Abstract
Background: Observational studies suggest an inverse association between whole-grain (WG) consumption and inflammation. However, evidence from interventional studies is limited, and few studies have included measurements of cell-mediated immunity.
Objective: We assessed the effects of diets rich in WGs compared with refined grains (RGs) on immune and inflammatory responses, gut microbiota, and microbial products in healthy adults while maintaining subject body weights.
Design: After a 2-wk provided-food run-in period of consuming a Western-style diet, 49 men and 32 postmenopausal women [age range: 40–65 y, body mass index (in kg/m2) <35] were assigned to consume 1 of 2 provided-food weight-maintenance diets for 6 wk.
Results: Compared with the RG group, the WG group had increased plasma total alkyresorcinols (a measure of WG intake) (P < 0.0001), stool weight (P < 0.0001), stool frequency (P = 0.02), and short-chain fatty acid (SCFA) producer Lachnospira [false-discovery rate (FDR)-corrected P = 0.25] but decreased pro-inflammatory Enterobacteriaceae (FDR-corrected P = 0.25). Changes in stool acetate (P = 0.02) and total SCFAs (P = 0.05) were higher in the WG group than in the RG group. A positive association was shown between Lachnospira and acetate (FDR-corrected P = 0.002) or butyrate (FDR-corrected P = 0.005). We also showed that there was a higher percentage of terminal effector memory T cells (P = 0.03) and LPS-stimulated ex vivo production of tumor necrosis factor-α (P = 0.04) in the WG group than in the RG group, which were positively associated with plasma alkylresorcinol concentrations.
Conclusion: The short-term consumption of WGs in a weight-maintenance diet increases stool weight and frequency and has modest positive effects on gut microbiota, SCFAs, effector memory T cells, and the acute innate immune response and no effect on other markers of cell-mediated immunity or systemic and gut inflammation. This trial was registered at clinicaltrials.gov as NCT01902394.
Keywords: gut microbiota, healthy adults, immune, inflammation, whole grains
See corresponding editorial on page 545.
INTRODUCTION
Previous studies have shown that a dysregulated, prolonged overproduction of inflammatory cytokines is associated with cardiovascular diseases, type 2 diabetes, and certain cancers (1–3). Some observational studies have suggested that diets that are rich in whole grains (WGs)13 are inversely associated with these inflammation-related diseases and all-cause mortality (4–9). These proposed benefits of consuming WGs may relate to the fact that WGs are a rich source of vitamins, minerals, antioxidants, dietary fiber, lignans, β-glucans, inulin, phytochemicals, phytosterols, phytin, and sphingolipids (10). However, these beneficial WG constituents are substantially lost during processing to make refined-grain (RG) flour including losses in ferulic acid (93%); selenium (92%); antioxidant activity (89%); phenolic compounds and magnesium (83%); flavonoids, zinc, and vitamin E (79%); zeaxanthin (78%); fiber (58%); and lutein (51%) (11, 12).
WG consumption is also known to be associated with an increase in the healthy gut microbiota phenotype, as indicated by their richness and diversity (13–15), and in short-chain fatty acid (SCFA) production. Both gut microbiota and SCFAs are considered important for immune function and gut health (16, 17). Emerging in vivo evidence has suggested that immunologic dysregulation may result from a dysbiosis of the gut microbiota (18–20). The relations between WGs and microbiota, chronic disease and microbiota, and the corresponding immune and inflammatory indexes indicate that WG has a potential to favorably alter microbiota and to regulate immune and inflammatory responses (21, 22).
Several observational studies have shown an inverse association between diets that are high in WGs and a decrease in C-reactive protein (CRP) and the soluble TNF-α receptor TNF-R2 in diabetic women (23) and a decrease in CRP in healthy adults (24–26), but discrepancy exists (27, 28). The limited interventional trials have reported inconsistent findings of a decrease in inflammatory markers IL-8 (29), IL-6 (30), and TNF-α (31) or no change in IL-6 and CRP (32–35). In addition, none of the studies evaluated cell-mediated immune responses, thereby limiting the ability to assess the overall impact of WG on immune and inflammatory responses. Several factors may explain these discrepancies such as differences in participant BMI ranges, sedentary lifestyles, and low WG habitual intake. Furthermore, none of the studies completely controlled the diet of participants. This is an important factor because day-to-day variations in dietary components can potentially affect inflammation, thereby making it difficult to identify the true effect of WGs. To this end, in the current study, we controlled the diet by providing all meals to participants in both groups, which served to minimize the interference from the variation in dietary intake. Because weight loss could affect both immune and inflammatory markers, the diets that were used in our study were designed to maintain participant weight throughout the intervention period.
Thus, the objective of this 6-wk randomized controlled trial was to assess the effect of WGs compared with RGs within the context of a weight-maintenance diet on markers of systemic inflammation, phenotype and functional aspects of the immune system, gut microbiota and SCFAs, as well as stool weight, water content, pH, and frequency of bowel movements in healthy adults with BMI (in kg/m2) that ranged from normal to obese.
METHODS
Study design, recruitment, and enrollment
This randomized, controlled, parallel-design human trial was conducted between May 2012 and September 2014 after being approved by the Institutional Review Board at Tufts Medical Center and Tufts University Health Sciences Campus (Institutional Review Board 10110) and was registered at clinicaltrials.gov as NCT01902394. Participants were recruited from the Jean Mayer USDA Human Nutrition Research Center on Aging (HNRCA) at Tufts University Volunteer Recruitment Services database and with advertisements posted in Tufts Medical Center clinics, local newspapers, bulletin boards, media sources, and via the HNRCA website. Interested individuals were first prescreened via an online or telephone questionnaire. Qualified individuals were invited to an onsite full-screening visit that was conducted by experienced research study staff at the Metabolic Research Unit at the HNRCA. The full screening included a health history and dietary habit interviews, a fiber-screening questionnaire, and standard blood tests. Participants were deemed eligible according to the following these criteria: men and women aged 40–65 y (women must have been >1 y postmenopausal or had both ovaries removed if premenopausal); BMI from 20 to 35; creatinine concentration ≤1.5 mg/dL; serum glutamic oxaloacetic transaminase, glutamic pyruvic transaminase, and total bilirubin ≤2 times the upper limit of the normal range; fasting glucose concentration <125 mg/dL; hematocrit ≥32%; white blood cell count ≥1.8 × 103/mm3; platelet count ≥100 × 103/mm3; consent to be randomly assigned; and a report of the consumption of a low-fiber diet (men: <7 g/1000 kcal; women: <8 g/1000 kcal) for ≥2 wk before enrollment. Participants with higher fiber consumption were invited to participate if they were willing to reduce habitual daily fiber intake ≤2 wk before enrollment. In addition, participants were included if they were willing to consume only the study foods provided.
Additional exclusion criteria were based on participant weight change, dietary commitment, supplement use, use of certain medications, and health conditions. Participants were excluded if they had a self-reported weight change >4 kg within the past 6-mo or if they were participating in a weight-loss program. Dietary exclusions included a vegetarian diet and not willing to stop taking probiotics, multivitamins, and supplements including fish oil or n–3 fatty acids and herbal supplements for 30 d before and during study participation. Calcium and vitamin D supplements were allowed. In addition to these criteria, participants were excluded if they consumed >2 drinks alcohol/d or were not willing to abstain from drinking alcohol during the study and if they had food allergies or other issues with foods that would preclude intake of study diets. The medication exclusion criteria included the use of antibiotics within the past 3 mo, the use of nonsteroidal anti-inflammatory medications or antihistamines, or the inability to discontinue the use of these substances for 72 h before the first-day blood draw until 48 h after receipt of a delayed-type hypersensitivity (DTH) skin-test implant, and the use of immunosuppressive drugs. Exclusions for health conditions included a diagnosis of autoimmune diseases, active cancer or history of cancer within the past 5 y except for nonmelanoma skin cancer, uncontrolled major illnesses such as cardiovascular disease, and a history of inflammatory bowel disease or gastrointestinal disorders. All participants gave written informed consent before participating in the study and received a stipend.
