Abstract
Background: Peripheral arterial disease (PAD) is a costly source of morbidity and mortality among older persons in the United States. Dietary intake plays a role in the development of atherosclerotic cardiovascular disease; however, few studies have examined the relation of food intake or dietary patterns with PAD.
Objective: We examined the relation between habitual dietary intake at midlife and incident PAD over ∼20 y of follow-up.
Design: Among 14,082 participants enrolled in the ARIC (Atherosclerosis Risk in Communities) Study initially free of PAD, dietary intake was assessed at baseline in 1987–1989 by using a modified Harvard food-frequency questionnaire. Food groups were created, and principal components analysis was used to develop “healthy” and “Western” dietary patterns; both were categorized into quintiles or quartiles. Incident PAD was determined by an ankle-brachial index <0.9 assessed at 2 subsequent examinations and hospital discharge codes through 2012. Multivariate-adjusted Cox proportional hazards regression was used.
Results: During a mean follow-up of 19.9 y, 1569 participants developed incident PAD. In models adjusted for demographic characteristics, behaviors, and food groups, the HRs (95% CIs) for incident PAD increased across quintiles of meat consumption [quintile 1: reference, quintile 2: 1.38 (1.16, 1.65), quintile 3: 1.38 (1.16, 1.65), quintile 4: 1.45 (1.20, 1.74), quintile 5: 1.66 (1.36, 2.03); P-trend <0.001]. Compared with those who drank no alcohol, those who had 1–6 drinks/wk had a lower risk of incident PAD [0.78 (0.68, 0.89)]. For coffee, ≥4 cups/d compared with none was inversely associated with incident PAD [quintile 5 compared with quintile 1: 0.84 (0.75, 1.00); P-trend = 0.014]. There was no association between other food groups or patterns and incident PAD.
Conclusions: In this prospective cohort study, greater meat consumption was associated with a higher risk, and moderate alcohol consumption was associated with a lower risk of incident PAD. Whether these associations are causal remains to be seen. This trial was registered at clinicaltrials.gov as NCT00005131.
Keywords: dietary patterns, food groups, meat, alcohol, peripheral arterial disease, cardiovascular disease
INTRODUCTION
In the United States, peripheral arterial disease (PAD)5, caused primarily by atherosclerosis, affects 8.5 million adults aged ≥40 y, with similar proportions of men and women (1, 2). PAD is frequently underdiagnosed, yet its total costs in the United States exceed $21 billion/y (3). Because PAD is associated with reduced functional capacity and quality of life, as well as increased risk of limb amputation and death (1), additional information on the preventable causes of PAD is needed.
Although smoking (including intensity, total dose, and duration of smoking cessation), diabetes, hypertension, and dyslipidemia are important risk factors for PAD (1, 4), the role of diet in the development of PAD is not well documented. Several dietary factors support a strong causal link with coronary artery disease, including the protective effect of the Mediterranean diet, a high-quality diet, a “prudent” diet, vegetables, and nuts and the harmful effect of the “Western” diet, trans-fatty acids, and high–glycemic index or load (5). Cross-sectional and case-control studies have suggested that PAD is inversely associated with several nutrients, including fiber from cereal (6–8), vegetable oil (9), vitamin A (8, 10), vitamin C (8, 11–13), vitamin D (14–16), vitamin E (7–9, 13), and folate (8, 10, 17–19), although the relation may not be causal. Recent research on dietary patterns and food groups has found that increased nut intake is cross-sectionally associated with lower PAD prevalence (20), whereas higher meat consumption has been associated with a lower mean ankle-brachial index (ABI) (7).
Few longitudinal studies have examined the relation between major food groups or a healthy-diet pattern with risk of developing incident PAD. No association of intake of fruit and vegetables with PAD in men has been found (21), although alcohol intake has been inversely related (22–24). Additionally, a randomized controlled trial found that those who followed a Mediterranean-diet pattern were less likely to develop PAD, suggesting a causal link (25). A high-quality diet may reduce oxidative stress and endothelial dysfunction and improve erythrocyte deformability, blood viscosity, and oxygen perfusion, which may reduce the number of incident PAD cases (26). Therefore, the objective of this study was to determine the long-term prospective association of food groups—including meat, dairy, whole and refined grains, fruit, vegetables, nuts, and beverages—and dietary patterns with risk of incident PAD in a biethnic community cohort.
METHODS
The ARIC (Atherosclerosis Risk in Communities) Study (NCT00005131) is a multicenter, community-based, prospective cohort study designed to investigate causes of atherosclerosis, its clinical outcomes, and variations in cardiovascular disease risk factors (27). The study involves 4 communities: Forsyth County, North Carolina; Jackson, Mississippi; selected suburbs of Minneapolis, Minnesota; and Washington County, Maryland. Recruitment took place in 1987–1989, and the study cohort included 15,972 predominantly black and white men and women who were aged 45–64 y at baseline. Five visits have now taken place, in 1987–1989 (visit 1), 1990–1992 (visit 2), 1993–1995 (visit 3), 1996–1998 (visit 4), and 2011–2013 (visit 5). We excluded from the study participants with prevalent PAD as defined by ABI <0.90 (n = 1125), those who were neither black nor white as well as blacks from the Minnesota and Maryland centers (n = 103), and those with implausible energy intake, which was defined as <500 or >3500 kcal for women and <700 or >4500 kcal for men (n = 392). The final analytic sample was 14,082. The Institutional Review Boards at all participating centers approved the study procedures, and all participants signed consent forms before enrollment.
Exposure
Dietary intake was assessed at visit 1 with the use of a modified 66-item Harvard food-frequency questionnaire (FFQ), including additional questions about fish intake (28). For each food item, frequency of intake was queried according to 9 predefined categories, and standard portion sizes were assumed. We created food groups, including meat (hamburger, red meat, hot dogs, sausage, bacon, liver), fish and seafood (canned tuna fish, fish, shrimp, lobster, scallops), poultry (chicken, turkey), dairy (milk, yogurt, ice cream, cheese), fruits (apples, pears, bananas, oranges, fruit juice, peaches, apricots, plums, other fruits), vegetables (green beans, cabbage, broccoli, cauliflower, brussels sprouts, sweet potatoes, squash, carrots, corn, dark leafy vegetables, peas, lima beans, beans, lentils, tomatoes, potatoes), whole grains (whole grain bread, whole grain cold cereal), refined grains (refined bread, refined cold cereal, refined rice and pasta, biscuits, dessert, snacks), nuts, coffee, tea, alcohol, sugar-sweetened beverages (regular soda and fruit drinks), and diet soda. In addition, 29 food groups were used to derive 2 dietary patterns through principal components analysis. Both the food groups and the dietary patterns were categorized into quintiles or quartiles, depending on the distribution.
