Abstract
Background: Current obesity theories suggest that the repeated intake of highly palatable high-sugar foods causes adaptions in the striatum, parietal lobe, and prefrontal and visual cortices in the brain that may serve to perpetuate consumption in a feed-forward manner. However, the data for humans are cross-sectional and observational, leaving little ability to determine the temporal precedence of repeated consumption on brain response.
Objective: We tested the impact of regular sugar-sweetened beverage intake on brain and behavioral responses to beverage stimuli.
Design: We performed an experiment with 20 healthy-weight individuals who were randomly assigned to consume 1 of 2 sugar-sweetened beverages daily for 21 d, underwent 2 functional MRI sessions, and completed behavioral and explicit hedonic assessments.
Results: Consistent with preclinical experiments, daily beverage consumption resulted in decreases in dorsal striatal response during receipt of the consumed beverage (r = −0.46) and decreased ventromedial prefrontal response during logo-elicited anticipation (r = −0.44). This decrease in the prefrontal response correlated with increases in behavioral disinhibition toward the logo of the consumed beverage (r = 0.54; P = 0.02). Daily beverage consumption also increased precuneus response to both juice logos compared with a tasteless control (r = 0.45), suggesting a more generalized effect toward beverage cues. Last, the repeated consumption of 1 beverage resulted in an explicit hedonic devaluation of a similar nonconsumed beverage (P < 0.001).
Conclusions: Analogous to previous reports, these initial results provide convergent data for a role of regular sugar-sweetened beverage intake in altering neurobehavioral responses to the regularly consumed beverage that may also extend to other beverage stimuli. Future research is required to provide evidence of replication in a larger sample and to establish whether the neurobehavioral adaptations observed herein are specific to high-sugar and/or nonnutritive-sweetened beverages or more generally related to the repeated consumption of any type of food. This trial was registered at clinicaltrials.gov as NCT02624206.
Keywords: reward, dorsal striatum, habit-based decision making, sugar-sweetened beverage, obesity, fMRI, ventromedial prefrontal cortex
INTRODUCTION
Sugar-sweetened beverage (SSB) intake is considered to contribute to obesity because SSBs contain a high sugar content and few healthful nutrients and elicit weak satiation signaling (1–3). On a typical day, 63–80% of individuals consume an SSB, contributing a mean of 7–13% of calories consumed daily (4–6). Sugar consumption signals the release of dopamine and opioids in the striatum at a large magnitude (7) and subsequently induces reinforcing feelings of pleasure (8). The striatum plays a key role in value-based learning, encoding hedonic valuation (i.e., reward), and motivated behavior (8–10). The robust ability of sugar in engaging reward-related brain regions while simultaneously providing nonhealthful calories has led theorists to propose that high-sugar food consumption is hedonically motivated, perpetuating consumption in a feed-forward manner (11).
Prominent brain-based models of obesity have proposed that overeating is underpinned by efforts to overcome anhedonia caused by dysfunctional dopamine circuitry (12) and via compromised behavioral control toward food (13, 14). In support of this theory, a reduced striatal response during palatable food intake is seen in obesity (15) and is inversely related to weight gain (16). Moreover, obesity is associated with low metabolism in prefrontal cortex regions implicated in executive functioning (14) and deficits in prefrontal region response during food-based impulsivity tasks (17). An alternative brain-based theory of obesity proposes that enhanced incentive motivation (i.e., “wanting”) for food drives overeating (18). In support of the incentive sensitization theory, obese individuals have shown increased striatal and parietal response to food cues (15, 19) and increased wanting of energy-dense foods during behavioral tasks (20, 21). However, the initial neuroadaptations associated with habitual consumption before weight gain that underlie aberrant obesity-related brain response patterns are unknown. It is important to note that human neuroimaging studies have reported an inverse relation between striatal response and the intake of a specified food independent of weight status. For example, frequent ice cream consumption was related to a reduced striatal and prefrontal response during the receipt of milkshake in lean adolescents (22), a finding supported by additional reports (23, 24). Furthermore, objective measures of elevated caloric intake have been shown to increase visual cortex and parietal response during cue-elicited anticipation of a palatable food (25). Last, regular Coke consumers have shown increased visual cortex and parietal response during exposure to Coke advertisements relative to their nonconsuming counterparts independent of weight status (26). These studies showed similar patterns when comparing obese and lean individuals but also suggest that the neurobehavioral patterns precede the accumulation of excess adipose tissue and thus may serve as risk factors for perpetuating intake. Although these observational data support the theory that regular high-sugar intake may contribute to neuroadaptations to frequently consumed foods and associated stimuli, it is vital to experimentally test these processes to determine the underlying cause of the responses.
To address this gap, a randomized controlled trial (NCT02624206) was performed to test whether the daily consumption of a branded high-sugar beverage over a 3-wk period would affect fMRI measures of brain response during beverage intake, logo-elicited anticipation of the beverage, behavioral disinhibition toward beverage logos, and perceptual hedonic ratings of the beverages. It was hypothesized that regular high-sugar beverage intake would result in 1) a specified decreased striatal and prefrontal response during beverage receipt, 2) increased visual cortex and parietal response during logo-elicited anticipation of the assigned beverage, and 3) increased behavioral disinhibition toward the assigned beverage logo compared with a nonconsumed but otherwise similar beverage.
