in the Invecchiare InCHIANTI study. Anal Chim Acta 2011;704:110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roura E, Andrés-Lacueva C, Estruch R, Lamuela-Raventós RM. Total polyphenol intake estimated by a modified Folin-Ciocalteu assay of urine. Clin Chem 2006;52:749–52. [DOI] [PubMed] [Google Scholar]
- 10.Medina-Remón A, Barrionuevo-González A, Zamora-Ros R, Andres-Lacueva C, Estruch R, Martínez-González M-Á, Diez-Espino J, Lamuela-Raventos RM. Rapid Folin-Ciocalteu method using microtiter 96-well plate cartridges for solid phase extraction to assess urinary total phenolic compounds, as a biomarker of total polyphenols intake. Anal Chim Acta 2009;634:54–60. [DOI] [PubMed] [Google Scholar]
- 11.Zamora-Ros R, Rabassa M, Cherubini A, Urpi-Sarda M, Bandinelli S, Ferrucci L, Andres-Lacueva C. High concentrations of a urinary biomarker of polyphenol intake are associated with decreased mortality in older adults. J Nutr 2013;143:1445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattila P, Pihlava J-m, Hellström J. Contents of phenolic acids, alkyl- and alkenylresorcinols, and avenanthramides in commercial grain products. J Agric Food Chem 2005;53:8290–5. [DOI] [PubMed] [Google Scholar]
- 13.Moco S, Martin F, Rezzi S. Metabolomics view on but microbiome modulation by polyphenol-rich foods. J Proteome Res 2012;11:4781–90. [DOI] [PubMed] [Google Scholar]
- 14.Rechner AR, Smith M, Kuhnle G, Gibson G, Debnam E, Srai S. Colonic metabolism of dietary polyphenols: influence of structure on microbial fermentation products. Free Radic Biol Med 2004;36:212–25. [DOI] [PubMed] [Google Scholar]
- 15.Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr 2000;130(Suppl):2073S–85S. [DOI] [PubMed] [Google Scholar]
- 16.Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans: review of 97 bioavailability studies. Am J Clin Nutr 2005;81(Suppl):230S–42S. [DOI] [PubMed] [Google Scholar]
- 17.Zamora-Ros R, Knaze V, Luján-Barroso L, Romieu I, Scalbert A, Slimani N, Hjartåker A, Engeset D, Skeie G, Overvad K, et al. Differences in dietary intakes, food sources and determinants of total flavonoids between Mediterranean and non-Mediterranean countries participating in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br J Nutr 2013;109:1498–507. [DOI] [PubMed] [Google Scholar]
- 18.Johnson EJ. The role of carotenoids in human health. Nutr Clin Care 2002;5:56–65. [DOI] [PubMed] [Google Scholar]
- 19.Carlsen MH, Karlsen A, Lillegaard ITL, Gran JM, Drevon CA, Blomhoff R, Andersen LF. Relative validity of fruit and vegetable intake estimated from an FFQ, using carotenoid and flavonoid biomarkers and the method of triads. Br J Nutr 2011;105:1530–8. [DOI] [PubMed] [Google Scholar]
- 20.Al-Delaimy WK, Slimani N, Ferrari P, Key T, Spencer E, Johansson I, Johansson G, Mattisson I, Wirfalt E, Sieri S, et al. Plasma carotenoids as biomarkers of intake of fruits and vegetables: ecological-level correlations in the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur J Clin Nutr 2005;59:1397–408. [DOI] [PubMed] [Google Scholar]