Outcome
Incident PAD was defined by a new ABI measure of <0.90 at either visit 3 or 4, or a hospital discharge diagnosis of PAD, a leg amputation, or a leg revascularization procedure (leg endarterectomy, aorto-iliac-femoral bypass surgery, or leg bypass surgery) through 2012. An ABI was measured on the full sample at visit 1 but only on a random sample at visits 3 and 4 (32.3% and 49.6% of visit attendees, respectively) (29). To measure ABI, trained staff used the Dinamap 1846 automated oscillometric device to measure ankle blood pressure at the posterior tibial artery in a randomly selected leg and brachial blood pressure in the right arm, with the participant in the supine position (30). ABI was defined as the ratio of the ankle systolic blood pressure to the brachial blood pressure.
Cohort participants were contacted annually by phone to identify all hospitalizations, coupled with surveillance of local hospitals for discharges for cardiovascular diagnoses. Hospitalized PAD was defined by the following International Classification of Diseases, 9th edition (ICD-9) codes: 00.55 (insertion of drug-eluting peripheral vessel stents), 38.18 (leg endarterectomy), 39.25 (aorto-iliac-femoral bypass), 39.29 (leg bypass surgery), 39.50 (angioplasty or atherectomy of other noncoronary vessels), 39.90 (insertion of non–drug-eluting peripheral vessel stents), 84.11 (toe amputation), 84.12 (foot amputation), 84.15 (below-knee amputation), 84.17 (above-knee amputation), 440.21 (atherosclerosis of native arteries of the extremities with intermittent claudication), 440.22 (atherosclerosis of native arteries of the extremities with rest pain), 440.23 (atherosclerosis of native arteries of the extremities with ulceration), 440.24 (atherosclerosis of native arteries of the extremities with gangrene), 443.9 (peripheral arterial disease, unspecified), and 785.4 (gangrene) through 2012 (29).
Confounding variables
Covariates were assessed at visit 1. Sociodemographic characteristics included self-reported age, race/ethnicity, sex, and field center. Height was measured to the nearest centimeter while standing. Behavioral factors included smoking [both status (i.e., current, former, or never) and pack-years] and physical activity as measured by the Baecke questionnaire sport index (31).
Analysis
Descriptive statistics were calculated for demographics, clinical values, and dietary intake, stratified by sex. Cox proportional hazards models were used to model time to incident PAD events relative to baseline dietary intake. Person-time was calculated by using time from the baseline examination until a PAD event, loss to follow-up, death, or 31 December 2012. The proportional hazards assumption was checked by using interactions with time and tests of correlations of the residuals, and no major violations were detected. Tests for linear trend were conducted by modeling exposures as continuous. Interactions between diet and age, sex, and race/ethnicity on PAD were tested by using cross-product terms and stratification, as appropriate. Principal components analysis was used to derive dietary patterns from 29 food groups by using the principal extraction method. Varimax rotation was used to remove correlation for greater interpretability. Based on the eigenvalues, interpretability, and previous ARIC diet articles (32, 33), 2 factors were chosen: the Western diet pattern and the healthy diet pattern. SAS version 9.3 (SAS Institute) was used to analyze the data.
We explored a series of models. The first model controlled for age, race/ethnicity, sex, field center, education, height, and energy intake (continuous). Model 2 added physical activity (continuous), smoking status, and pack-years. For the models with food groups as the exposures, model 3 was added, which additionally controlled for meat, dairy, fruits and vegetables, whole grains, and refined grains as continuous variables.
RESULTS
The mean age at visit 1 was 54.1 y, and 55.0% of the sample was female. As shown in Table 1, women at baseline on average were younger, engaged in less physical activity, consumed fewer calories, and consumed fewer servings of refined grains, meat, coffee, and regular soda than men. Relative to men, a greater percentage of women identified as black, having a high school education, and being never smokers.
TABLE 1.
Participant characteristics by sex at baseline: the Atherosclerosis Risk in Communities Study (1987–2012)1
| Men (N = 6341) | Women (N = 7741) | |
| Age, y | 54.5 ± 5.82 | 53.7 ± 5.7 |
| Race/ethnicity | ||
| Black | 1379 (21.7)3 | 2288 (29.6) |
| White | 4962 (78.3) | 5453 (70.4) |
| Education | ||
| Less than high school | 1489 (23.5) | 1747 (22.6) |
| High school | 2287 (36.1) | 3464 (44.7) |
| More than high school | 2553 (40.3) | 2520 (32.6) |
| Smoking | ||
| Current | 1700 (26.8) | 1858 (24.0) |
| Former | 2828 (44.6) | 1743 (22.5) |
| Never | 1812 (28.6) | 4132 (53.4) |
| Pack-years | 22.5 ± 24.7 | 9.9 ± 16.3 |
| Physical activity, 1–5 scale | 2.6 ± 0.8 | 2.3 ± 0.8 |
| Daily calorie intake, kcal | 1796.5 ± 645.1 | 1494.4 ± 535.6 |
| Alcohol, drinks/wk | 5.1 ± 9.3 | 1.5 ± 3.7 |
| Height, cm | 176.2 ± 6.6 | 162.4 ± 6.0 |
| Type 2 diabetes | 580 (9.2) | 737 (9.6) |
| Hypertension | 2121 (33.6) | 2663 (34.6) |
| HDL, mg/dL | 44.5 ± 13.9 | 57.6 ± 17.2 |
| LDL, mg/dL | 139.2 ± 37.1 | 135.5 ± 40.7 |
| Food groups, servings/d | ||
| Meat | 1.2 ± 0.8 | 0.9 ± 0.7 |
| Fish and seafood | 0.3 ± 0.3 | 0.3 ± 0.3 |
| Poultry | 0.3 ± 0.3 | 0.4 ± 0.3 |
| Dairy | 1.7 ± 1.4 | 1.5 ± 1.2 |
| Fruits | 1.8 ± 1.5 | 2.2 ± 1.6 |
| Vegetables | 2.0 ± 1.3 | 2.1 ± 1.2 |
| Whole grains | 1.3 ± 1.3 | 1.2 ± 1.1 |
| Refined grains | 2.8 ± 1.9 | 2.1 ± 1.5 |
| Nuts | 0.1 ± 0.3 | 0.1 ± 0.2 |
| Coffee | 2.0 ± 2.1 | 1.6 ± 2.0 |
| Tea | 0.5 ± 1.0 | 0.6 ± 1.1 |
| Sugar-sweetened beverages | 0.6 ± 1.0 | 0.5 ± 0.9 |
| Diet soda | 0.5 ± 0.9 | 0.6 ± 1.0 |
Some totals may not equal the total N for each group due to missing data.
Mean ± SD (all such values).
n; percentage in parentheses (all such values).
We identified 1569 incident cases of PAD over a mean of 19.9 y of follow-up. The crude incidence rate was 5.6 cases/1000 person-years. PAD cases were similarly distributed in men (12.3%) and women (10.2%) and in blacks (12.4%) and whites (10.7%). A total of 37.8% of PAD cases were current smokers. Of these cases, incident PAD was identified by ICD-9 codes for 64.7%, whereas for 35.3% it was identified by ABI at visit 3 or 4. A list of ICD-9 codes used can be found in Supplemental Table 1.