METHODS
Study design and participants
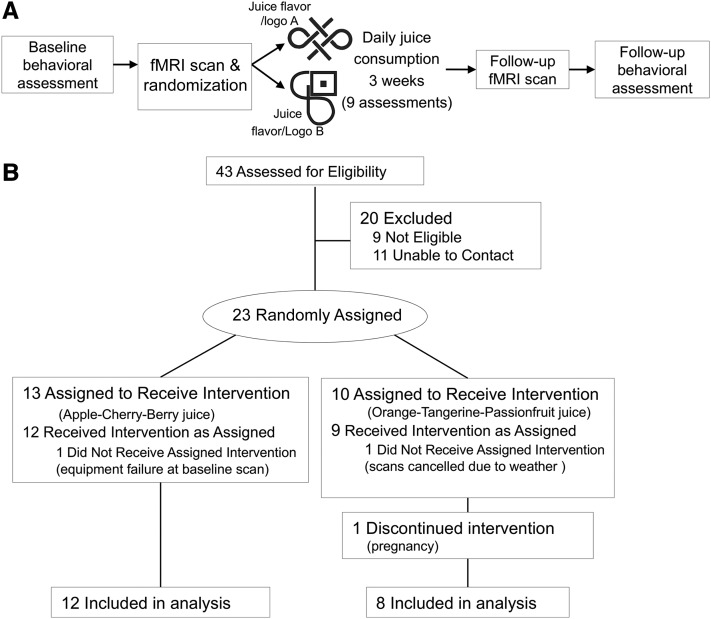
We assigned participants to consume 1 of 2 novel-flavored high-sugar beverages daily for a 3-wk intervention period with the use of a block randomization pattern based on sex. The study included fMRI and behavioral assessments at baseline and postintervention, i.e., follow-up and 9 study visits during the intervention period (Figure 1A). A convenience sample of 20 healthy-weight young adults [n = 10 women and 10 men; mean age: 23.3 ± 3.4 y; mean BMI (in kg/m2): 22.1 ± 1.9; ethnicity: 5% Hispanic, 10% Asian/Pacific Islanders, and 85% European Americans] completed the protocol (Figure 1B). Exclusion criteria were a BMI <18.5 or >26.5, sweetened (artificial or caloric) beverage consumption >6 times/wk, nicotine use >6 times/wk, psychoactive medications or drugs >1 time/mo, pregnancy, head injury with a loss of consciousness, substantial cognitive impairment, major medical problems, and endorsement of disordered eating or current Axis I psychiatric disorder. Participants provided written informed consent, and the methods and procedures were approved by the University of North Carolina at Chapel Hill Institutional Review Board.
FIGURE 1.
Individual participant study timeline (A) and flow of all samples through the intervention (B).
Intervention
To provide an active control (assigned beverage compared with nonassigned beverage), participants were randomly assigned to consume 1 of the 2 juice conditions stratified by sex. After completing the baseline behavioral and neuroimaging assessments, participants began the intervention period, during which they consumed their assigned branded juice daily for a 21-d period. During the intervention, participants came to the laboratory on Mondays, Wednesdays, and Fridays (9 total assessments) to consume a portion of that day’s juice in the laboratory, complete perceptual hedonic measures, have their weight assessed, pick up the following day’s juice, and return the empty bottles from the previous day(s). On Fridays, participants were given 2 bottles to take home and instructed to consume 1 bottle/d and to return the bottles on the following Monday. Empty bottles were returned to increase compliance. Upon completion of the intervention, participants did not consume juice for 24 h and then completed the follow-up neuroimaging assessment. The follow-up behavioral assessment occurred within 3 d of the end of the intervention.
Before initiating the study, beverages and logos were tested in the local population to ensure equal palatability, novelty, willingness to consume juice flavors, and uniqueness of the beverage logos. Beverages were prepared in our laboratory with the use of 100% fruit juices and eat-to-drink juice blends (V8 V-fusion refreshers). Beverages were designed to exclude high-fructose corn syrup or artificial sweeteners, artificial flavors, colors, or preservatives and be calorically equivalent to soft drinks, caffeine- and texture-free, and generally novel in flavor. The 2 juices selected for the study were apple-cherry-berry and orange-tangerine-passion fruit. The 10-fl oz (296-mL) bottles of juice presented daily contained 133 kcal and 31 g sugar, with the only identifier a sticker of the associated logo (Figure 1A). The juice logos were drawn from a pool from a previous study that evaluated aspects of cue-potentiated food intake (27). Logos selected for this study were confirmed to be novel via pilot testing and were similar in visual complexity, black and white, conveyed no information beyond a unique symbol, and were easily distinguishable from one another. Images of the logos on the bottles can be seen in Supplemental Figure 1.
Neuroimaging
Juice receipt and logo-elicited anticipation paradigm
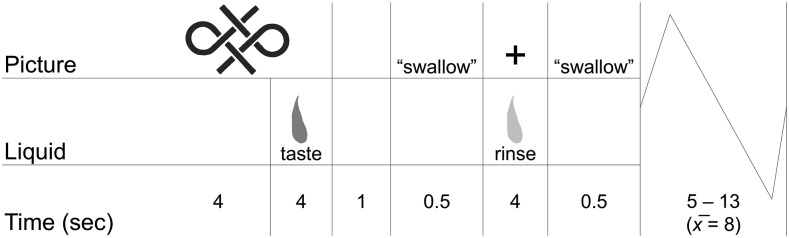
The juice fMRI paradigm assessed evoked a BOLD response to the receipt of both juices and a tasteless solution, as well as logo-elicited anticipation of each of the 3 tastes (the 2 juices and tasteless solution). The paradigm (Figure 2) was controlled by in-house scripts written in MATLAB (MathWorks). The visual stimuli were the 2 juice logos, a similar logo preceding the tasteless solution, and a fixation cross. Each logo signaled the impending delivery of 0.5 mL of the associated juice, with the fixation cross otherwise presented. Juices were delivered with the use of individual programmable pumps that were connected to a manifold that fit into the participants’ mouths and delivered the tastes without cross-contamination. Juice administration was followed by an uncued rinse of the tasteless solution and swallow cue. A jitter ranging from 5 to 13 s (mean: 8 s) followed each trial. In total, 27 repeats of each of the 3 tastes (totaling 81 taste events) and 27 repeats of each of the logos (totaling 81 logo events) were administered over three ∼12-min runs.