- 21.Svilaas A, Sakhi AK, Andersen LF, Svilaas T, Ström EC, Jacobs DR, Ose L, Blomhoff R. Intakes of antioxidants in coffee, wine, and vegetables are correlated with plasma carotenoids in humans. J Nutr 2004;134:562–7. [DOI] [PubMed] [Google Scholar]
- 22.Craft NE. Carotenoid reversed-phase high-performance liquid chromatography methods: reference compendium. Methods Enzymol 1992;213:185–205. [DOI] [PubMed] [Google Scholar]
- 23.Al-Delaimy WK, Natarajan L, Sun X, Rock C, Pierce J, Pierce J, Faerber S, Newman V, Flatt S, Kealey S. Reliability of plasma carotenoid biomarkers and its relation to study power. Epidemiology 2008;19:338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Sohemy A, Baylin A, Kabagambe E, Ascherio A, Spiegelman D, Campos H. Individual carotenoid concentrations in adipose tissue and plasma as biomarkers of dietary intake. Am J Clin Nutr 2002;76:172–9. [DOI] [PubMed] [Google Scholar]
- 25.Rock CL, Swendseid ME, Jacob RA, McKee RW. Plasma carotenoid levels in human subjects fed a low carotenoid diet. J Nutr 1992;122:96–100. [DOI] [PubMed] [Google Scholar]
- 26.Ocké MC, Kaaks RJ. Biochemical markers as additional measurements in dietary validity studies: application of the method of triads with examples from the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr 1997;65(Suppl):1240S–5S. [DOI] [PubMed] [Google Scholar]
- 27.Daures JP, Gerber M, Scali J, Astre C, Bonifacj C, Kaaks R. Validation of a food-frequency questionnaire using multiple-day records and biochemical markers: application of the triads method. J Epidemiol Biostat 2000;5:109–15. [PubMed] [Google Scholar]
- 28.Fraser GE, Butler TL, Shavlik D. Correlations between estimated and true dietary intakes: using two instrumental variables. Ann Epidemiol 2005;15:509–18. [DOI] [PubMed] [Google Scholar]
- 29.Butler TL, Fraser GE, Beeson WL, Knutsen SF, Herring RP, Chan J, Sabaté J, Montgomery S, Haddad E, Preston-Martin S, et al. Cohort profile: the Adventist Health Study-2 (AHS-2). Int J Epidemiol 2008;37:260–5. [DOI] [PubMed] [Google Scholar]
- 30.Jaceldo-Siegl K, Knutsen S, Sabate J, Beeson W, Chan J, Herring R, Butler T, Haddad E, Bennett H, Montgomery S, et al. Validation of nutrient intake using an FFQ and repeated 24 h recalls in black and white subjects of the Adventist Health Study-2 (AHS-2). Public Health Nutr 2010;13:812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fraser G. Diet, life expectancy, and chronic disease: studies of Seventh-Day Adventists and other vegetarians. New York: Oxford University Press; 2003. [Google Scholar]
- 32.Neveu V, Perez-Jimenez J, Vos F, Crespy V, du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart D, et al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford) 2010;2010:bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhagwat S, Haytowitz DB, Holden JM. Database for flavonoid content of selected foods. Release 3.1. 2014. Beltsville (MD): USDA. [cited 2015 Dec 15]. Available from: https://www.ars.usda.gov/ARSUserFiles/80400525/Data/Flav/Flav_R03-1.pdf.
- 34.Bhagwat S, Haytowitz DB, Holden JM. USDA database for the isoflavone content of selected foods. Release 2.0. 2008. Beltsville (MD): USDA. [cited 2015 Dec 15]. Available from: https://www.ars.usda.gov/ARSUserFiles/80400525/Data/isoflav/Isoflav_R2.pdf.