Table 2 shows HRs (95% CIs) of incident PAD for each food group. In fully adjusted models, we found few statistically significant associations between the food groups and risk of incident PAD. Compared with participants in the lowest quintile, the HRs for incident PAD increased across quintiles of meat consumption [quintile 5 compared with quintile 1: 1.66 (1.36, 2.03); P-trend < 0.001]. For alcohol, 1–6 drinks/wk (compared with 0 drinks) was associated with a lower risk of incident PAD [0.78 (0.68, 0.89)], but for ≥7 drinks/wk the magnitude of the association was much smaller and not statistically significant [0.89 (0.76, 1.04)]. For coffee, there was a small, statistically significant inverse association with incident PAD [quintile 5 compared with quintile 1: 0.84 (0.75, 1.00); P-trend = 0.014]. Sugar-sweetened beverages were associated with lower risk of PAD and diet soda with higher risk of PAD. However, when stratified by diabetes status at baseline (Supplemental Table 2), there was no relation between sugar-sweetened beverages and PAD. For those with diabetes, those who drank more diet soda were at increased risk of incident PAD, but there was no association between diet soda and PAD among those without diabetes. Overall, there was no association between consumption of fish and seafood, poultry, dairy, fruits, vegetables, whole grains, refined grains, nuts, and other beverages with incident PAD. There was no effect modification by sex.
TABLE 2.
Adjusted HRs (95% CIs) for food groups and risk of incident peripheral arterial disease: the Atherosclerosis Risk in Communities Study (1987–2012)1
| Events, n | Study participants, n | Model 12 | Model 23 | Model 34 | Model 45 | |
| Meat, servings/d | NR | |||||
| ≤0.42 | 237 | 2877 | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) |
| 0.43–0.74 | 307 | 2757 | 1.39 (1.17, 1.65) | 1.33 (1.12, 1.58) | 1.38 (1.16, 1.65) | 1.30 (1.09, 1.55) |
| 0.75–1.10 | 318 | 2805 | 1.40 (1.18, 1.67) | 1.31 (1.10, 1.55) | 1.38 (1.16, 1.65) | 1.27 (1.06, 1.52) |
| 1.11–1.56 | 331 | 2798 | 1.46 (1.22, 1.74) | 1.33 (1.11, 1.59) | 1.45 (1.20, 1.74) | 1.26 (1.04, 1.52) |
| ≥1.57 | 376 | 2845 | 1.60 (1.32, 1.93) | 1.47 (1.22, 1.78) | 1.66 (1.36, 2.03) | 1.41 (1.15, 1.73) |
| P-trend | <0.001 | <0.001 | <0.001 | 0.009 | ||
| Fish and seafood, servings/d | NR | |||||
| 0–0.07 | 314 | 2752 | 1 (referent) | 1 (referent) | 1 (referent) | |
| 0.14 | 261 | 2429 | 0.96 (0.81, 1.13) | 0.97 (0.82, 1.15) | 0.97 (0.82, 1.15) | |
| 0.21–0.28 | 431 | 3972 | 0.95 (0.82, 1.11) | 0.98 (0.84, 1.14) | 0.97 (0.84, 1.13) | |
| 0.35–0.50 | 259 | 2291 | 0.99 (0.84, 1.18) | 1.04 (0.88, 1.23) | 1.04 (0.87, 1.23) | |
| >0.50 | 304 | 2638 | 1.02 (0.87, 1.21) | 1.11 (0.94, 1.31) | 1.10 (0.92, 1.30) | |
| P-trend | 0.693 | 0.172 | 0.238 | |||
| Poultry, servings/d | NR | |||||
| 0–0.07 | 230 | 1933 | 1 (referent) | 1 (referent) | 1 (referent) | |
| 0.14 | 432 | 3764 | 0.98 (0.83, 1.15) | 0.97 (0.83, 1.15) | 0.96 (0.82, 1.13) | |
| 0.21–0.28 | 170 | 1581 | 0.95 (0.78, 1.16) | 1.01 (0.83, 1.24) | 1.00 (0.82, 1.23) | |
| 0.43 | 423 | 4017 | 0.88 (0.75, 1.04) | 0.96 (0.81, 1.13) | 0.95 (0.80, 1.12) | |
| ≥0.50 | 314 | 2787 | 1.00 (0.84, 1.20) | 1.12 (0.94, 1.34) | 1.12 (0.94, 1.35) | |
| P-trend | 0.545 | 0.272 | 0.274 | |||
| Dairy, servings/d | NR | |||||
| ≤0.56 | 322 | 2613 | 1 (referent) | 1 (referent) | 1 (referent) | |
| 0.57–1.13 | 320 | 2956 | 0.87 (0.75, 1.02) | 0.93 (0.79, 1.09) | 0.94 (0.80, 1.10) | |
| 1.14–1.56 | 307 | 2788 | 0.87 (0.74, 1.02) | 0.95 (0.81, 1.12) | 0.96 (0.81, 1.13) | |
| 1.57–2.49 | 299 | 2906 | 0.81 (0.69, 0.96) | 0.89 (0.75, 1.05) | 0.90 (0.76, 1.07) | |
| ≥2.50 | 321 | 2819 | 0.93 (0.78, 1.11) | 1.03 (0.86, 1.23) | 1.09 (0.90, 1.30) | |
| P-trend | 0.291 | 0.978 | 0.648 | |||
| Fruits, servings/d | NR | |||||
| ≤0.78 | 328 | 2852 | 1 (referent) | 1 (referent) | 1 (referent) | |
| 0.79–1.42 | 301 | 2750 | 0.95 (0.81, 1.11) | 1.04 (0.89, 1.22) | 1.03 (0.88, 1.21) | |
| 1.43–2.07 | 328 | 2852 | 0.98 (0.84, 1.15) | 1.13 (0.96, 1.32) | 1.13 (0.96, 1.33) | |
| 2.08–3.00 | 279 | 2792 | 0.81 (0.69, 0.96) | 1.01 (0.85, 1.19) | 1.02 (0.86, 1.21) | |
| >3.00 | 333 | 2836 | 0.90 (0.77, 1.06) | 1.15 (0.97, 1.36) | 1.19 (1.00, 1.42) | |
| P-trend | 0.062 | 0.202 | 0.102 | |||
| Vegetables, servings/d | NR | |||||
| ≤1.08 | 313 | 2819 | 1 (referent) | 1 (referent) | 1 (referent) | |