Sample-size calculations and random assignment
Sample-size calculations were based on available data for DTH (36, 37), key inflammatory cytokines (IL-6 and TNF-α) (38), selected gut microbiota (Bifidobacteria and Lactobacillus) (38), and natural killer (NK) cell cytotoxicity (38). The largest sample size needed was n = 37/group to detect a significant difference at P < 0.05 for DTH with 80% power. This sample size was increased to n = 40/group to account for a 10% dropout rate on the basis of studies at the center in this age group. Participants were randomly assigned to the WG or RG group with the use of block random assignment with stratification by BMI (20–25, 25–30, and 30–35), age (40–55 and 55–65 y), sex, and race (Caucasian, African American, Asian American, Hispanic, and other). The statistician, who had no contact with participants and had no role in the data collection, assigned the random-assignment coding for the WG and RG groups.
Study diets
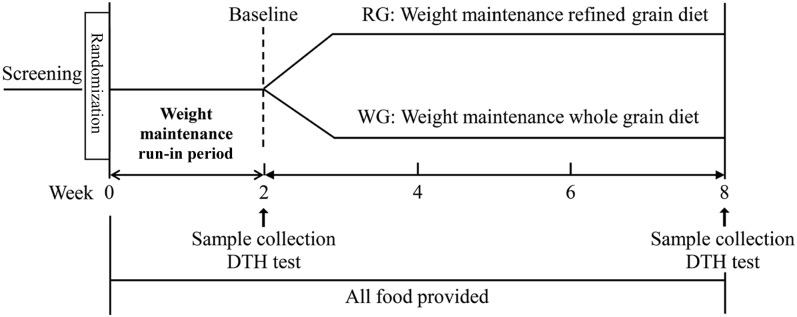
All randomly assigned participants underwent a 2-wk run-in phase in which they were provided with a Western-style diet (high in saturated fats, red meats, simple carbohydrates, and processed or refined foods and low in fresh fruit and vegetables, WGs, seafood, and poultry). The purpose of the run-in period was to minimize the effect of habitual diet intake before starting the experimental diets. Total daily caloric intake of each participant was initially calculated with the use of the Harris-Benedict formula and was adjusted for physical activity when necessary to maintain the current body weights of subjects. Participants were instructed to maintain their current physical activity levels throughout the study. All meals were based on the USDA Dietary Guidelines 2010, which recommends that 50–55% of energy is derived from carbohydrates, 15–20% of energy is derived from protein, and 25–30% of energy is derived from fat (39). After completion of the run-in phase, participants were assigned to the following experimental diets: an RG diet (8 g/1000 kcal) and a WG diet (16 g/1000 kcal), respectively (Figure 1). The targeted fiber intake that was provided by the WG diet met the recommended Dietary Guidelines for Americans (35 g/d), whereas the fiber intake from the RG diet was slightly above the average intake in adults.
FIGURE 1.
Schematic outline of the study protocol. DTH, delayed-type hypersensitivity; RG, refined grain; WG, whole grain.
The diets were similar in composition with the exception of the source of grain. The WG group received all grains from WG sources, and the RG group received all grains from RG-containing foods. Otherwise, the diets were matched for servings of fruit, vegetables, and protein (e.g., turkey meatloaf with 100% whole-wheat bread crumbs with mixed vegetables or turkey meatloaf with 100% white-bread crumbs with mixed vegetables). Six 240-mL glasses of water or calorie-free drinks were recommended daily. The study dietitian developed 3-d menu cycles at 3 caloric levels (2000, 2500, and 3000 kcal) that used commonly available ingredients and food items. Calories were adjusted (plus or minus) from these calorie amounts on the basis of participants’ weight fluctuations.
Food was prepared with the use of standardized recipes by trained staff at the Metabolic Research Unit of the HNRCA and was picked up by participants 3 times/wk. Meals were packaged with reheating instructions and a food checklist. To maintain body weight within ±1 kg of baseline weight, body weight was measured 2–3 times/wk and calorie amounts were adjusted if necessary. Participants were asked to complete the food checklist, whereby they indicated the foods that were not consumed each day, and to return the completed checklist on a weekly basis. Subjects were also requested to report the consumption of any additional food that was not provided by the study. Although we discouraged the consumption of additional food, we tried to accommodate the preferences of subjects. If they were unhappy with a meal and would check off that they did not eat that item, our dietitian would ask them what other food on the menu they would like to eat more of and would try to incorporate that food into the future menu with adjustment of all nutrients to maintain the study requirements. Actual intake of foods was determined by subtracting the amounts of foods that were not consumed, as indicated on the food checklist, from the amounts of foods that were provided to participants plus the additional foods that were consumed. Participants’ actual intakes were analyzed with the use of the Nutrition Data System for Research software (version 2011; Nutrition Coordinating Center, University of Minnesota). Participants were also asked to record the presence and severity of 6 gastrointestinal symptoms on a weekly basis. Compliance was assessed via the measurement of plasma alkylresorcinol homologs (19:0, 21:0, and 23:0), which are WG biomarkers that are present mainly in the bran of WGs (40).
Sample collection for experimental analysis
Stool, 12-h fasting blood, and saliva samples were collected, and DTH tests were conducted at baseline (at or near the end of week 2 of the run-in period) and again at the end of dietary intervention phase (at or near the end of week 8 of the study). Participants were asked to stop the use of any anti-inflammatory medicines, including aspirin and antihistamines, for 72 h before blood collection and immunological testing.
Collection and processing of stool samples
Participants were provided with kits and instructions for stool collection, storage, and delivery to the HNRCA. Stool samples were collected in specimen containers and placed into plastic bags, surrounded by frozen gel packs, and delivered to the center in coolers. Participants were instructed to collect all stool samples daily during a 72-h period, record the date and time of collection, and deliver all stool samples within 24 h of production. The number of bowel movements and weights of samples were recorded by the study staff. From samples that were delivered within 24 h after production, 3 aliquots of 4 g were collected, and 2 aliquots were immediately stored at −80°C in polypropylene conical tubes for later analysis of SCFAs, cytokines, and IgA. The third aliquot was homogenized by vigorous stirring with a sterile spatula, and 5 × 200-mg aliquots were placed in sterile tubes (Eppendorf) and stored at −80°C for the later analysis of the microbiota composition. All stool samples that were collected within a 72-h window were pooled and used to determine aerobic and anaerobic bacteria, pH, and water contents. Stool anaerobic and aerobic counts were determined by taking two 0.5-g stool samples, adding each sample to 4.5 mL phosphate-buffered saline, and mixing vigorously. Each suspension was sequentially diluted to 1:10 to achieve dilutions between 1010 and 1012. Samples were plated onto petri dishes that contained anaerobic blood agar and were incubated for 48 h at 37°C in an anaerobic chamber or in a warm room under atmospheric conditions for anaerobic and aerobic bacteria cultures, respectively. CFUs on anaerobic and aerobic plates were counted, and the results were expressed as CFUs per gram of stool. The stool water content was determined by drying 2 g stool sample in an oven for 12–16 h at 140°C. The fecal water weight was determined by the difference between the predrying weight and postdrying weight. The percentage of stool water content was calculated as the result of fecal water being divided by the predrying weight and multiplied by 100. To measure the stool pH, 1 g stool was added to 1 mL distilled water, mixed thoroughly by vortex, and measured with a pH meter.
Saliva, blood, and plasma collection
Drinking straws were cut into 2-in pieces. Participants placed one end of the straw into the mouth while placing the other end into polypropylene vials. The allowance of saliva to pool in the mouth and tilting the head forward caused the saliva to travel from the mouth to the storage vials. Participants were asked to fill vials to a designated line, and the duration of the process was recorded as flow rates, which affected IgA concentrations (41). After collection, samples were stored at −80°C for a later analysis.
To account for day-to-day variations in ex vivo immunologic function assays, venous blood was collected into either heparin-treated (for culture) or EDTA-coated (for hematology and plasma isolation) tubes on 2 consecutive days after a 12-h fast.
Compositional analysis of fecal microbiota by high-throughput sequencing
DNA extraction from stool was done with the use of the QIAamp DNA Stool Mini kit (Qiagen) with the following modifications: Each 200 mg stool sample was thawed in a Qiagen stool extraction buffer (Qiagen), containing 50 μg lysozyme and 13.5 U lysostaphin (Sigma Corp.), that was resuspended by stirring the solution with a pipette tip, which was transferred to a tube that contained 500 mg 0.1-mm zirconium and silica beads (Biospec Products). The tubes were placed on a bead-beating adapter on a Vortex Genie (both from MoBio) for 5 min of bead beating at 4°C. The tubes were heated to 37°C for 10 min. After the addition of 200 μg proteinase K (Qiagen) and 20 μg RNase A (Sigma-Aldrich), the tubes were heated to 70°C for 10 min, which was followed by an additional 5 min of bead beating. Tubes were centrifuged, and the supernatant fluid was added to a tube that contained an InhibitEx tablet from the Qiagen kit (Qiagen). The remaining procedure was conducted according to the manufacturer’s protocol. Stool extractions were carried out in batches of n = 15 with a no-stool extraction control included in each batch to detect any potential cross-contamination.