FIGURE 2.
Sample epoch timing for the juice and anticipation fMRI paradigm.
MRI acquisition parameters
Scanning was performed with the use of a Siemens Tim Trio MRI scanner. A 32-channel coil acquired data from the entire brain. Functional scans used a T2*-weighted gradient, single-shot, echo-planar imaging sequence (echo time: 30 ms; repetition time: 2000 ms; flip angle: 80°) with an in-plane resolution of 3 × 3 mm2 (64 × 64 matrix; 192 × 192-mm2 field of view). Thirty-two 3-mm slices (interleaved acquisition, no skip) were acquired along the AC-PC transverse oblique plane as determined by the midsagittal section. Structural scans were collected with the use of an inversion-recovery T1-weighted sequence (magnetization-prepared rapid gradient echo) along the AC-PC plane. High-resolution structural MRI sequences (field of view: 256 × 256 mm2; matrix: 256 × 256; thickness: 1.0 mm; number of slices: ∼160) were acquired.
Neuroimaging data processing and statistical analysis
Neuroimaging data were preprocessed and analyzed primarily with SPM12 in MATLAB version 8.5 for Mac OS X. Anatomic images were segmented and normalized to Montreal Neurological Institute space with the use of the DARTEL toolbox. Functional images were slice-timing corrected, adjusted for variation in magnetic field distortion, realigned to the mean functional volume, coregistered with the anatomic images, normalized to Montreal Neurological Institute space with the use of DARTEL, and smoothed to a 6-mm Gaussian full-width half maximum. The z-normalized global brain activation >3 SDs from the mean of the run or >1 mm of the composite movement were flagged as outliers and deweighted during individual-level model estimation.
Individual-level t maps were constructed to compare the activation in response to juice receipt and logo-elicited anticipation. These individual contrasts were entered into second-level flexible factorial models that accounted for the between- and within-subject nature of the data. However, although a test of a time-by-condition interaction provides data on whether there is a simultaneous effect of these conditions, one cannot determine the directionality of effects or relative impact of each condition without pairwise comparisons. As such, to probe interactions with a priori hypotheses, positive and negative time-by-condition interactions were tested, and the results were saved as masks (P < 0.05) and then applied as a filter to the results from a priori paired sample t tests (28). This approach ensured that only activity significant within the interaction was tested in the pairwise tests. It did not restrict the number of voxels that were compared; whole-brain corrections were used throughout. Data presented are from the masked paired sample t tests for the responses to the logo and juice receipt for the specific effect of juice consumption, e.g., (pre)assigned juice receipt − (pre)juice not assigned receipt compared with (post)juice assigned receipt − (post)juice not assigned receipt. To examine the more general effects of juice consumption, contrasts were used that collapsed across both juice stimuli and were compared with the tasteless solution stimuli [(pre)receipt of both juices − (pre)tasteless solution receipt compared with (post)receipt of both juices − (post)tasteless solution receipt]. The latter contrast was preformed post hoc. Statistical significance was calculated with the use of the 3dFWHMx/3DClustSim modules in AFNI version 16.3.09. Monte Carlo simulations of random noise were run through the functional data of the whole brain that indicated activity surviving a threshold of P < 0.005; k ≥ 22 was statistically significant when corrected for multiple comparisons across the whole brain (P-corr < 0.05). This correction reflects comparisons across the whole brain. The masking approach to ensure data were active in both the time-by-condition interaction and directional t tests did not reduce the number of comparisons and thus did not affect the family-wise correction techniques. In brain-behavioral correlation analyses, highly leveraging data points (Cook’s d > 1) were excluded; this criterion was applied to 1 data point in post hoc brain-behavioral correlations. Significant neuroimaging results presented were not attenuated when controlling for hunger, phase of menstrual cycle, sex, and/or sweetened beverage consumption over the previous 2 wk, which was very low (mean: 0.091 ± 0.25 beverages/d). Cohen’s d was calculated as a measure of pre- and posteffect size, which was then transformed to r because this is a universal measure of effect size (29). Extended methods and additional results (main effects of logo exposure and juice receipt) are shown in Supplemental Figures 2 and 3. Study protocol, raw imaging data, unthresholded statistical parametric maps, and analysis scripts are publicly available at http://www.NIBLunc.org.
Behavioral and perceptual measures
Behavioral logo task
Motor disinhibition in response to the beverage and control logos was measured with the use of the behavioral logo task similar to a traditional go or no-go task. Participants were instructed to respond as quickly and accurately as possible by pressing the keyboard when shown the target logo but to withhold their responses until the other logos were presented. Participants performed the task twice, each time depicting one of the beverage logos as the target logo at both the preintervention and follow-up assessments. The order of the target logo presentation was counterbalanced across participants. Each task consisted of 48 trials. For each trial, a picture of the target logo (go trial: 75% occurrence) or similar logos (no-go trial: 25% occurrence) was presented for 500 ms. Trials were separated by a fixation cross that was presented at intervals ranging from 2 to 6 s. Stimuli were presented, and reaction times and commission and omission errors were recorded and calculated with the use of Presentation version 9 (Neurobehavioral Systems).
Perceptual hedonic scales
Explicit perceived pleasantness of and desire to consume the 2 juices was measured with the use of the adapted labeled hedonic scales (30) at all assessments. Scales were tailored to pleasantness of and desire to consume the beverages and were anchored from least imaginable to most imaginable (−100 to 100, respectively, with neutral = 0).