| 1.12–1.55 | 320 | 2819 | 0.99 (0.85, 1.16) | 1.02 (0.87, 1.19) | 1.01 (0.86, 1.19) | |
| 1.56–2.13 | 295 | 2802 | 0.94 (0.80, 1.11) | 0.99 (0.84, 1.16) | 0.99 (0.84, 1.16) | |
| 2.13–2.91 | 307 | 2825 | 0.95 (0.80, 1.11) | 1.01 (0.85, 1.19) | 1.01 (0.86, 1.20) | |
| >2.91 | 334 | 2817 | 0.98 (0.83, 1.17) | 1.06 (0.89, 1.26) | 1.07 (0.90, 1.28) | |
| P-trend | 0.674 | 0.579 | 0.504 | |||
| Whole grains, servings/d | NR | |||||
| ≤0.21 | 354 | 2800 | 1 (referent) | 1 (referent) | 1 (referent) | |
| 0.28–0.80 | 332 | 2981 | 0.94 (0.81, 1.09) | 0.99 (0.85, 1.16) | 0.99 (0.85, 1.15) | |
| 0.86–1.08 | 275 | 2504 | 0.90 (0.77, 1.06) | 1.02 (0.87, 1.20) | 1.02 (0.87, 1.20) | |
| 1.14–2.00 | 324 | 3106 | 0.89 (0.77, 1.04) | 1.08 (0.92, 1.27) | 1.08 (0.92, 1.27) | |
| >2.00 | 284 | 2691 | 0.89 (0.76, 1.05) | 1.08 (0.92, 1.28) | 1.10 (0.93, 1.31) | |
| P-trend | 0.114 | 0.210 | 0.158 | |||
| Refined grains, servings/d | NR | |||||
| ≤1 | 301 | 2821 | 1 (referent) | 1 (referent) | 1 (referent) | |
| 1.01–1.62 | 292 | 2795 | 0.95 (0.81, 1.12) | 0.97 (0.82, 1.14) | 0.98 (0.84, 1.16) | |
| 1.63–2.35 | 312 | 2831 | 0.97 (0.82, 1.14) | 0.98 (0.83, 1.16) | 1.02 (0.86, 1.21) | |
| 2.36–3.51 | 321 | 2820 | 0.93 (0.78, 1.10) | 0.91 (0.77, 1.08) | 0.97 (0.81, 1.16) | |
| ≥3.52 | 343 | 2815 | 0.94 (0.78, 1.14) | 0.92 (0.76, 1.11) | 1.03 (0.84, 1.26) | |
| P-trend | 0.503 | 0.279 | 0.894 | |||
| Nuts, servings | NR | |||||
| Almost never | 704 | 5780 | 1 (referent) | 1 (referent) | 1 (referent) | |
| 1–3/mo | 435 | 4253 | 0.90 (0.79, 1.01) | 0.93 (0.82, 1.05) | 0.94 (0.83, 1.06) | |
| 1/wk | 219 | 2154 | 0.87 (0.74, 1.01) | 0.92 (0.78, 1.07) | 0.93 (0.80, 1.09) | |
| ≥2/wk | 211 | 1893 | 0.95 (0.81, 1.12) | 1.01 (0.86, 1.19) | 1.04 (0.89, 1.23) | |
| P-trend | 0.213 | 0.711 | 0.996 | |||
| Coffee, servings | NR | |||||
| 0 | 439 | 3762 | 1 (referent) | 1 (referent) | 1 (referent) | |
| 1/mo to 6/wk | 198 | 1937 | 0.89 (0.75, 1.05) | 0.89 (0.75, 1.05) | 0.89 (0.75, 1.06) | |
| 1/d | 319 | 2734 | 0.93 (0.80, 1.07) | 0.89 (0.77, 1.03) | 0.89 (0.77, 1.03) | |
| 2–3/d | 346 | 3262 | 0.94 (0.81, 1.09) | 0.82 (0.71, 0.95) | 0.83 (0.72, 0.97) | |
| ≥4/d | 267 | 2387 | 1.13 (0.97, 1.33) | 0.84 (0.71, 0.99) | 0.84 (0.75, 1.00) | |
| P-trend | 0.348 | 0.009 | 0.014 | |||
| Tea, servings | NR | |||||
| Almost never | 635 | 5378 | 1 (referent) | 1 (referent) | 1 (referent) | |
| 1/mo to 1/wk | 324 | 3076 | 0.90 (0.79, 1.03) | 0.94 (0.82, 1.08) | 0.94 (0.82, 1.08) | |
| 2–6/wk | 246 | 2271 | 0.92 (0.79, 1.07) | 0.99 (0.85, 1.16) | 0.99 (0.85, 1.16) | |
| ≥1/d | 364 | 3357 | 0.91 (0.79, 1.04) | 0.96 (0.84, 1.10) | 0.96 (0.84, 1.09) | |
| P-trend | 0.101 | 0.550 | 0.529 | |||
| Sugar-sweetened beverages, servings | NR | |||||
| Almost never | 506 | 4440 | 1 (referent) | 1 (referent) | 1 (referent) | |
| 1–3/mo | 169 | 1611 | 0.84 (0.70, 1.00) | 0.86 (0.72, 1.03) | 0.87 (0.73, 1.04) | |
| 1/wk | 233 | 2069 | 0.85 (0.72, 0.99) | 0.89 (0.76, 1.04) | 0.89 (0.76, 1.05) | |
| 2–6/wk | 283 | 2822 | 0.71 (0.61, 0.82) | 0.71 (0.61, 0.83) | 0.74 (0.63, 0.86) | |
| ≥1/d | 377 | 3136 | 0.82 (0.70, 0.95) | 0.79 (0.68, 0.92) | 0.86 (0.73, 1.00) | |
| P-trend | <0.001 | <0.001 | 0.005 | |||
| Diet soda, servings | NR | |||||
| Almost never | 714 | 6365 | 1 (referent) | 1 (referent) | 1 (referent) | |
| 1/mo to 1/wk | 249 | 2207 | 1.09 (0.94, 1.26) | 1.21 (1.04, 1.41) | 1.19 (1.02, 1.38) | |
| 2–6/wk | 220 | 2314 | 0.93 (0.80, 1.09) | 1.06 (0.91, 1.24) | 1.02 (0.87, 1.20) | |
| ≥1/d | 385 | 3195 | 1.25 (1.10, 1.42) | 1.35 (1.19, 1.54) | 1.31 (1.15, 1.49) | |
| P-trend | 0.007 | <0.001 | <0.001 | |||
| Alcohol, drinks/wk | NR | |||||
| 0 | 1012 | 8560 | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) |
| 1–6 | 290 | 3291 | 0.85 (0.74, 0.97) | 0.77 (0.67, 0.88) | 0.78 (0.68, 0.89) | 0.86 (0.75, 0.99) |
| ≥7 | 260 | 2203 | 1.09 (0.94, 1.26) | 0.84 (0.72, 0.98) | 0.89 (0.76, 1.04) | 1.00 (0.85, 1.18) |
| P-trend | 0.813 | 0.011 | 0.024 |
NR, model not run.
Adjusted for age, sex, race/ethnicity, field center, education, height, and energy intake.
Adjusted as for model 1 plus for physical activity, baseline smoking status, and pack-years.
Adjusted as for model 2 plus for meat, dairy, fruits, vegetables, whole grains, and refined grains.
Adjusted as for model 3 plus for diabetes status, hypertension status, and HDL and LDL cholesterol.