Amplicons of the V4 region of the bacterial 16S ribosomal DNA were generated with primers according to Caporaso et al. (42). Each amplicon reaction was carried out in triplicate with a template control; the success of the reaction (band with template; no band with a negative control) was confirmed by running the reaction products on an agarose gel. DNA concentrations of all successful amplicon reactions were measured with the use of the Quant-iT ds DNA Assay Kit, High Sensitivity (Invitrogen). Amplicons were pooled in equimolar amounts, which was followed by the purification of the pool on Angencourt AMpure XP beads (Beckman Coulter) according to the manufacturer’s protocol. Pools were submitted to the Tufts University Core Facility for 250-bp paired-end sequencing on a MiSeq sequencer (Illumina).
Marker-gene sequencing data were processed with the use of the Quantitative Insights Into Microbial Ecology package (43). Reads (results of nucleotide sequences) were clustered at 97% identity and assigned taxonomy by open reference with the use of the Greengenes reference database (greengenes.lbl.gov) (44) and USEARCH program v6.1 (drive5.com) (45). Sequences from all samples were subsampled at a sample-sequencing depth of 19,490 sequences to control for the differential sequencing effort. Within-sample biodiversity was measured with the use of whole-tree phylogenetic diversity. To quantify differences in microbial compositions between participants over time, weighted and unweighted UniFrac (a distance metric used for comparing biological communities) (46) distances between all pairs of samples was calculated.
Stool SCFAs
Stool SCFAs were determined on the basis of the method described by Tomcik et al. (47) with a slight modifications. Undiluted stool samples were removed from storage at −80°C and freeze dried for 5–7 d. Stool samples were ground to a fine powder with the use of a mortar and pestle. Because the extraction yield of SCFAs can be increased by decreasing the stool homogenate pH (48), each stool sample (0.1 g) was suspended in 1% phosphoric acid and homogenized by shaking for 30 min, which was followed by centrifugation for 15 min at 7000 × g at 4°C. The liquid fraction was decanted and filtered through a 0.2-μm polysulfone membrane (Sigma-Aldrich) and stored at −80°C for later extraction. Fecal samples were spiked with internal standards actetate-D4 and butyrate-D7 before being extracted by ethyl acetate; samples were derivatized with 200 μL of 100 mmol pentaflourobenzyl bromide acetone solution/L with 100 μL trimethylamine (both from Sigma-Aldrich) as the catalyst (49) for 1 h at 60°C. SCFAs were analyzed with the use of gas chromatography–mass spectrometry. A multipoint standard curve was established for quantitative analyses of acetate, propionate, and butyrate, respectively. Known SCFA concentrations were linearly regressed to the ratio of the SCFA:inflammatory score (IS) AUC. Concentrations were quantified with the use of the ratio of each analyte to the internal standard in the equation from the standard curve.
Stool cytokines
Stool cytokines were analyzed as previously described (50) with a slight modification. Briefly, stool samples were stored at −80°C, were freeze dried for 5–7 d, and ground to a fine powder with the use of a mortar and pestle. Stools were diluted in phosphate-buffered saline at a 1:4 ratio and centrifuged at 20,000 × g. Samples were filtered through centrifuge tube filters (Sigma-Aldrich) to remove particles; filtered samples were used to quantify stool cytokine and IgA. Stool cytokines [interferon γ (IFN-γ), TNF-α, IL-6, IL-7, and transforming growth factor β] were measured with the use of a high-sensitivity ELISA, and stool IgA was measured with the use of a regular ELISA (eBioscience) according to the manufacturer’s instructions.
Measurement of DTH response
The DTH skin test was conducted as previously described by Hamer et al. (7). The 3 recall skin antigens Tetanus toxoid (Tetanus toxoid USP; Aventis Pasteur), Candida albians (Candin; Allermed Laboratories), and Trichophyton species (Trichophyton mentagrophytes in conjunction with Trichophyton rubrum; Hollister-Stier Laboratories) and a negative control (0.9% normal saline) (Bound Tree Medical) were injected intradermally at separate sites on the volar surface of the forearm. Induration of the skin response was read 24 and 48 h later, and a positive reaction was defined as a mean ≥5 mm. The number of positive responses and a composite score of the responses to all antigens were recorded.
Complete blood count and differential count
Whole blood that was collected in EDTA-coated tubes was used to analyze complete blood counts and differential counts with the use of an automated hematology analyzer (ABX Penta 60+ ABX Diagnostics) according to the manufacturer’s instructions. Samples were analyzed in duplicate by the Nutrition Evaluation Laboratory at the HNRCA in the “complete blood count + 5 population differential count” mode. The mean was calculated and used for the statistical analysis.
Lymphocyte subsets in peripheral blood
To determine the lymphocyte phenotype, whole blood surface staining for different white blood cell markers was conducted with the use of fluorescent-conjugated antibodies according to previously published methods (51). A 3- or 4-color flow cytometry analysis was performed for a panel that consisted of anti-CD3 (total T cells), CD4 (helper T cells), and CD8 (cytotoxic T cells) as well as their naive and memory subpopulations (which were determined by corresponding patterns in the expression of CD45RA, CD45RO, and CD62L), CD19 (B cells), CD16 and CD56 (NK cells), and CD4 and CD25 (regulatory T cells). Antibodies and their corresponding isotype controls were added according to the manufacturer’s protocol. All samples were run on an Accuri C6 flow cytometer (BD Biosciences), and acquired data were analyzed with the use of Flowjo version 10 software (TreeStar).
Plasma alkylresorcinols, cytokines, and LPS-binding protein
To assess adherence to the dietary regimen, plasma concentrations of the individual alkylresorcinol homologs 19:0, 21:0, and 23:0 were measured with the use of gas chromatography–mass spectrometry as previously described with minor modifications (52). The alkylresorcinol quantification was calculated by introducing a multipoint standard curve for each alkylresorcinol (19:0, 21:0, and 23:0). Concentrations were calculated with the use of the ratio of each alkylresorcinol to the internal standard.
Plasma concentrations of TNF-α, IL-6, IL-8, IL-1β, and LPS-binding protein (LBP) were analyzed with the use of electrochemiluminescence assays (Meso Scale Discovery) according to the manufacturer’s instructions. The data acquired were analyzed with the use of the Meso Scale Discovery Workbench 3.0 Data Analysis Toolbox (Meso Scale Discovery). For a few samples, because their IL-1β concentrations were below the detection limit, we set 50% of the detection limit as their estimated values (53). Samples were analyzed in triplicates, and the means were used for the statistical analysis. An overall IS was computed from plasma concentrations of TNF-α, IL-6, IL-8, and IL-1β as previously described (54). Briefly, individual cytokines were ranked, standardized as z scores, and summed to compute a score (IS) that was representative of the overall inflammation.
Lymphocyte proliferation
The ability of lymphocytes to proliferate was assessed by quantifying the incorporation of [3H]-thymidine after stimulation with phytohemagglutinin, concanavalin A (Con A), or antibodies against CD3 (T cell receptor) and CD28 (T cell co-receptor) (anti-CD3 and anti-CD28) with the use of a modified whole blood culture protocol (55). Venous blood that was collected in 2 consecutive days was diluted to a final 1:10 ratio [volume:volume (vol:vol)] with Roswell Park Memorial Institute (RPMI) 1640 media (Sigma-Aldrich), which was supplemented with penicillin (100 U/mL), streptomycin (100 μg/mL), HEPES (25 mmol/L), and glutamine (2 mmol/L). This complete RPMI-1640 media was used in the whole study unless indicated otherwise. Diluted blood in round-bottom 96-well cell culture plates was incubated in triplicated wells per treatment of 72 h in the presence of phytohemagglutinin at 2, 5, or 50 μg/mL, Con A at 5, 25, or 50 μg/mL, or immobilized anti-CD3 (eBioscience) at 1, 5, or 10 μg/mL and soluble anti-CD28 at 1 μg/mL. The culture condition for cell function measurements in this study was an atmosphere of 37°C, 5% CO2, and 95% humidity unless indicated otherwise. Cultures were pulsed with 0.5 μCi [3H]-thymidine (Perkin Elmer) during the last 4 h of incubation. Cells were harvested onto glass-fiber mats (Wallac) with the use of a Perkin Elmer cell harvester (Perkin Elmer). Cell proliferation was quantified as the amount of [3H]-thymidine that was incorporated into the DNA, which was determined with the use of liquid-scintillation counting with a Micro Beta 2 MicroPlate counter (Perkin Elmer). Results are expressed as mean counts per minute that were averaged from both days of venous blood collection at baseline (week 2) and 6 wk postintervention (week 8).