- 35.Arapitsas P. Identification and quantification of polyphenolic compounds from okra seeds and skins. Food Chem 2008;110:1041–5. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura K, Kayahara H, Tian S. Analysis of phenolic compounds in white rice, brown rice, and germinated brown rice. J Agric Food Chem 2004;52:4808–13. [DOI] [PubMed] [Google Scholar]
- 37.Spencer JP, Abd El Mohsen M, Minihane A, Mathers J. Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research. Br J Nutr 2008;99:12–22. [DOI] [PubMed] [Google Scholar]
- 38.Pérez-Jiménez J, Hubert J, Hooper L, Cassidy A, Manach C, Williamson G, Scalbert A. Urinary metabolites as biomarkers of polyphenol intake in humans: a systematic review. Am J Clin Nutr 2010;92:801–9. [DOI] [PubMed] [Google Scholar]
- 39.Jaceldo-Siegl K, Fraser GE, Chan J, Franke A, Sabaté J. Validation of soy protein estimates from a food-frequency questionnaire with repeated 24-h recalls and isoflavonoid excretion in overnight urine in a Western population with a wide range of soy intakes. Am J Clin Nutr 2008;87:1422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol 2010;5:1003–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fraser GE, Jaceldo-Siegl K, Henning S, Fan J, Knutsen S, Haddad E, Sabaté J, Beeson L, Bennett H. Biomarkers of dietary intake are correlated with corresponding measures from repeated dietary recalls and food-frequency questionnaires in the Adventist Health Study-2. J Nutr 2016;146:586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaceldo-Siegl K, Fan J, Sabate J, Knutsen SF, Haddad E, Beeson WL, Herring RP, Butler TL, Bennett H, Fraser GE. Race-specific validation of food intake obtained from a comprehensive FFQ: the Adventist Health Study-2. Public Health Nutr 2011;14:1988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65:1220S–8S. [DOI] [PubMed] [Google Scholar]
- 44.Willett WC. Nutritional epidemiology. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
- 45.Efron B, Tibshirani R. An introduction to the bootstrap. New York: Chapman and Hall; 1993. [Google Scholar]
- 46.Gonzalez S, Fernandez M, Cuervo A, Lasheras C. Dietary intake of polyphenols and major food sources in an institutionalised elderly population. J Hum Nutr Diet 2014;27:176–83. [DOI] [PubMed] [Google Scholar]
- 47.Ovaskainen M-L, Törrönen R, Koponen JM, Sinkko H, Hellström J, Reinivuo H, Mattila P. Dietary intake and major food sources of polyphenols in Finnish adults. J Nutr 2008;138:562–6. [DOI] [PubMed] [Google Scholar]
- 48.Burkholder-Cooley N, Rajaram S, Haddad E, Fraser G, Jaceldo-Siegl K. Comparison of polyphenol intakes according to distinct dietary patterns and food sources in the Adventist Health Study-2 cohort. Br J Nutr 2016;115:2162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kesteloot H, Joossens J. On the determinants of the creatinine clearance: a population study. J Hum Hypertens 1996;10:245–9. [PubMed] [Google Scholar]
- 50.Wruss J, Lanzerstorfer P, Huemer S, Himmelsbach M, Mangge H, Hoglinger O, Weghuber D, Weghuber J. Differences in pharmacokinetics of apple polyphenols after standardized oral consumption of unprocessed apple juice. Nutr J 2015;14: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olthof MR, Hollman P, Buijsman M, van Amelsvoort J, Katan M. Chlorogenic acid, quercetin-3-rutinoside and black tea phenols are extensively metabolized in humans. J Nutr 2003;133:1806–14. [DOI] [PubMed] [Google Scholar]
- 52.Mennen LI, Sapinho D, Ito H, Bertrais S, Galan P, Hercberg S, Scalbert A. Urinary flavonoids and phenolic acids as biomarkers of intake for polyphenol-rich foods. Br J Nutr 2006;96:191–8. [DOI] [PubMed] [Google Scholar]
- 53.Saura-Calixto F, Goñi I. Antioxidant capacity of the Spanish Mediterranean diet. Food Chem 2006;94:442–7. [Google Scholar]
- 54.Orlich MJ, Jaceldo-Siegl K, Sabaté J, Fan J, Singh P, Fraser GE. Patterns of food consumption among vegetarians and non-vegetarians. Br J Nutr 2014;112:1644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eroglu A, Harrison EH. Carotenoid metabolism in mammals, including man: formation, occurrence, and function of apocarotenoids. J Lip Res 2013;54:1719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]