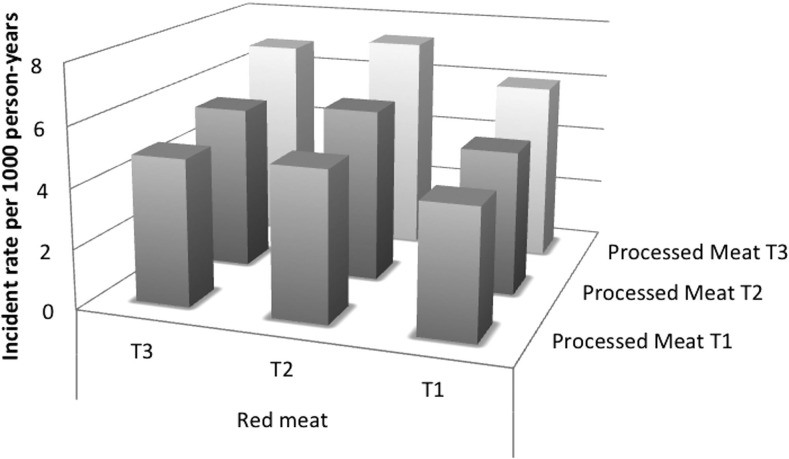
Table 3 shows HRs (95% CIs) for meat subgroups and risk of incident PAD. Compared with participants in the lowest quintile, the HR for incident PAD in the highest quintile of red meat consumption was 1.26 (1.04, 1.51) and 1.31 (1.10, 1.55) for processed meat consumption. Results were similar with adjustment for additional confounders. Incidence rates for PAD by processed meat and red meat consumption can be found in Figure 1. Incidence rates were lower at the lowest tertiles of consumption.
TABLE 3.
Adjusted HRs (95% CIs) for meat subgroups and risk of incident peripheral arterial disease: the Atherosclerosis Risk in Communities Study (1987–2012)
| Events, n | Study participants, n | Model 11 | Model 22 | Model 33 | Model 44 | |
| Red meat,5 servings/d | ||||||
| ≤0.18 | 244 | 2530 | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) |
| 0.21–0.32 | 266 | 2685 | 1.05 (0.88, 1.24) | 1.01 (0.85, 1.20) | 1.03 (0.87, 1.23) | 0.99 (0.82, 1.18) |
| 0.35–0.57 | 411 | 3355 | 1.32 (1.13, 1.56) | 1.27 (1.08, 1.49) | 1.32 (1.12, 1.56) | 1.24 (1.05, 1.47) |
| 0.61–0.78 | 320 | 2620 | 1.31 (1.10, 1.56) | 1.29 (1.08, 1.54) | 1.38 (1.15, 1.65) | 1.27 (1.06, 1.52) |
| ≥0.79 | 328 | 2892 | 1.26 (1.04, 1.51) | 1.23 (1.02, 1.49) | 1.35 (1.11, 1.64) | 1.16 (0.95, 1.42) |
| P-trend | 0.001 | 0.002 | <0.001 | 0.016 | ||
| Processed meat,6 servings/d | ||||||
| ≤0.07 | 299 | 3203 | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) |
| 0.14 | 171 | 1758 | 1.00 (0.83, 1.21) | 0.95 (0.78, 1.15) | 0.97 (0.80, 1.17) | 0.95 (0.78, 1.15) |
| 0.21–0.42 | 379 | 3492 | 1.16 (1.00, 1.36) | 1.10 (0.94, 1.28) | 1.14 (0.97, 1.33) | 1.07 (0.91, 1.26) |
| 0.43–0.80 | 330 | 2734 | 1.24 (1.05, 1.46) | 1.11 (0.94, 1.31) | 1.16 (0.98, 1.37) | 1.05 (0.88, 1.25) |
| ≥0.86 | 390 | 2895 | 1.31 (1.10, 1.55) | 1.17 (0.99, 1.40) | 1.25 (1.05, 1.49) | 1.13 (0.95, 1.36) |
| P-trend | <0.001 | 0.031 | 0.005 | 0.127 |
Adjusted for age, sex, race/ethnicity, field center, education, height, and energy intake.
Adjusted as for model 1 plus for physical activity, baseline smoking status, and pack-years.
Adjusted as for model 2 plus for dairy, fruits, vegetables, refined grains, and whole grains.
Adjusted as for model 3 plus for diabetes status, hypertension status, and HDL and LDL cholesterol.
Hamburger, meat sandwich, or red meat as main dish.
Hot dogs, sausages, or bacon.
FIGURE 1.
Incident rates for PAD by tertiles of red meat and processed meat intake. The x axis indicates tertiles of processed meat intake. The y axis indicates tertiles of red meat intake. The z axis indicates the incident rate per 1000 person-years. PAD, peripheral arterial disease; T, tertile.
The rotated factor pattern from the principal components analysis can be found in Table 4. There were no significant associations of either the Western diet pattern (factor 1) or the healthy diet pattern (factor 2) with incident PAD after demographic adjustments. (Table 5). However, with additional adjustment for physical activity, smoking status, and pack-years, there was a linear trend whereby higher consumption of the healthy dietary pattern was associated with increased risk of incident PAD.
TABLE 4.
Rotated factor pattern from principal components analysis
| Value | |
| Factor 1 (Western diet pattern) | |
| Processed meats | 0.61 |
| Fried food | 0.61 |
| Refined grains | 0.61 |
| Red meat | 0.57 |
| Eggs | 0.47 |
| Dessert | 0.44 |
| High-fat dairy | 0.40 |
| Sugar-sweetened beverages | 0.37 |
| Fat | 0.34 |
| Sweets | 0.33 |
| Legumes | 0.33 |
| Potatoes | 0.30 |
| Ice cream | 0.28 |
| Other vegetables | 0.26 |
| Nuts and peanut butter | 0.21 |
| Coffee | 0.16 |
| Tomatoes | 0.14 |
| Tea | 0.12 |
| Dark-leaf vegetables | 0.08 |
| Carotene vegetables | 0.01 |
| Fruit juice | 0.00 |
| Diet soda | −0.02 |
| Fish and seafood | −0.03 |
| Poultry | −0.03 |
| Cruciferous vegetables | −0.04 |
| Fruits, excluding fruit juice | −0.08 |
| Whole grains | −0.08 |
| Low-fat dairy | −0.10 |
| Yogurt | −0.17 |
| Factor 2 (healthy diet pattern) | |
| Cruciferous vegetables | 0.61 |
| Fruits, excluding fruit juice | 0.58 |
| Carotene vegetables | 0.57 |
| Other vegetables | 0.53 |
| Fish and seafood | 0.47 |
| Poultry | 0.44 |
| Dark-leaf vegetables | 0.43 |
| Whole grains | 0.38 |
| Tomatoes | 0.38 |
| Legumes | 0.35 |
| Low-fat dairy | 0.31 |
| Yogurt | 0.28 |
| Fruit juice | 0.26 |
| Potatoes | 0.24 |
| Nuts and peanut butter | 0.23 |
| Fat | 0.20 |
| Diet soda | 0.12 |
| Tea | 0.07 |
| High-fat dairy | 0.05 |
| Red meat | 0.02 |
| Dessert | 0.01 |
| Ice cream | 0.01 |
| Sweets | −0.01 |
| Eggs | −0.03 |
| Coffee | −0.07 |
| Processed meat | −0.08 |
| Refined grains | −0.09 |
| Fried food | −0.13 |
| Sugar-sweetened beverages | −0.17 |
TABLE 5.