Nonimaging statistical analysis
Data from baseline and follow-up behavioral and perceptual measures were analyzed with the use of PROC MIXED implemented in SAS version 9.4 (SAS Institute). These data were entered into 2 × 2 repeated-measure mixed models, including assignment-by-time interactions. Significant interactions (P < 0.05) were probed with least-squared mean comparisons. Reaction times from the behavioral logo task were log-transformed to reduce the influence of outliers and nonnormality of the data (31). No significant differences at baseline between the 2 experimental groups (assigned juice or logo compared with nonassigned juice or logo) or the 2 juices (apple- compared with orange-based juice) were observed (P = 0.13–0.87). Perceptual hedonic juice ratings and body mass data from the 9 intervention assessments were analyzed in random-intercept growth-curve models implemented in SAS version 9.4 to model change over time.
RESULTS
BOLD response to anticipatory beverage logos and beverage receipt
Examination of specified effect of consumption
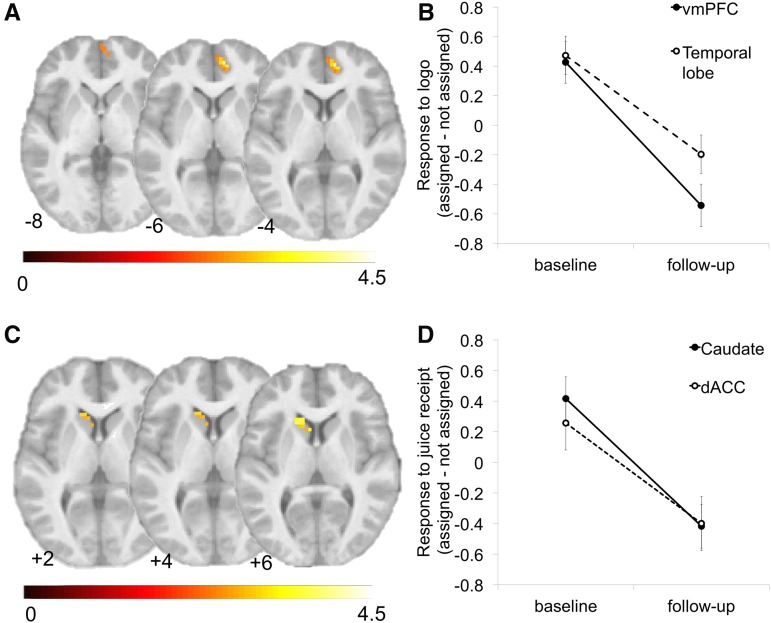
The daily intake of the assigned beverage significantly reduced BOLD response during the exposure of the anticipatory logo of the assigned beverage in the ventromedial prefrontal cortex (vmPFC) compared with the nonassigned logo (z = −4.0; k = 33; r = −0.44) (Table 1, Figure 3A, B) and temporal lobe (z = −4.3; k = 35; r = −0.53) (Table 1, Figure 3B). Similarly, the daily intake of the assigned beverage significantly reduced BOLD response during the receipt of the assigned beverage in the caudate (z = −3.8; k = 27; r = −0.46) (Table 1, Figure 3C, D) and dorsal anterior cingulate cortex (z = −3.1; k = 24; r = −0.41) compared with the nonassigned beverage (Table 1, Figure 3D). Analyses of baseline-only data confirmed no significant differences in BOLD response during beverage receipt or to the anticipatory logo when directly comparing the 2 juices and their associated logos independently of study assignment [(pre)apple-cherry-berry compared with (pre)orange-tangerine-passion fruit) and when based on assignment [(pre)assigned compared with (pre)nonassigned].
TABLE 1.
Changes in BOLD response as a function of daily SSB intake over a 3-wk period (n = 20)1
| Hemisphere | x, y, z2 | k | Peak z value | r3 | |
| Specified effect of consumption | |||||
| (Pre)assigned logo receipt − (pre)logo not assigned receipt − (post)assigned logo receipt − (post)logo not assigned receipt | |||||
| Temporal lobe | Left | −54, −21, −15 | 35 | −4.3 | −0.53 |
| vmPFC | Right | 9, 51, 6 | 33 | −4.0 | −0.44 |
| 3, 51, 6 | −2.9 | ||||
| (Pre)assigned juice receipt − (pre)juice not assigned receipt − (post)juice assigned receipt − (post)juice not assigned receipt | |||||
| Caudate | Right | −18, 21, 9 | 27 | −3.8 | −0.46 |
| −6, 15, 6 | −3.0 | ||||
| −3, 15, −3 | −2.7 | ||||
| dACC | Left | 15, 36, 15 | 24 | −3.1 | −0.41 |
| 12, 30, 21 | −3.1 | ||||
| 9, 27, 12 | −2.8 | ||||
| General effect of consumption4 | |||||
| (Pre)receipt of both juice logos − (pre)tasteless solution logo receipt − (post)receipt of both juice logos − (post)tasteless solution logo receipt | |||||
| Parietal lobe (precuneus) | Right | −9, −54, 42 | 35 | 3.6 | 0.45 |
| −6, −45, 45 | 3.4 |
Significant values for the whole brain corrected for multiple comparisons for the pre- and postchange at P-corr < 0.05 are presented. The significant activity presented is masked from flexible factorial models that test the assignment-by-time interaction, and values were significant when tested in paired sample t tests to account for the between- and within-subject nature of the data. dACC, dorsal anterior cingulate cortex; SSB, sugar-sweetened beverage; vmPFC, ventromedial prefontal cortex.
Coordinates presented in Montreal Neurological Institute space.
Cohen’s d was calculated as a measure of pre- and posteffect size, which was then transformed to r because this is a universal measure of effect size (29).