Ex vivo cytokine production
The ability of immune cells to produce cytokines was assessed under dynamic conditions by stimulation with LPS or anti-CD3 and anti-CD28 with the use of a modified whole blood culture technique (56). Venous blood, which was collected on 2 consecutive days, was diluted to a final ratio of 1:5 (vol:vol) or 1:3.6 (vol:vol) with the RPMI-1640 media. To determine T cell cytokine IFN-γ, IL-2, and IL-4 production, diluted blood was stimulated with immobilized anti-CD3 (5 μg/mL) and soluble anti-CD28 (2 μg/mL) in round-bottom 96-well cell culture plates for 48 h. To measure the production of inflammatory cytokines IFN-γ, TNF-α, IL-1β, IL-6, and IL-8, diluted blood was stimulated with LPS (1 μg/mL) in flat-bottom 24-well cell culture plates for 24 h. Supernatant fluid was collected from these cultures for the cytokine analysis.
All cytokines, except IFN-γ, were measured, and data were analyzed with the use of Meso Scale Discovery system as described previously for plasma cytokine analysis. Because it was not possible to attain the dilution to measure IFN-γ along with the other cytokines, IFN-γ was measured with the use of a regular ELISA with agents from BD Biosciences and a plate reader (BioTek Instruments). In a few cases, IFN-γ, IL-10, IL-4, and IL-2 concentrations were below detection limits; therefore, one-half of the detection-limit values were recorded as estimated concentrations (57). Results from both days were averaged, and mean values were used for the statistical analysis.
Isolation of peripheral blood mononuclear cells and NK cell activity analysis
An NK cell activity assay was conducted with the use of a modified flow-cytometry method on the basis of a previously reported method (58). Briefly, peripheral blood mononuclear cells (PBMCs) were isolated with the use of Ficoll-Histopaque gradient centrifugation (Sigma-Aldrich) and suspended in RPMI-1640 and 5% fetal bovine serum at 2 × 106 cells/mL. PBMCs were incubated in the presence of allophycocyanin-conjugated anti-CD56 and phycoerythrin-conjugated anti-CD16 monoclonal antibodies (both from eBioscience) according to the manufacturer’s instructions. Human chronic myelogenous leukemia cells K562 (American Type Culture Collection CCL-243) were cultured in RPMI-1640 media and 10% fetal bovine serum. K562 cells, at 1 × 105 cells/mL, were incubated in the presence of 1 μM carboxyflourescein succinimidyl ester. Effector cells (PBMCs) were co-incubated with target cells (K562 cells) at effector:target ratios of 100:1, 50:1, 25:1, and 12.5:1. The spontaneous death of target cells was determined with the use of a culture without PBMCs. All samples were run in triplicate. After 3 h of co-incubation, 5 μL 7-aminoactinomycin D (eBioscience) was added to each tube to stain dead cells, which was incubated in the dark and on ice for an additional 10 min. Samples were analyzed on an Accuri C6 flow cytometer (BD Biosciences). The percentage of NK cells in PBMCs was also determined with the use of flow cytometry. The percentage of cytotoxic activity was calculated with the use of the following equation:
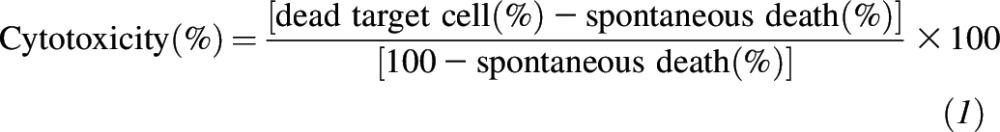 |
Salivary secretory IgA
Salivary secretory IgA was measured with the use of an indirect competitive immunoassay kit according to the manufacturer’s protocol (Salimetrics). Samples were read with the use of an ELISA plate reader, and output was collected and analyzed with the use of Gen5 software (BioTek Instruments). Samples were run in duplicate, and mean values were calculated for each sample.
Blood lipid profile
Blood lipid profile outcomes (cholesterol, triglycerides, HDL, LDL, and VLDL) were measured, and the full processing methods were described elsewhere (M Meydani, M Thomas, JB Barnett, SM Vanegas, O Chen, G Dolnikowski, S Jonnalagadda, E Saltzman, S Roberts, SN Meydani, unpublished data, April 2016). Briefly, cholesterol, triglyceride, and HDL were measured with the use of an enzymatic, calorimetric endpoint assay. LDL was calculated if the triglyceride concentration was <400 mg/dL, or LDL was measured directly if the triglyceride concentration was ≥400 mg/dL. VLDL was calculated with the use of Friedewald’s formula (59).
Statistical analysis
An analysis of the clinical variables was carried out with the use of SAS software (release 9.2; SAS Institute Inc.). Data are presented as means ± SEMs. Both the change from baseline to postintervention within each group (ΔRG or ΔWG) and the difference of this change between groups (ΔWG minus ΔRG) were reported. Normality tests were performed, and transformations were done when needed before the statistical analysis. The comparisons conducted include those between baseline and postintervention in each diet group, between the 2 diet groups at each time point, and between groups for the change from baseline to postintervention. A general linear model was used to analyze the data. Variables specified in the model were BMI, age, sex, and baseline values of dependent variables because of their influence on the microbiota and immune and inflammatory responses. A correlation analysis between outcomes that were significantly (P < 0.05) affected by the diet and alkylresorcinols was corrected for measures at baseline, BMI, age, and sex. The DTH response was the primary outcome, and other measures were secondary outcomes. Within a category (such as immune or fecal microbiota) and the same class (primary or secondary) of outcomes, multiple comparisons were taken into account for calculating the levels of significance (P values). Results were considered significant at P < 0.05 or as a trend if P < 0.1.
Differences in relative abundances of taxonomic groups that were summarized at the levels of both the phylum and genus were examined. Comparisons that were conducted included those between baseline and postintervention in each diet group, between the 2 diet groups at each time point, and between groups for the change from baseline to postintervention. These comparisons were conducted with the use of a linear model in the statistical package R (60) and controlling for age, BMI, and sex. P values were corrected for the total number of comparisons with the use of a false-discovery rate (FDR) correction, whereby significance was determined according to FDR-adjusted P values <0.25. We chose the FDR of 0.25 for these analyses because of the large number of tests.
α-Diversity values for each sample were described with the use of the whole-tree phylogenetic diversity variable. α-Diversity values were verified to be normally distributed with the use of the Shapiro-Wilks test, and within- and between-group analyses were carried out with the use of a paired t test.
Weighted and unweighted UniFrac measures of between-sample ecologic variation (β diversity) were used to test the hypothesis that participants were more similar to other subjects within the same treatment group. This test was performed with the use of the Adonis function in the vegan package in R software (60) similar to that performed in the permutational multivariate ANOVA analysis.
A statistical analysis of associations between the relative abundances of common taxa (those that were present in ≥10% of samples) and clinical metadata covariates of interest were performed with the use of a generalized linear regression in the statistical package R while controlling for age, BMI, and sex. The significance of associations was corrected for the total number of taxa comparisons with the use of a FDR correction. In addition, correlation tests (with the use of Spearman correlation) were conducted to control for outliers and were corrected for the total number of comparisons with the use of the FDR. Associations were identified as significant if they had FDR-adjusted P values <0.25 for both generalized linear regression and Spearman correlation results.