Adjusted HRs (95% CIs) for quintiles of principal components analysis–derived diet patterns and risk of incident peripheral arterial disease: the Atherosclerosis Risk in Communities Study (1987–2012)
| Events, n | Study participants, n | Model 11 | Model 22 | |
| Western diet pattern | ||||
| 1 | 281 | 2813 | 1 (referent) | 1 (referent) |
| 2 | 288 | 2814 | 1.00 (0.84, 1.18) | 1.03 (0.87, 1.22) |
| 3 | 323 | 2813 | 1.11 (0.93, 1.33) | 1.14 (0.96, 1.37) |
| 4 | 328 | 2814 | 1.13 (0.93, 1.38) | 1.16 (0.95, 1.42) |
| 5 | 344 | 2813 | 1.15 (0.89, 1.48) | 1.23 (0.95, 1.59) |
| P-trend | 0.148 | 0.070 | ||
| Healthy diet pattern | ||||
| 1 | 337 | 2813 | 1 (referent) | 1 (referent) |
| 2 | 315 | 2814 | 1.01 (0.87, 1.19) | 1.13 (0.96, 1.33) |
| 3 | 310 | 2813 | 1.03 (0.87, 1.21) | 1.20 (1.02, 1.42) |
| 4 | 318 | 2814 | 1.07 (0.91, 1.26) | 1.32 (1.11, 1.56) |
| 5 | 284 | 2813 | 0.95 (0.80, 1.12) | 1.19 (1.00, 1.42) |
| P-trend | 0.783 | 0.011 |
Adjusted for age, sex, race/ethnicity, field center, education, height, and energy intake.
Adjusted as for model 1 plus for physical activity, baseline smoking status, and pack-years.
To explore this counterintuitive association, we examined interaction by smoking status. There was not a significant interaction by smoking status (P-interaction = 0.16); however, when we stratified by smoking status we observed variation in the estimates. Specifically, comparing quintile 5 and quintile 1 of the healthy dietary pattern, the risk of incident PAD was higher in former smokers (1.48 (1.09, 2.02)] than in current smokers [1.18 (0.88, 1.59)] or in never smokers [0.97 (0.71, 1.33)].
Several sensitivity analyses were conducted to test the robustness of the results. When participants with ABI >1.4 were removed from the sample, no appreciable changes were detected. Additionally, no changes were detected when only those participants with PAD defined by ICD-9 codes were counted as cases.
DISCUSSION
The main finding from this prospective, community-based study was a moderately strong positive association between meat consumption and incident PAD. Additionally, low alcohol intake and coffee consumption were associated with a lower risk of incident PAD. These results provide some of the first prospective evidence of a relation between diet and incident PAD.
Our finding that greater meat consumption was associated with higher PAD risk is consistent with previous findings from a cross-sectional study in which greater meat consumption was associated with lower ABI (7). Greater meat consumption has been linked to increased risk of other cardiovascular diseases (34, 35), including stroke, but not coronary artery disease in the ARIC Study (36, 37). Also, supporting our finding, red and processed meat have consistently been associated with other cardiovascular events and risk factors. Red and processed meat have also been positively associated with cardiovascular disease mortality (35). Processed meat, but not red meat, has been positively associated with incident coronary artery disease and diabetes (38).
There are several potential pathways by which meat consumption may influence PAD. The high levels of sodium and nitrate preservatives in processed meats may raise blood pressure, which may increase the risk of incident PAD (39). Meta-analyses have shown consistent and positive associations between meat and another risk factor for PAD, type 2 diabetes, as was found in this study (data not shown) (40). Additionally, intake of heme iron has been consistently associated with type 2 diabetes (34).
In this study, 1–6 drinks/wk, but not ≥7 drinks, was associated with a lower risk of incident PAD. This is consistent with evidence from several other cohort studies, in which moderate alcohol consumption was associated with a lower risk of incident PAD (22–24). Moderate alcohol has also been inversely associated with other cardiovascular diseases in many observational and experimental studies (41, 42), although not always in the ARIC Study (43, 44). Alcohol consumption may affect the lipid profile, especially by raising HDL cholesterol and improving insulin sensitivity, which may reduce the incidence of cardiovascular diseases, including PAD (45–47). However, the inverse association between alcohol and PAD in this study may be due to residual confounding, because cardiovascular disease risk factors, such as diabetes, physical inactivity, obesity, and poor general health, are more common among nondrinkers than moderate drinkers (48).
Additionally, coffee had a small, statistically significant inverse association with incident PAD. Previous research on coffee consumption and cardiovascular disease has also detected inverse associations, although some have also been U-shaped (49). Coffee consumption has been associated with higher insulin sensitivity and lower risk of type 2 diabetes, a major risk factor for PAD (50). However, those with prevalent disease related to PAD may have altered their coffee intake, confounding the association.
Although no association was found between dietary patterns and incident PAD in demographic-adjusted models, there was a linear trend for the association between the healthy-diet pattern and incident PAD once behaviors were added as confounders. However, this relation varied by smoking status, a major risk factor for PAD. When stratified, higher consumption of the healthy-diet pattern was associated with a greater risk of incident PAD among former smokers but not among current or never smokers. Participants who were former smokers may have changed their diet in response to another health issue, and thus these findings likely do not represent an etiologic association between a healthy-diet pattern and incident PAD.
In this study, intakes of dairy, whole and refined grains, fruit, vegetables, and nuts, among others, were not associated with incident PAD. Food groups have been examined in relation to PAD in few previous studies, although associations between dietary intake of nutrients and PAD have been studied more extensively (6–18). In a large sample of adults who completed vascular screening tests, nut consumption was inversely associated with prevalent PAD (20). Fruit and vegetable consumption in a large cohort of male health professionals was not associated with incident PAD over 12 y of follow-up (21). Dietary patterns have also been examined in relation to PAD with mixed results. A case-control study of people with diabetes (51) found an inverse association between the Mediterranean diet and PAD, but an inverse association was not statistically significant because of the low number of PAD cases in a large European cohort (52). Importantly, the PREDIMED (Primary Prevention of Cardiovascular Disease with a Mediterranean Diet) randomized clinical trial (25) found that those randomly assigned to the Mediterranean diet had a significantly lower rate of incident PAD than those in the low-fat diet group.
This study has several limitations. Dietary intake was assessed by using a self-report, 66-item FFQ, so there is random and systematic measurement error in the exposure. This error likely biased results toward the null and made it more difficult to detect significant effects. However, the validated FFQ was administered by trained interviewers according to standardized procedures (28), and some ARIC food groups or dietary patterns have been associated significantly with other cardiovascular disease risk factors and outcomes (32, 53, 54). Because there were only 66 items in this instrument, energy intake is likely underestimated, so residual confounding may explain some of the meat-PAD relation. Additionally, dietary intake was measured in the late 1980s and may not be representative of contemporary dietary intake. For the outcome, ABI was measured only on a randomly selected subset of the ARIC Study population at visits 3 and 4, so some participants with asymptomatic PAD may have been missed. Additionally, ABI was measured only on one randomly selected leg, so some participants with unilateral PAD may have been misclassified (29). Information on ABI is also available only during the first 9 y of the follow-up period, and thereafter incident cases were identified only through hospital surveillance. The use of administrative data, such as hospitalization data, to identify PAD cases is known to have high specificity but low sensitivity (55). Thus, we likely under-ascertained PAD cases. A large number of statistical tests were performed in this study, so it is possible that the meat-PAD relation is due to chance, although associations were found between meat subgroups and PAD. Additionally, residual confounding may have remained despite our attempts at adjustment. This study also has several strengths, including its longitudinal design, enrollment of >14,000 black and white men and women, the large number of PAD cases, standardized method of data collection, comprehensive assessment of potential confounding factors, and 20 y of follow-up.