No significant effects were observed with the (pre)receipt of both juices − (pre)tasteless solution receipt − (post)receipt of both juices − (post)tasteless solution receipt contrast.
FIGURE 3.
Alterations in brain response as a function of daily beverage consumption. (A) Changes in brain response during exposure to the assigned anticipatory logo in the vmPFC presented in axial slices and (B) parameter estimates of brain response graphically represented at baseline and follow-up. (C) Changes in brain response during the intake of the assigned sugar-sweetened beverage in the caudate presented in axial slices and (D) parameter estimates of brain response graphically represented at baseline and follow-up. The color bands in panels A and C reflect the t value of the BOLD response. dACC, dorsal anterior cingulate cortex; vmPFC, ventromedial prefontal cortex.
Examination of the general effect of consumption
The daily intake of the assigned beverage significantly increased BOLD response during the exposure of both juice logos compared with the logo of the tasteless solution in the precuneus (z = 3.6; k = 35; r = 0.45) (Table 1). No significant effect of daily consumption was observed when assessing the receipt of both juices compared with tasteless solution receipt. No significant intervention effects and/or time were observed when assessing tasteless solution receipt compared with baseline and logo-elicited anticipation of the tasteless solution compared with baseline.
Behavioral and explicit hedonic measures
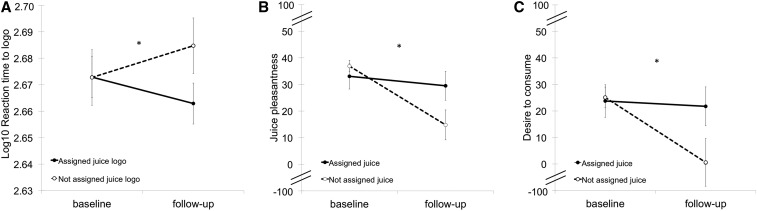
The daily consumption of the branded beverages resulted in changes in reaction time toward the beverage logos (F = 4.0; P = 0.04; r = 0.31 (Figure 4A), driven by a trend of faster reaction times toward the assigned logo from baseline to follow-up (t = −1.9; P = 0.07). No changes in omission and commission error rates as a function of the intervention were observed (P = 0.67–0.75). Post hoc correlation analyses revealed that the changes in reaction times were positively associated with changes in vmPFC response to the assigned logos (r = 0.54; P = 0.02), suggesting the decrease in prefrontal response to the assigned logo underpinned faster (i.e., lower) reaction times in the behavioral task toward the same logo. No other brain-behavior correlations were observed in the hypothesized striatal and prefrontal regions (P > 0.05).
FIGURE 4.
Alterations in behavioral responses and explicit hedonic ratings as a function of daily beverage consumption. Significant changes in (A) reaction times toward juice logos, (B) juice pleasantness, and (C) desire to consume juice from baseline to follow-up are shown. The full scales in panels B and C ranging from −100 to 100 (with 0 indicating neutral) were truncated for display purposes.
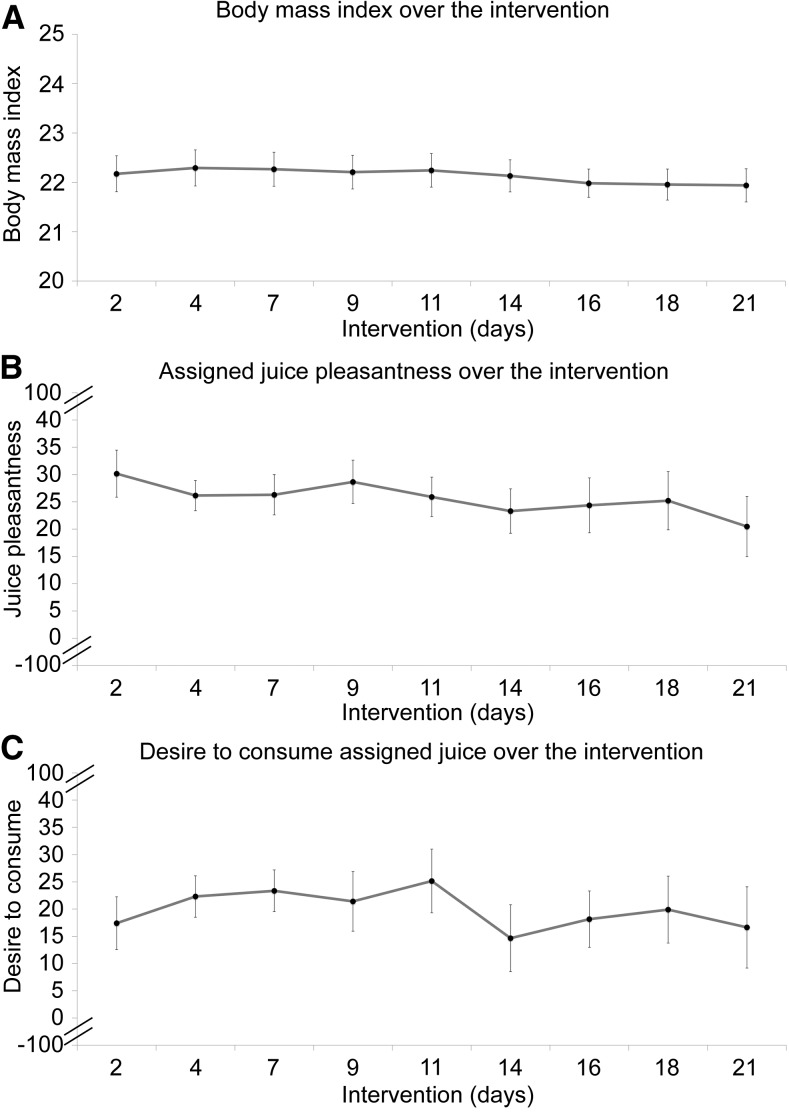
A significant change in explicit perceptual ratings of beverage pleasantness was observed as a function of the intervention (F = 8.7; P < 0.01) (Figure 4B). This effect was driven by a decrease in pleasantness of the nonassigned beverage (t = −4.2; P < 0.001) from baseline to follow-up. Similar effects were observed in reported desire to consume the beverages (F = 6.4; P = 0.01) (Figure 4C), in which a significant decrease in desire in nonassigned juice was observed (t = −3.1; P < 0.01). There were no differences in pleasantness or desire to consume the juices at baseline (P = 0.86–0.99). No statistically significant changes were observed in BMI (β: −0.01; SE: 0.006; P = 0.10) (Figure 5A), perceptual hedonic ratings for pleasantness of the assigned juice (β: −0.36; SE: 0.32; P = 0.26) (Figure 5B), or desire to consume the assigned juice (β: −0.19; SE: 0.35; P = 0.59) (Figure 5C) over the intervention period.