RESULTS
Recruitment, enrollment, and baseline comparisons
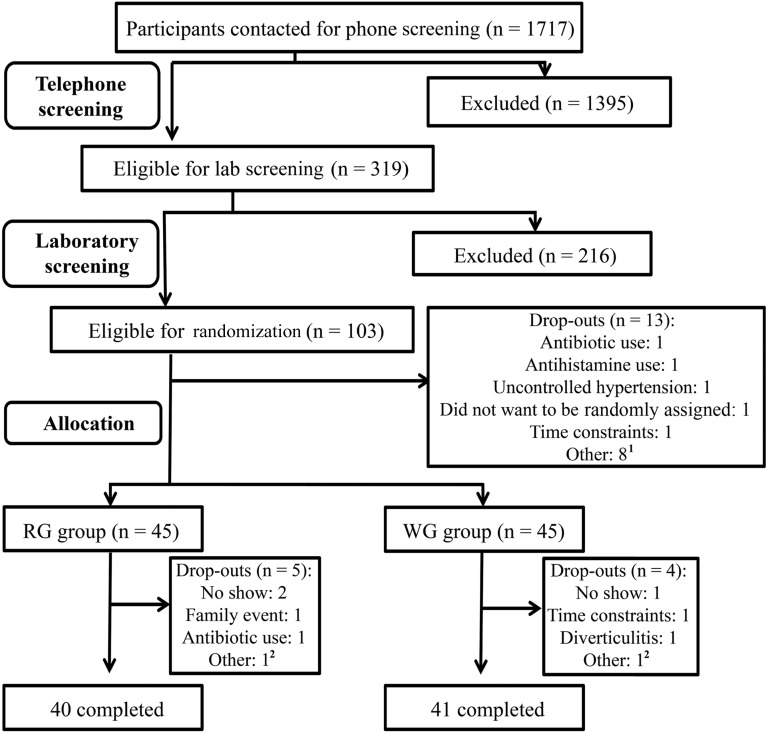
Initially, 1714 participants were prescreened via a telephone or online screening questionnaire and were deemed eligible on the basis of age, BMI, postmenopausal status, chronic illness, medication use, and willingness to be randomly assigned. Of these subjects, 319 men and women were screened at the HNRCA for eligibility on the basis of laboratory exclusion criteria. A total of 103 qualified volunteers were enrolled and randomly assigned, and 81 of these individuals completed the study with 40 subjects (25 men and 15 women) in the RG group and 41 subjects (24 men and 17 women) in the WG group (Figure 2). Of 22 participants who dropped out, 13 individuals dropped out during the run-in phase, and 9 individuals dropped out after random assignment (n = 5 from the RG group, and n = 4 from the WG group) because of personal reasons that were unrelated to the intervention including the need to take antibiotics, time constraints, family events, no shows, and a diverticulitis diagnosis by the primary care physician. Sample sizes reported for different outcome measurements varied slightly, which reflected missing samples that were due to the reasons including below the detection limit, accidental loss of samples during processing, and a technical failure in the analysis. However, there were no samples that were purposely excluded from the statistical analysis. There was no difference in sex, age, BMI, race, education, marital status, or occupation at baseline between groups (Table 1) or in blood lipid profiles (data not shown). Furthermore, there was no significant difference in the change in triglycerides, LDLs, HDLs, or VLDLs between WG or RG groups (data not shown), but a significantly smaller decrease (from baseline to postintervention) in total cholesterol was shown in the WG group than in the RG group (3.61 ± 3.43 compared with 11.30 ± 3.88 mg/dL; P < 0.05).
FIGURE 2.
Consolidated Standards of Reporting Trials diagram. 1Other reasons included an unwillingness to eat all study foods, current participation in other studies, and being over the BMI range at the start date but after signing consent. 2Unwilling to eat all study foods. RG, refined grain; WG, whole grain.
TABLE 1.
Baseline characteristics of participants1
| Characteristic | RG group | WG group |
| Subjects, n | 40 | 41 |
| Sex, M/F, n | 25/15 | 24/17 |
| Age, y | 54 ± 0.79 (41–65)2 | 55 ± 0.94 (40–65) |
| BMI, kg/m2 | 26 ± 0.47 (20–33) | 26 ± 0.47 (20–34) |
| Race, n | ||
| White | 21 | 23 |
| Black | 9 | 9 |
| Asian American | 3 | 6 |
| Other | 7 | 3 |
| Education, n (%) | ||
| Graduate of professional school | 14 (37) | 14 (34) |
| 4 y of college | 17 (45) | 15 (37) |
| <4 y of college | 6 (16) | 9 (22) |
| Other | 1 (3) | 3 (7) |
| Marital status, n (%) | ||
| Married | 16 (42) | 12 (29) |
| Single | 14 (37) | 17 (41) |
| Divorced | 6 (16) | 8 (20) |
| Separated | 1 (3) | 3 (7) |
| Widowed | 1 (3) | 1 (2) |
| Occupation, n (%) | ||
| Service | 6 (17) | 12 (32) |
| Technical | 6 (17) | 1 (3) |
| Professional | 6 (17) | 5 (13) |
| Retired | 3 (8) | 3 (8) |
| Unemployed | 2 (5) | 2 (5) |
| Other | 13 (36) | 15 (39) |
There were no statistical differences between groups on the basis of Student’s t test or Fisher’s exact test. RG, refined grain; WG, whole grain.
Mean ± SEM; range in parentheses (all such values).
Dietary intake and adherence
Body weight was maintained within 1 kg of baseline values according to the study design. At baseline and at the end of the intervention, mean ± SD body weights were 74.7 ± 12.0 compared with 74.7 ± 12.4 kg in the WG group and 75.4 ± 12.0 compared with 74.9 ± 11.7 kg in the RG group. There was no difference in energy intake or total fat intake over the study period both within each group and between groups (Supplemental Table 1). There was a negligible between-group difference in macronutrient intake with a WG-group compared with RG-group increase by 3%, a decrease by 2%, and an increase by 0.4% for carbohydrate, protein, and PUFAs, respectively. We observed increased intake of cholesterol in the RG group; this finding resulted in a small difference in cholesterol intake when the change between WG and RG groups was compared. Observed differences in micronutrient intake reflected the nutritional composition of the WG food and the manufacturer’s fortification of products that contained refined cereals and white flour. Compared with the RG group, intakes of iron, magnesium, zinc, and selenium were higher in the WG group; however, intakes of vitamin D, thiamin, niacin, and folate were lower.
The consumption of WGs, as expected, resulted in an increase in total dietary fiber intake in the WG group than in the RG group. Over the 6-wk intervention phase, the WG group had daily reported consumption of 207 ± 39 g WGs and 40 ± 5 g fiber compared with 0 g WGs and 21 ± 3 g fiber in the RG group. There was a small increase in soluble fiber intake in RG and WG groups at the follow-up time point compared with at baseline. In contrast, insoluble fiber intake did not increase in the RG group but doubled in the WG group. In this study, wheat was the major contributor of WG intake; therefore, insoluble fiber was the major source of dietary fiber. Incidences of self-reported gastrointestinal symptoms did not differ between groups (Supplemental Table 2).
Alkylresorcinols are phenolic lipids that are present in the bran of wheat and rye, and thus, their plasma concentrations are suitable biomarkers of WG wheat consumption. Plasma concentrations of alkylresorcinol between groups were not different at baseline. Increased WG consumption was accompanied by increases in total alkylresorcinols and individual alkylresorcinols (19:0, 21:0, and 23:0) (all P < 0.0001) in the WG group (Table 2). Plasma alkylresorcinol concentrations of 19:0, 21:0, and 23:0 and total alkylresorcinols in the WG group after the intervention were 6-, 9-, 5-, and 7-fold higher (all P < 0.0001), respectively, than the concentrations at baseline. The larger increase (9-fold) in plasma concentrations of 21:0 relative to that of other alkylresorcinols in the WG group reflected the fact that wheat was the main component of the WG diet in the study. As expected, no changes in plasma alkylresorcinol concentrations were shown in the RG group after the intervention phase, which confirmed participant adherence.
TABLE 2.