Overall, this study found that greater meat intake was associated with greater risk of incident PAD, whereas low alcohol intake and coffee consumption were associated with a lower risk of incident PAD. It is not clear whether these associations are causal. Public health strategies are needed to help Americans choose foods that promote cardiovascular health.
Acknowledgments
The authors’ responsibilities were as follows—RPO, PLL, and LMS: designed the research; RPO: analyzed the data and had primary responsibility for the final content; and all authors: wrote the manuscript and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: ABI, ankle-brachial index; ARIC, Atherosclerosis Risk in Communities; FFQ, food-frequency questionnaire; ICD-9, International Classification of Diseases, 9th edition; PAD, peripheral arterial disease.
REFERENCES
- 1.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, et al. . ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006;113:e463–654. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, et al. . Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- 3.Mahoney EM, Wang K, Cohen DJ, Hirsch AT, Alberts MJ, Eagle K, Mosse F, Jackson JD, Steg PG, Bhatt DL, et al. . One-year costs in patients with a history of or at risk for atherothrombosis in the United States. Circ Cardiovasc Qual Outcomes 2008;1:38–45. [DOI] [PubMed] [Google Scholar]
- 4.Joosten MM, Pai JK, Bertoia ML, Rimm EB, Spiegelman D, Mittleman MA, Mukamal KJ. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA 2012;308:1660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med 2009;169:659–69. [DOI] [PubMed] [Google Scholar]
- 6.Merchant AT, Hu FB, Spiegelman D, Willett WC, Rimm EB, Ascherio A. Dietary fiber reduces peripheral arterial disease risk in men. J Nutr 2003;133:3658–63. [DOI] [PubMed] [Google Scholar]
- 7.Donnan PT, Thomson M, Fowkes FG, Prescott RJ, Housley E. Diet as a risk factor for peripheral arterial disease in the general population: the Edinburgh Artery Study. Am J Clin Nutr 1993;57:917–21. [DOI] [PubMed] [Google Scholar]
- 8.Naqvi AZ, Davis RB, Mukamal KJ. Nutrient intake and peripheral artery disease in adults: key considerations in cross-sectional studies. Clin Nutr 2014;33:443–7. [DOI] [PubMed] [Google Scholar]
- 9.Antonelli-Incalzi R, Pedone C, McDermott MM, Bandinelli S, Miniati B, Lova RM, Lauretani F, Ferrucci L. Association between nutrient intake and peripheral artery disease: results from the InCHIANTI study. Atherosclerosis 2006;186:200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lane JS, Magno CP, Lane KT, Chan T, Hoyt DB, Greenfield S. Nutrition impacts the prevalence of peripheral arterial disease in the United States. J Vasc Surg 2008;48:897–904. [DOI] [PubMed] [Google Scholar]
- 11.Leng GC, Horrobin DF, Fowkes FG, Smith FB, Lowe GD, Donnan PT, Ells K. Plasma essential fatty acids, cigarette smoking, and dietary antioxidants in peripheral arterial disease. A population-based case-control study. Arterioscler Thromb 1994;14:471–8. [DOI] [PubMed] [Google Scholar]
- 12.Langlois M, Duprez D, Delanghe J, De Buyzere M, Clement DL. Serum vitamin C concentration is low in peripheral arterial disease and is associated with inflammation and severity of atherosclerosis. Circulation 2001;103:1863–8. [DOI] [PubMed] [Google Scholar]
- 13.Klipstein-Grobusch K, den Breeijen JH, Grobbee DE, Boeing H, Hofman A, Witteman JC. Dietary antioxidants and peripheral arterial disease: the Rotterdam Study. Am J Epidemiol 2001;154:145–9. [DOI] [PubMed] [Google Scholar]
- 14.Melamed ML, Muntner P, Michos ED, Uribarri J, Weber C, Sharma J, Raggi P. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol 2008;28:1179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reis JP, Michos ED, von Muhlen D, Miller ER 3rd. Differences in vitamin D status as a possible contributor to the racial disparity in peripheral arterial disease. Am J Clin Nutr 2008;88:1469–77. [DOI] [PubMed] [Google Scholar]
- 16.Zagura M, Serg M, Kampus P, Zilmer M, Eha J, Unt E, Lieberg J, Cockcroft JR, Kals J. Aortic stiffness and vitamin D are independent markers of aortic calcification in patients with peripheral arterial disease and in healthy subjects. Eur J Vasc Endovasc Sur 2011;42:689–95. [DOI] [PubMed] [Google Scholar]
- 17.Wilmink AB, Welch AA, Quick CR, Burns PJ, Hubbard CS, Bradbury AW, Day NE. Dietary folate and vitamin B6 are independent predictors of peripheral arterial occlusive disease. J Vasc Surg 2004;39:513–6. [DOI] [PubMed] [Google Scholar]
- 18.Bunout D, Petermann M, Hirsch S, de la Maza P, Suazo M, Barrera G, Kauffman R. Low serum folate but normal homocysteine levels in patients with atherosclerotic vascular disease and matched healthy controls. Nutrition 2000;16:434–8. [DOI] [PubMed] [Google Scholar]
- 19.Bertoia ML, Pai JK, Cooke JP, Joosten MM, Mittleman MA, Rimm EB, Mukamal KJ. Plasma homocysteine, dietary B vitamins, betaine, and choline and risk of peripheral artery disease. Atherosclerosis 2014;235:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heffron SP, Rockman CB, Gianos E, Guo Y, Berger JS. Greater frequency of nut consumption is associated with lower prevalence of peripheral arterial disease. Prev Med 2015;72:15–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hung HC, Merchant A, Willett W, Ascherio A, Rosner BA, Rimm E, Joshipura KJ. The association between fruit and vegetable consumption and peripheral arterial disease. Epidemiology 2003;14:659–65. [DOI] [PubMed] [Google Scholar]
- 22.Mukamal KJ, Kennedy M, Cushman M, Kuller LH, Newman AB, Polak J, Criqui MH, Siscovick DS. Alcohol consumption and lower extremity arterial disease among older adults: the cardiovascular health study. Am J Epidemiol 2008;167:34–41. [DOI] [PubMed] [Google Scholar]
- 23.Camargo CA Jr, Stampfer MJ, Glynn RJ, Gaziano JM, Manson JE, Goldhaber SZ, Hennekens CH. Prospective study of moderate alcohol consumption and risk of peripheral arterial disease in US male physicians. Circulation 1997;95:577–80. [DOI] [PubMed] [Google Scholar]
- 24.Djoussé L, Levy D, Murabito JM, Cupples LA, Ellison RC. Alcohol consumption and risk of intermittent claudication in the Framingham Heart Study. Circulation 2000;102:3092–7. [DOI] [PubMed] [Google Scholar]
- 25.Ruiz-Canela M, Estruch R, Corella D, Salas-Salvado J, Martinez-Gonzalez MA. Association of Mediterranean diet with peripheral artery disease: the PREDIMED randomized trial. JAMA 2014;311:415–7. [DOI] [PubMed] [Google Scholar]
- 26.Carrero JJ, Grimble RF. Does nutrition have a role in peripheral vascular disease? Br J Nutr 2006;95:217–29. [DOI] [PubMed] [Google Scholar]