FIGURE 5.
The impact of daily beverage consumption on body mass and hedonic ratings throughout the intervention. No significant changes were observed in (A) body mass and (B and C) perceptual hedonic ratings of the assigned juice over the 3-wk intervention period. The full scales shown in panels B and C ranging from −100 to 100 (with 0 indicating neutral) were truncated for display purposes.
DISCUSSION
Frequent SSB consumption has been previously implicated in increased energy intake and body mass and as a predictor of future weight gain (1, 2). The daily consumption of branded high-sugar beverages in this study resulted in decreases in the neural response to specified consumed beverage stimuli in frontostriatal brain regions. Consistent with reports that have shown decreases in the striatal response to taste in obesity and weight gain (15, 16), the repeated intake of an SSB herein resulted in decreases in the striatal response to that beverage. These data are also consistent with previous reports that showed that habitual eating behavior was related to decreases in the response to those foods independent of weight status (22, 23). These data collectively indicate that the regular consumption of an SSB plays a causal role in the downregulation of striatal response during the intake of that beverage. Although a specific response to the logo of the beverage consumed was not found in this study, a general increased response to the juice logos was observed in brain regions thought to encode salience of stimuli. These data may suggest that the repeated consumption of an SSB may increase the valuation of its logo and logos representing a similar beverage, theoretically increasing the risk for consumption akin to the incentive sensitization theory (18). Last, these neuroimaging results were bolstered by increased behavioral disinhibition toward the assigned logo and an explicit hedonic devaluation of the nonassigned beverage that theoretically may serve to prime individuals to perpetuate the intake of the previously consumed beverage in a habit-like pattern.
The dorsal striatum and vmPFC exhibit activity involved in hedonic valuation (8, 32) and goal-directed decision making (33–35). As such, the decreases in frontostriatal brain response observed herein may reflect patterns observed with a change in value-based decision making, specifically indicating a shift from a goal-directed (model-based) system toward a habit-based (model-free) system (33–35). In the habitual system, behaviors seek to repeat actions that were previously rewarding and are more efficient; however, these behaviors are less sensitive to reinforcement resulting directly from the action (36, 37). The data and interpretations herein are consistent with preclinical experiments that have reported that the repeated intake of palatable food decreased striatal responsiveness, and this decrease was associated with insensitivity to negative reinforcement during food intake, i.e., compulsive eating (38). In humans, habit-based decision making has been implicated in compulsive repetitive actions, including binge eating (39) and addiction (40). It has also been suggested to underlie impulsivity (41). The reduction in vmPFC response during the presentation of the consumed beverage logo herein correlated with faster behavioral responses toward that logo. It is important to note that no changes in the error rates of the behavioral logo task were observed, indicating that individuals were not displaying less cognitive control toward the logos. The neurobehavioral adaptations seen herein could mean that individuals are shifting toward habit-based processing of the assigned stimuli, e.g., more efficient behavioral action, which given the assigned daily consumption nature of the study, is not such a remarkable notion. However, the degree to which these processes occur in individuals, the characteristics of stimuli, and number of exposures needed to create neuroadaptations that create habits are highly relevant to obesity prevention. This report represents the first step to my knowledge in fully understanding these processes.
Because the hypothesis of a specified increased response to an assigned compared with nonassigned beverage logo was not supported, a post hoc examination was performed of both juice stimuli collapsed compared with tasteless solution at baseline and follow-up to test for more general effects of juice consumption. The precuneus, which is located in the parietal lobe, has been shown to increase during exposure to familiar relative to nonfamiliar brands and logos (26, 42, 43), which is in line with the notion that this region most commonly responds to emotionally salient stimuli independent of valance (44). Burger and Stice (22) observed a similar response pattern, in which the reduced striatal response to palatable food receipt was specified to the consumption of that food, when overeating, in general, was associated with an increased precuneus response to cue-elicited anticipation of palatable food receipt (25). Collectively, these reports and the results reported herein suggest that, in healthy-weight individuals, the reduced striatal response to food intake may be very specified to the foods repeatedly consumed, whereas increases to the precuneus response to anticipatory stimuli may be more generalized.
The observed alterations in brain response and behavioral disinhibition were coupled with an explicit hedonic devaluation of the nonconsumed beverage. The hedonic devaluation of a nonconsumed but otherwise similar beverage has also been demonstrated in preclinical studies in which repeatedly consumed rewards were preferred over nonconsumed rewards (45). The hedonic targeting of a consumed reward by the explicit devaluation of an alternate, similar reward may be implicated in habitual behavior by creating specificity to promote repeated actions. Moreover, consistent with previous experiments (20, 21), no significant increase in explicit hedonic ratings of the assigned beverage as a function of repeated consumption was observed. Together, the increase in behavioral disinhibition toward the consumed beverage without an explicit change in hedonic valuation of that beverage may reflect a dissociation between wanting and liking of a repeatedly consumed reward, supporting previous experiments (20, 21) and the obesity theory (18).