Alkylresorcinol concentrations of participants at baseline (week 2) and follow-up (week 8)1
| RG group |
WG group |
|||||||||
| Measure | Baseline | Follow-up | ΔRG | P | Baseline | Follow-up | ΔWG | P | ΔWG − ΔRG | P |
| 19:0, nmol/L | 12.24 ± 2.74 | 12.68 ± 1.88 | 0.44 ± 2.42 | 0.16 | 8.59 ± 1.31 | 64.17 ± 7.64 | 55.59 ± 7.29 | <0.0001 | 55.15 ± 7.76 | <0.0001 |
| 21:0, nmol/L | 11.31 ± 2.30 | 14.06 ± 2.43 | 2.75 ± 2.84 | 0.08 | 12.30 ± 2.39 | 116.97 ± 15.55 | 104.67 ± 13.82 | <0.0001 | 101.90 ± 14.27 | <0.0001 |
| 23:0, nmol/L | 3.07 ± 0.58 | 3.86 ± 0.72 | 0.79 ± 0.81 | 0.75 | 3.14 ± 0.69 | 16.89 ± 2.76 | 13.75 ± 2.29 | <0.0001 | 12.96 ± 2.46 | <0.0001 |
| Total alkylresorcinol, nmol/L | 26.62 ± 4.10 | 30.60 ± 3.76 | 3.98 ± 4.96 | 0.90 | 24.02 ± 3.65 | 198.03 ± 24.27 | 174.01 ± 21.89 | <0.0001 | 170.00 ± 22.70 | <0.0001 |
All values are means ± SEMs. n = 40 in the RG group, and n = 41 in the WG group. There were no differences between groups at baseline. P values were obtained with the use of an ANCOVA and controlling for the baseline measure, BMI, age, and sex. RG, refined grain; WG, whole grain.
Stool weight, frequency, pH, water content, and SCFAs
We observed an increase in bowel-movement frequency (P = 0.03) and stool weight (P = 8.39 × 10−8) in the WG group (Table 3) with no change in the RG group, which resulted in between-group differences in bowel-movement frequency (P = 0.02) and stool weight (P = 1.77 × 10−7). Changes in stool acetate (ΔWG compared with ΔRG mean ± SEM: 2.13 ± 1.36 mmol/L; P = 0.02) and total SCFA (2.70 ± 2.25 mmol/L; P = 0.05) were larger in the WG group than in the RG group mainly because of a decline in the RG group (Table 4). There was also a weak correlation between acetate and 19:0 (r = 0.19, P = 0.02) and total alkylresorcinol (r = 0.19, P = 0.02), which suggested that the difference in changes between the 2 groups was a reflection of the difference in WG intake.
TABLE 3.
Stool characteristics of participants at baseline (week 2) and follow-up (week 8)1
| RG group |
WG group |
|||||||||
| Measure | Baseline | Follow-up | ΔRG | P | Baseline | Follow-up | ΔWG | P | ΔWG − ΔRG | P |
| Stool in 72 h, n | 3.62 ± 0.24 | 3.46 ± 0.26 | −0.15 ± 0.20 | 0.33 | 3.78 ± 0.30 | 4.34 ± 0.32 | 0.56 ± 0.25 | 0.03 | 0.76 ± 0.32 | 0.02 |
| Stool water content, % | 72.11 ± 1.11 | 72.79 ± 1.13 | 0.86 ± 1.44 | 0.39 | 71.35 ± 1.46 | 74.57 ± 1.19 | 3.23 ± 1.53 | 0.01 | 2.36 ± 2.11 | 0.15 |
| Stool weight, g/d | 105.05 ± 8.57 | 95.83 ± 8.57 | −9.22 ± 8.07 | 0.26 | 116.60 ± 11.25 | 178.68 ± 12.79 | 62.07 ± 9.96 | <0.0001 | 69.30 ± 12.89 | <0.0001 |
| Aerobic, CFUs × 107 | 1.66 ± 5.78 | 9.19 ± 3.18 | −7.32 ± 6.28 | 0.13 | 8.48 ± 3.17 | 4.79 ± 1.33 | −3.69 ± 3.18 | 0.12 | 3.63 ± 6.84 | 0.32 |
| Anaerobic, CFUs × 109 | 1.34 ± 3.19 | 2.19 ± 8.89 | 8.14 ± 8.97 | 0.13 | 3.38 ± 1.16 | 1.21 ± 5.12 | −2.18 ± 1.01 | 0.12 | 2.99 ± 1.36 | 0.31 |
| Stool pH | 6.78 ± 0.05 | 6.81 ± 0.05 | 0.01 ± 0.05 | 0.05 | 6.77 ± 0.06 | 6.75 ± 0.04 | −0.03 ± 0.06 | 0.04 | −0.07 ± 0.08 | 0.31 |
All values are means ± SEMs. n = 40 in the RG group, and n = 41 in the WG group. There were no differences between groups at baseline. P values were obtained with the use of an ANCOVA and controlling for the baseline measure, BMI, age, and sex. RG, refined grain; WG, whole grain.
TABLE 4.
Fecal water SCFA concentrations of participants at baseline (week 2) and follow-up (week 8)1
| RG group |
WG group |
|||||||||
| Measure | Baseline | Follow-up | ΔRG | P | Baseline | Follow-up | ΔWG | P | ΔWG − ΔRG | P |
| Acetate, mmol/L | 20.73 ± 1.11 | 19.3 ± 0.92 | −1.43 ± 0.96 | 0.03 | 21.63 ± 1.17 | 22.34 ± 1.26 | 0.71 ± 0.98 | 0.19 | 2.13 ± 1.36 | 0.02 |
| Propionate, mmol/L | 5.19 ± 0.64 | 3.6 ± 0.5 | −1.59 ± 0.32 | <0.0001 | 5.37 ± 0.71 | 3.46 ± 0.46 | −1.91 ± 0.25 | <0.0001 | −0.32 ± 0.41 | 0.29 |
| Butyrate, mmol/L | 5.29 ± 0.8 | 4.5 ± 0.51 | −0.79 ± 0.89 | 0.14 | 5.43 ± 0.7 | 5.52 ± 0.91 | 0.09 ± 0.76 | 0.86 | 0.88 ± 1.18 | 0.25 |
| Total SCFAs, mmol/L | 31.21 ± 2.12 | 27.4 ± 1.62 | −3.81 ± 1.66 | 0.002 | 32.43 ± 2.1 | 31.32 ± 2.08 | −1.11 ± 1.51 | 0.65 | 2.70 ± 2.25 | 0.05 |
All values are means ± SEMs. n = 40 in the RG group, and n = 41 in the WG group. There were no differences between groups at baseline. P values were obtained with the use of an ANCOVA and controlling for the baseline measure, BMI, age, and sex. RG, refined grain; SCFA, short-chain fatty acid; WG, whole grain.
Fecal microbiota composition
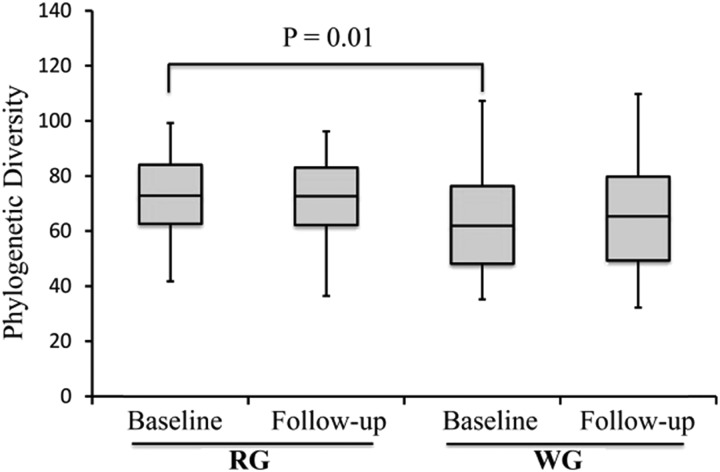
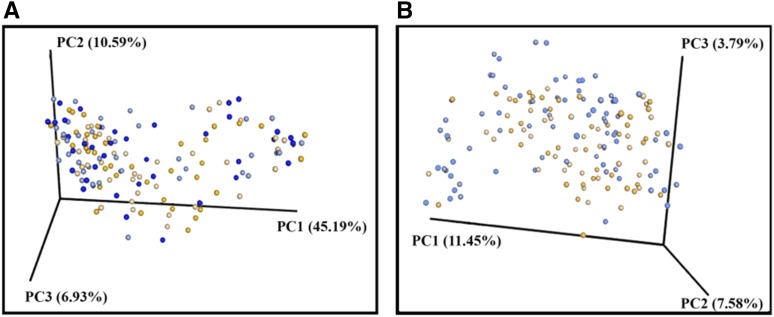
Samples were sequenced in 2 batches (number of samples per sequencing run: ∼100) to generate a good depth (number of sequences per sample) of sequencing per sample. We obtained an average of 189,971 sequences/sample and a total of 33,254,004 sequences that were assigned to barcodes. Reads were clustered into 5341 total operational taxonomic units at a 97% sequence identity, used in a taxonomic analysis, and classified into 10 bacterial phyla and 118 genera. Community complexities of WG and RG microbiota were compared according to α (intraindividual) diversity and β (interindividual) diversity. There were differences in α diversity at baseline; however, these differences were no longer observed at the end of the intervention (Figure 3). Although we observed a difference in unweighted UniFrac at baseline (P = 0.05), there was no difference in the weighted UniFrac analysis (Figure 4). This result may have implied the presence of small populations of bacteria, and when their abundance was taken into account, the differences were no longer observed.