- 27.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 28.Hu FB, Rimm E, Smith-Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr 1999;69:243–9. [DOI] [PubMed] [Google Scholar]
- 29.Wattanakit K, Folsom AR, Selvin E, Coresh J, Hirsch AT, Weatherley BD. Kidney function and risk of peripheral arterial disease: results from the Atherosclerosis Risk in Communities (ARIC) Study. J Am Soc Nephrol 2007;18:629–36. [DOI] [PubMed] [Google Scholar]
- 30.Weatherley BD, Chambless LE, Heiss G, Catellier DJ, Ellison CR. The reliability of the ankle-brachial index in the Atherosclerosis Risk in Communities (ARIC) study and the NHLBI Family Heart Study (FHS). BMC Cardiovasc Disord 2006;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 1982;36:936–42. [DOI] [PubMed] [Google Scholar]
- 32.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation 2008;117:754–61. [DOI] [PubMed] [Google Scholar]
- 33.Nettleton JA, Matijevic N, Follis JL, Folsom AR, Boerwinkle E. Associations between dietary patterns and flow cytometry-measured biomarkers of inflammation and cellular activation in the Atherosclerosis Risk in Communities (ARIC) Carotid Artery MRI Study. Atherosclerosis 2010;212:260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Z, Li S, Liu G, Yan F, Ma X, Huang Z, Tian H. Body iron stores and heme-iron intake in relation to risk of type 2 diabetes: a systematic review and meta-analysis. PLoS One 2012;7:e41641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abete I, Romaguera D, Vieira AR, Lopez de Munain A, Norat T. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: a meta-analysis of cohort studies. Br J Nutr 2014;112:762–75. [DOI] [PubMed] [Google Scholar]
- 36.Haring B, Misialek JR, Rebholz CM, Petruski-Ivleva N, Gottesman RF, Mosley TH, Alonso A. Association of dietary protein consumption with incident silent cerebral infarcts and stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke 2015;46:3443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haring B, Gronroos N, Nettleton JA, von Ballmoos MC, Selvin E, Alonso A. Dietary protein intake and coronary heart disease in a large community based cohort: results from the Atherosclerosis Risk in Communities (ARIC) study [corrected]. PLoS One 2014;9:e109552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation 2010;121:2271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes–an updated review of the evidence. Curr Atheroscler Rep 2012;14:515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aune D, Ursin G, Veierod MB. Meat consumption and the risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies. Diabetologia 2009;52:2277–87. [DOI] [PubMed] [Google Scholar]
- 41.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ 2011;342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ 2011;342:d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding J, Eigenbrodt ML, Mosley TH Jr, Hutchinson RG, Folsom AR, Harris TB, Nieto FJ. Alcohol intake and cerebral abnormalities on magnetic resonance imaging in a community-based population of middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) study. Stroke 2004;35:16–21. [DOI] [PubMed] [Google Scholar]
- 44.Fuchs FD, Chambless LE, Folsom AR, Eigenbrodt ML, Duncan BB, Gilbert A, Szklo M. Association between alcoholic beverage consumption and incidence of coronary heart disease in whites and blacks: the Atherosclerosis Risk in Communities Study. Am J Epidemiol 2004;160:466–74. [DOI] [PubMed] [Google Scholar]
- 45.Gaziano JM, Buring JE, Breslow JL, Goldhaber SZ, Rosner B, VanDenburgh M, Willett W, Hennekens CH. Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions, and decreased risk of myocardial infarction. N Engl J Med 1993;329:1829–34. [DOI] [PubMed] [Google Scholar]
- 46.Hendriks HF, Veenstra J, van Tol A, Groener JE, Schaafsma G. Moderate doses of alcoholic beverages with dinner and postprandial high density lipoprotein composition. Alcohol Alcohol 1998;33:403–10. [DOI] [PubMed] [Google Scholar]
- 47.Schrieks IC, Heil AL, Hendriks HF, Mukamal KJ, Beulens JW. The effect of alcohol consumption on insulin sensitivity and glycemic status: a systematic review and meta-analysis of intervention studies. Diabetes Care 2015;38:723–32. [DOI] [PubMed] [Google Scholar]
- 48.Naimi TS, Brown DW, Brewer RD, Giles WH, Mensah G, Serdula MK, Mokdad AH, Hungerford DW, Lando J, Naimi S, et al. . Cardiovascular risk factors and confounders among nondrinking and moderate-drinking U.S. adults. Am J Prev Med 2005;28:369–73. [DOI] [PubMed] [Google Scholar]
- 49.Ding M, Bhupathiraju SN, Satija A, van Dam RM, Hu FB. Long-term coffee consumption and risk of cardiovascular disease: a systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation 2014;129:643–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding M, Bhupathiraju SN, Chen M, van Dam RM, Hu FB. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: a systematic review and a dose-response meta-analysis. Diabetes Care 2014;37:569–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ciccarone E, Di Castelnuovo A, Salcuni M, Siani A, Giacco A, Donati MB, De Gaetano G, Capani F, Iacoviello L, Gendiabe I. A high-score Mediterranean dietary pattern is associated with a reduced risk of peripheral arterial disease in Italian patients with Type 2 diabetes. J Thromb Haemost 2003;1:1744–52. [DOI] [PubMed] [Google Scholar]
- 52.Hoevenaar-Blom MP, Nooyens AC, Kromhout D, Spijkerman AM, Beulens JW, van der Schouw YT, Bueno-de-Mesquita B, Verschuren WM. Mediterranean style diet and 12-year incidence of cardiovascular diseases: the EPIC-NL cohort study. PLoS One 2012;7:e45458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nettleton JA, Steffen LM, Loehr LR, Rosamond WD, Folsom AR. Incident heart failure is associated with lower whole-grain intake and greater high-fat dairy and egg intake in the Atherosclerosis Risk in Communities (ARIC) study. J Am Diet Assoc 2008;108:1881–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weng LC, Steffen LM, Szklo M, Nettleton J, Chambless L, Folsom AR. A diet pattern with more dairy and nuts, but less meat is related to lower risk of developing hypertension in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) study. Nutrients 2013;5:1719–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hong Y, Sebastianski M, Makowsky M, Tsuyuki R, McMurtry MS. Administrative data are not sensitive for the detection of peripheral artery disease in the community. Vasc Med 2016;21:331–6. [DOI] [PubMed] [Google Scholar]