Theorists have linked the neurobiology of obesity to drug addiction (46, 47), and several similarities exist. For example, the frontostriatal and behavioral adaptions seen herein are similar to those seen in addiction (46, 48), and habit-based decision making is thought to be a characteristic of addiction (40). However, this investigation does not address many features of this disorder, most notably ad libitum intake, escalation, or withdrawal; thus, it is inappropriate to draw conclusions regarding the addictive potential of SSBs from this study alone. Furthermore, because this study was limited by sample size and the absence of a pure (nonsugar-beverage) control group, it is viable that the neurobehavioral adaptions seen herein are evident with regular practice of any behavior that elicits a pleasurable response. As such, investigating whether the sugar content or flavor differentially affect these processes relative to a pure control group is warranted. Last, the design used in this study could not differentiate 2 key aspects of sugar, i.e., sweetness or nutrient content. Previous reports have indicated distinctive roles of sweet taste and nutritive value on intake response. In support of these reports, several studies have demonstrated differential brain response to nutritive and nonnutritive sweetener consumption in regions implicated in reward and taste processing, typically showing that responses to nonnutritive sweeteners show decreased engagement of these brain regions (49–51). Given the relatively high prevalence of nonnutritive sweetener consumption predominantly via diet soft drinks (52) and the lack of clear evidence of the impact of nonnutritive sweetener consumption on energy intake and weight regulation (53), a direct investigation of the relative impact of repeated consumption of sweet taste compared with nutrient content is warranted. These study limitations do not detract from the notion that the adaptive processes seen herein are a function of repeated SSB consumption and are largely supported by the aforementioned preclinical and human studies.
In conclusion, the repeated intake of an SSB beverage resulted in specified decreases in response in frontostriatal brain regions coupled with increased behavioral disinhibition toward the consumed beverage logo. The precuneus response to high-sugar beverage logos also generally increased after consumption. Last, a distinct and explicit hedonic devaluation of a similar nonconsumed beverage was observed. These data emerged in an experimental free-living setting and without changes in body mass, providing vital information regarding the temporal precedence of aberrant neurobehavioral response patterns seen in obesity and establishing previously undetected consequences of the daily consumption of branded high-sugar beverages. These findings are supported by previous reports (16, 20–23, 33–35, 38, 39) and in line with predominant food-reward obesity theories (12–14, 18). Collectively, these preliminary data indicate that neurobehavioral adaptations can arise from the repeated intake of a highly rewarding beverage, which may reflect the initial unfolding of patterns previously associated with habitual overeating and obesity. Although previous studies have identified similar response patterns as risk factors for increased consumption, future research in larger samples is required to establish whether the neurobehavioral adaptations seen herein serve to increase the risk to maintain or perpetuate consumption.
Acknowledgments
I thank Jenny Gilbert, Pranish Kantak, and Grace Shearrer of the Neuropsychology of Ingestive Behavior Laboratory (NIBL) and Weili Lin and Amber Leinwand of Biomedical Research Imaging Center (BRIC) at UNC-CH for their assistance in data collection.
The sole author had responsibility for all parts of the manuscript and reported no conflicts of interest.
REFERENCES
- 1.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004;292:927–34. [DOI] [PubMed] [Google Scholar]
- 2.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet 2001;357:505–8. [DOI] [PubMed] [Google Scholar]
- 3.DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes Relat Metab Disord 2000;24:794–800. [DOI] [PubMed] [Google Scholar]
- 4.Bleich SN, Wang YC, Wang Y, Gortmaker SL. Increasing consumption of sugar-sweetened beverages among US adults: 1988–1994 to 1999–2004. Am J Clin Nutr 2009;89:372–381. [DOI] [PubMed] [Google Scholar]
- 5.Kit BK, Fakhouri THI, Park S, Nielsen SJ, Ogden CL. Trends in sugar-sweetened beverage consumption among youth and adults in the United States: 1999–2010. Am J Clin Nutr 2013;98:180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang YC, Coxson P, Shen YM, Goldman L, Bibbins-Domingo K. A penny-per-ounce tax on sugar-sweetened beverages would cut health and cost burdens of diabetes. Health Aff (Millwood) 2012;31:199–207. [DOI] [PubMed] [Google Scholar]
- 7.Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PLoS One 2007;2:e698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate from pleasure to aversion. Brain 2001;124:1720–33. [DOI] [PubMed] [Google Scholar]
- 9.Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors. Ann N Y Acad Sci 2008;1129:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci 2006;7:464–76. [DOI] [PubMed] [Google Scholar]
- 11.Lutter M, Nestler EJ. Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr 2009;139:629–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang G-J, Volkow ND, Fowler JS. The role of dopamine in motivation for food in humans: implications for obesity. Expert Opin Ther Targets 2002;6:601–9. [DOI] [PubMed] [Google Scholar]
- 13.Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr Psychiatry 2007;48:57–61. [DOI] [PubMed] [Google Scholar]
- 14.Volkow ND, Wang G, Telang F, Fowler JS, Thanos PK, Logan J, Alexoff D, Ding Y, Wong C, Ma Y, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage 2008;42:1537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol 2008;117:924–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci 2010;30:13105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage 2010;52:1696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berridge KC, Ho C-Y, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res 2010;1350:43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothemund Y, Preuschhof C, Bohner G, Bauknecht H-C, Klingebiel R, Flor H, Klapp BF. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage 2007;37:410–21. [DOI] [PubMed] [Google Scholar]