FIGURE 3.
α-Diversity comparisons of gut microbiota in stool samples that were collected at baseline and at the end of the intervention. Data are presented as box-and-whisker plots that show the distribution of data in quartiles. α Diversity did not significantly change from baseline after the intervention in either diet group as observed with the use of phylogenic diversity–richness metrics. The α diversity in stool samples that were collected at baseline was lower in the WG group (n = 38) than in the RG group (n = 39) (P = 0.01). RG, refined grain; WG, whole grain.
FIGURE 4.
β-Diversity comparisons of gut microbiota in stool samples that were collected at baseline and after the intervention. Data are shown from WG participants (n = 38) before the intervention (dark-blue dots) and after the intervention (light-blue dots) as well as from RG participants (n = 39) before the intervention (dark-orange dots) and after the intervention (light-orange dots). A PC analysis of weighted (A) and unweighted (B) UniFrac distances. A permutation-based ANOVA [Adonis function in the vegan package in R software (60); 99 permutations] with weighted UniFrac-distance metrics revealed that the variation in the gut microbiota community structure could not be explained by the intervention group either at baseline (P = 0.39) or after the intervention (P = 0.25). However, a similar analysis with the use of unweighted UniFrac metrics showed differences in the microbiota structure of WG and RG groups at P = 0.05 and P = 0.07 for baseline and after the intervention, respectively. PC, principal coordinate; RG, refined grain; WG, whole grain.
The bacterial composition was determined at the phyla and genera levels; however, family-level comparisons were made when genera-level identifications were not possible. There were no differences between groups when the change (ΔWG compared with ΔRG) of relative abundance at the phyla level was compared (Table 5). At the family level, there was a significant relative change toward a decrease in Enterobacteriaceae abundance in the WG group than in the RG group (ΔWG compared with ΔRG mean ± SEM: −0.07 ± 0.11%; FDR-adjusted P = 0.25) (Table 6). At the genera level, there was a significant relative change toward an increase in Lachnospira abundance in the WG group than in the RG group (ΔWG compared with ΔRG mean ± SEM: 1.04 ± 0.33; FDR-adjusted P = 0.25). In addition, there was a trend of relative change toward an increase in Roseburia abundance in the WG group than in the RG group (ΔWG compared with ΔRG mean ± SEML: 1.32 ± 0.33; FDR-adjusted P = 0.30). Furthermore, we observed a positive correlation of Lachnospira and Roseburia with both acetate and butyrate at week 8 (FDR-adjusted P < 0.25, Spearman P < 0.25).
TABLE 5.
Relative abundance of bacteria phyla in stool samples of participants at baseline (week 2) and follow-up (week 8)1
| RG group |
WG group |
|||||||||
| Phylum | Baseline | Follow-up | ΔRG | P | Baseline | Follow-up | ΔWG | P | ΔWG − ΔRG | P |
| Firmicutes, % of total microbiota | 51.41 ± 3.00 | 48.61 ± 2.74 | −2.80 ± 2.87 | 0.97 | 43.29 ± 2.57 | 43.57 ± 2.33 | 0.28 ± 2.70 | 0.94 | 3.08 ± 2.75 | 0.94 |
| Bacteroidetes, % of total microbiota | 38.66 ± 2.85 | 45.33 ± 3.13 | 6.67 ± 0.43 | 0.91 | 49.13 ± 2.73 | 50.2 ± 2.33 | 1.07 ± 2.79 | 0.94 | −5.6 ± 1.61 | 0.97 |
| Proteobacteria, % of total microbiota | 2.96 ± 0.65 | 2.32 ± 0.29 | −0.64 ± 0.49 | 0.91 | 3.30 ± 0.53 | 3.05 ± 0.43 | −0.25 ± 0.48 | 0.94 | 0.39 ± 0.49 | 0.97 |
| Actinobacteria, % of total microbiota | 1.74 ± 0.43 | 1.56 ± 0.33 | −0.18 ± 0.39 | 0.91 | 1.59 ± 0.44 | 1.05 ± 0.28 | −0.54 ± 0.36 | 0.94 | −0.36 ± 0.37 | 0.89 |
| Tenericutes, % of total microbiota | 1.45 ± 0.54 | 0.73 ± 0.26 | −0.72 ± 0.42 | 0.91 | 0.73 ± 0.31 | 0.71 ± 0.28 | −0.02 ± 0.29 | 0.94 | 0.70 ± 0.43 | 0.89 |
| Verrucomicrobia, % of total microbiota | 0.45 ± 0.11 | 0.71 ± 0.28 | 0.26 ± 0.27 | 0.91 | 0.26 ± 0.1 | 0.39 ± 0.14 | 0.13 ± 0.12 | 0.94 | −0.13 ± 0.20 | 0.94 |
| Cyanobacteria, % of total microbiota | 0.63 ± 0.58 | 0.01 ± 0.01 | −0.62 ± 0.01 | 0.97 | 0.13 ± 0.1 | 0.07 ± 0.06 | −0.06 ± 1.09 | 0.94 | 0.56 ± 0.55 | 0.97 |
| Fusobacteria, % of total microbiota | 1.75 ± 1.74 | 0.02 ± 0.01 | −1.73 ± 0.01 | 0.93 | 1.07 ± 0.61 | 0.72 ± 0.44 | −0.35 ± 0.74 | 0.94 | 1.38 ± 0.38 | 0.94 |
| Lentisphaerae, % of total microbiota | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.01 ± 0.02 | 0.91 | 0.07 ± 0.07 | 0.02 ± 0.01 | −0.05 ± 0.04 | 0.94 | −0.06 ± 0.03 | 0.89 |
All values are means ± SEMs. n = 40 in the RG group, and n = 39 in the WG group. There were no differences between groups at baseline. P values were obtained with the use of a linear model; covariates in the model included age, BMI, and sex with false-discovery rate correction for multiple testing. RG, refined grain; WG, whole grain.
TABLE 6.
Relative abundance of bacteria genera in stool samples of participants at baseline (week 2) and follow-up (week 8)1
| RG group |
WG group |
|||||||||
| Phylum | Baseline | Follow-up | ΔRG | P | Baseline | Follow-up | ΔWG | P | ΔWG − ΔRG | P |
| Lachnospira, % of total microbiota | 2.16 ± 0.37 | 1.31 ± 0.26 | −0.85 ± 0.31 | 0.99 | 1.71 ± 0.26 | 1.90 ± 0.41 | 0.19 ± 0.34 | 1.00 | 1.04 ± 0.33 | 0.25 |
| Roseburia, % of total microbiota | 1.88 ± 0.29 | 1.48 ± 0.19 | −0.40 ± 0.24 | 0.99 | 1.89 ± 0.38 | 2.81 ± 0.46 | 0.92 ± 0.42 | 1.00 | 1.32 ± 0.33 | 0.30 |
| Enterobacteriaceae, % of total microbiota | 0.05 ± 0.02 | 0.09 ± 0.04 | 0.04 ± 0.03 | 0.99 | 0.33 ± 0.17 | 0.30 ± 0.20 | −0.03 ± 0.19 | 1.00 | −0.07 ± 0.11 | 0.25 |
All values are means ± SEMs. n = 40 in the RG group, and n = 39 in the WG group. There were no differences between groups at baseline. P values were obtained with the use of a linear model; covariates in the model included age, BMI, and sex with false-discovery rate correction for multiple testing. RG, refined grain; WG, whole grain.
Salivary and stool IgA and stool cytokines
The WG intervention had no effect on the salivary IgA concentration or rate or on stool IgA (Supplemental Table 3) or stool cytokine concentrations (Table 7).
TABLE 7.