- 20.Temple JL, Bulkley AM, Badawy RL, Krause N, McCann S, Epstein LH. Differential effects of daily snack food intake on the reinforcing value of food in obese and nonobese women. Am J Clin Nutr 2009;90:304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark EN, Dewey AM, Temple JL. Effects of daily snack food intake on food reinforcement depend on body mass index and energy density. Am J Clin Nutr 2010;91:300–8. [DOI] [PubMed] [Google Scholar]
- 22.Burger KS, Stice E. Frequent ice cream consumption is associated with reduced striatal response to receipt of an ice cream-based milkshake. Am J Clin Nutr 2012;95:810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green E, Murphy C. Altered processing of sweet taste in the brain of diet soda drinkers. Physiol Behav 2012;107:560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudenga KJ, Small DM. Amygdala response to sucrose consumption is inversely related to artificial sweetener use. Appetite 2012;58:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burger KS, Stice E. Elevated energy intake is correlated with hyperresponsivity in attentional, gustatory, and reward brain regions while anticipating palatable food receipt. Am J Clin Nutr 2013;97:1188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burger KS, Stice E. Neural responsivity during soft drink intake, anticipation, and advertisement exposure in habitually consuming youth. Obesity (Silver Spring) 2014;22:441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ridley-Siegert TL, Crombag HS, Yeomans MR. Whether or not to eat: a controlled laboratory study of discriminative cueing effects on food intake in humans. Physiol Behav 2015;152:347–53. [DOI] [PubMed] [Google Scholar]
- 28.Poldrack RA, Mumford JA, Nichols TE. Handbook of functional MRI data analysis. Cambridge (United Kingdom): Cambridge University Press; 2011. [Google Scholar]
- 29.Cohen J. Statistical power analysis for the behavioral sciences. New York: Routledge; 1988. [Google Scholar]
- 30.Lim J, Wood A, Green BG. Derivation and evaluation of a labeled hedonic scale. Chem Senses 2009;34:739–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whelan R. Effective analysis of reaction time data. Psychol Rec 2010;58:9. [Google Scholar]
- 32.Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage 2003;19:1709–15. [DOI] [PubMed] [Google Scholar]
- 33.Daw ND, O’Doherty JP, Dayan P, Seymour B, Dolan RJ. Cortical substrates for exploratory decisions in humans. Nature 2006;441:876–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jocham G, Klein TA, Ullsperger M. Dopamine-mediated reinforcement learning signals in the striatum and ventromedial prefrontal cortex underlie value-based choices. J Neurosci 2011;31:1606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Wit S, Corlett PR, Aitken MR, Dickinson A, Fletcher PC. Differential engagement of the ventromedial prefrontal cortex by goal-directed and habitual behavior toward food pictures in humans. J Neurosci 2009;29:11330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci 2008;9:545–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daw ND, O’Doherty JP. Multiple systems for value learning. In Glimcher PW, Fehr E, editors. Neuroeconomics: decision making and the brain. 2nd ed Amsterdam (Netherlands): Elsevier; 2013. p. 393–410. [Google Scholar]
- 38.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci 2010;13:635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voon V, Derbyshire K, Rück C, Irvine MA, Worbe Y, Enander J, Schreiber LRN, Gillan C, Fineberg NA, Sahakian BJ, et al. Disorders of compulsivity: a common bias towards learning habits. Mol Psychiatry 2015;20:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Everitt BJ, Robbins TW. Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychology 2016;67:23–50. [DOI] [PubMed] [Google Scholar]
- 41.Hogarth L, Chase HW, Baess K. Impaired goal-directed behavioural control in human impulsivity. Q J Exp Psychol (Hove); 2012;65:305–16. [DOI] [PMC free article] [PubMed]
- 42.Stoll M, Baecke S, Kenning P. What they see is what they get? An fMRI-study on neural correlates of attractive packaging. J Consum Behav 2008;7:342–59. [Google Scholar]
- 43.Schaefer M, Rotte M. Thinking on luxury or pragmatic brand products: brain responses to different categories of culturally based brands. Brain Res 2007;1165:98–104. [DOI] [PubMed] [Google Scholar]
- 44.Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci 1999;22:310–6. [DOI] [PubMed] [Google Scholar]
- 45.Carelli RM, West EA. When a good taste turns bad: neural mechanisms underlying the emergence of negative affect and associated natural reward devaluation by cocaine. Neuropharmacology 2014;76:360–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci 2011;12:638–51. [DOI] [PubMed] [Google Scholar]
- 47.Volkow ND, Wang G-J, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc B-Biological Sci 2008;363:3191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feil J, Sheppard D, Fitzgerald PB, Yücel M, Lubman DI, Bradshaw JL. Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neurosci Biobehav Rev; 2010;35:248–75. [DOI] [PubMed]
- 49.McCutcheon JE, Beeler JA, Roitman MF. Sucrose‐predictive cues evoke greater phasic dopamine release than saccharin‐predictive cues. Synapse 2012;66:346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frank GKW, Oberndorfer TA, Simmons AN, Paulus MP, Fudge JL, Yang TT, Kaye WH. Sucrose activates human taste pathways differently from artificial sweetener. Neuroimage 2008;39:1559–69. [DOI] [PubMed] [Google Scholar]
- 51.Haase L, Cerf-Ducastel B, Murphy C. Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. Neuroimage 2009;44:1008–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sylvetsky AC, Welsh JA, Brown RJ, Vos MB. Low-calorie sweetener consumption is increasing in the United States. Am J Clin Nutr 2012;96:640–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr 2009;89:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]