Stool water cytokine concentrations of participants at baseline (week 2) and follow-up (week 8)1
| RG group |
WG group |
|||||||||
| Measure | Baseline | Follow-up | ΔRG | P | Baseline | Follow-up | ΔWG | P | ΔWG − ΔRG | P |
| IFN-γ, pg/mL | 0.37 ± 0.19 | 0.21 ± 0.06 | −0.16 ± 0.16 | 0.63 | 0.14 ± 0.03 | 0.24 ± 0.14 | 0.10 ± 0.15 | 0.61 | 0.27 ± 0.22 | 0.97 |
| IL-17, pg/mL | 1.72 ± 0.73 | 3.17 ± 1.80 | 1.46 ± 1.08 | 0.22 | 1.32 ± 0.298 | 1.51 ± 0.23 | 0.19 ± 0.18 | 0.16 | −1.27 ± 1.11 | 0.88 |
| TNF-α, pg/mL | 1.76 ± 0.31 | 2.11 ± 0.38 | 0.35 ± 0.29 | 0.54 | 2.76 ± 0.52 | 2.63 ± 0.47 | −0.14 ± 0.60 | 0.74 | −0.49 ± 0.63 | 0.82 |
| IL-6, pg/mL | 0.34 ± 0.13 | 0.29 ± 0.08 | −0.05 ± 0.12 | 0.09 | 0.603 ± 0.23 | 0.43 ± 0.15 | −0.17 ± 0.17 | 0.14 | −0.12 ± 0.20 | 0.89 |
| TGF-β, pg/mL | 0.02 ± 0.01b | 0.08 ± 0.06 | 0.06 ± 0.06 | 0.83 | 0.097 ± 0.04c | 0.18 ± 0.09 | 0.09 ± 0.08 | 0.07 | 0.03 ± 0.10 | 0.25 |
All values are means ± SEMs. n = 19–39 in the RG group, and n = 18–38 in the WG group. There were differences between groups at baseline. P values were obtained with the use of an ANCOVA and controlling for the baseline measure, BMI, age, and sex. IFN-γ, interferon γ RG, refined grain; TGF-β, transforming growth factor β WG, whole grain.
DTH skin test
There was no within- or between-group difference in the total number of positive antigens or total diameter of induration that was measured at 48 h after implantation (Table 8). There was no difference in DTH changes between RG and WG groups. However, we observed an increase in the number of positive antigens and total diameter of induration in response to Trichophyton in both groups (Table 8), which might have been a reflection of the repeated administration of the antigen.
TABLE 8.
Delayed-type hypersensitivity of participants at baseline (week 2) and follow-up (week 8)1
| RG group |
WG group |
|||||||||
| Measure | Baseline | Follow-up | ΔRG | P | Baseline | Follow-up | ΔWG | P | ΔWG − ΔRG | P |
| Positive responses, n | 1.8 ± 0.1 | 2.0 ± 0.1 | 0.2 ± 0.1 | 0.05 | 1.7 ± 0.1 | 2.0 ± 0.1 | 0.3 ± 0.1 | 0.03 | 0.1 ± 0.2 | 0.88 |
| Total diameter, mm | 27.4 ± 2.5 | 31.0 ± 2.9 | 3.6 ± 3.2 | 0.10 | 24.7 ± 2.3 | 31.0 ± 2.2 | 5.9 ± 2.6 | 0.10 | 2.31 ± 4.1 | 0.90 |
| Tetanus, mm | 13.2 ± 1.3 | 13.9 ± 1.4 | 0.6 ± 1.5 | 0.70 | 12.6 ± 1.3 | 14.0 ± 1.2 | 1.3 ± 1.7 | 0.60 | 0.6 ± 2.3 | 0.94 |
| Candida, mm | 10.8 ± 1.3 | 11.6 ± 1.4 | 0.8 ± 1.3 | 0.60 | 11.4 ± 1.5 | 13.3 ± 1.4 | 1.8 ± 1.3 | 0.20 | 1.0 ± 1.9 | 0.51 |
| Trichophyton, mm | 3.6 ± 1.1 | 5.8 ± 1.4 | 2.1 ± 0.9 | 0.04 | 1.6 ± 0.8 | 3.8 ± 1.1 | 2.2 ± 0.8 | 0.03 | 0.1 ± 1.2 | 0.93 |
All values are means ± SEMs. n = 38–40 in the RG group, and n = 41 in the WG group. There were no differences between groups at baseline. P values were obtained with the use of an ANCOVA and controlling for the baseline measure, BMI, age, and sex. RG, refined grain; WG, whole grain.
White blood cell count differential, lymphocyte phenotype, and lymphocyte proliferation
No between-group differences occurred in total numbers of white blood cells, lymphocytes, monocytes, eosinophils, basophils, or neutrophils (Supplemental Table 4). WG intake did not influence the percentages of T lymphocytes, CD4+ T cells and subpopulations, CD8+ T cells and subpopulations, B cells, NK cells, NK T cells, or regulatory T cells (Table 9). There was a difference in the change of the percentage of total terminal effector memory T cells (ΔWG compared with ΔRG mean ± SEM: 2.68% ± 2.33%; P = 0.03). A partial correlation analysis showed a modest positive correlation between terminal effector memory cells and 19:0 (r = 0.22, P = 0.04), 21:0 (r = 0.33, P = 0.001), and total alkylresorcinols (r = 0.28, P = 0.01), thereby suggesting that the increase in terminal effector memory T cells was associated with an increase in WG intake.
TABLE 9.
Immune cell phenotype of participants at baseline (week 2) and follow-up (week 8)1
| RG group |
WG group |
|||||||||
| Cell type | Baseline | Follow-up | ΔRG | P | Baseline | Follow-up | ΔWG | P | ΔWG − ΔRG | P |
| Total T cells, % | 66.03 ± 1.78 | 63.36 ± 2.89 | −2.67 ± 3.47 | 0.71 | 62.42 ± 2.14 | 66.60 ± 1.43 | 4.18 ± 2.21 | 0.30 | 6.86 ± 4.11 | 0.33 |
| Total effector memory, % | 18.92 ± 1.71 | 16.92 ± 1.42 | −2.00 ± 1.69 | 0.11 | 21.12 ± 1.55 | 21.80 ± 2.01 | 0.68 ± 1.62 | 0.45 | 2.68 ± 2.33 | 0.03 |
| CM, % | 19.79 ± 1.71 | 22.55 ± 2.18 | 2.51 ± 2.17 | 0.29 | 21.21 ± 2.31 | 19.16 ± 1.71 | −2.05 ± 2.58 | 0.39 | −4.55 ± 3.39 | 0.18 |
| TEM, % | 28.31 ± 2.14 | 26.61 ± 2.26 | −1.70 ± 2.47 | 0.57 | 27.16 ± 1.74 | 30.02 ± 2.70 | 2.86 ± 3.11 | 0.29 | 4.56 ± 4.01 | 0.26 |
| Naive, % | 35.67 ± 2.09 | 38.52 ± 3.08 | 2.85 ± 3.11 | 0.33 | 36.79 ± 2.78 | 35.74 ± 2.63 | −1.10 ± 2.09 | 0.77 | −3.94 ± 3.71 | 0.37 |
| CD4, % | 47.93 ± 1.96 | 49.95 ± 1.79 | 2.02 ± 1.56 | 0.11 | 47.49 ± 1.75 | 47.10 ± 1.80 | −0.39 ± 1.19 | 0.72 | −2.4 ± 1.95 | 0.16 |
| CD4 CM, % | 19.96 ± 3.56 | 16.53 ± 3.35 | −3.64 ± 3.52 | 0.58 | 15.16 ± 3.19 | 15.32 ± 3.39 | 0.17 ± 4.02 | 0.62 | 3.81 ± 5.36 | 0.96 |
| CD4 EM, % | 19.64 ± 1.62 | 17.38 ± 1.72 | −1.62 ± 1.27 | 0.17 | 21.03 ± 1.62 | 21.61 ± 1.72 | 0.61 ± 1.73 | 0.49 | 2.23 ± 2.15 | 0.14 |
| CD4 TEM, % | 26.92 ± 3.77 | 28.74 ± 3.52 | 2.56 ± 3.06 | 0.39 | 26.31 ± 2.84 | 30.81 ± 3.01 | 4.50 ± 3.55 | 0.12 | 1.94 ± 4.70 | 0.63 |
| CD4 naive, % | 34.15 ± 2.36 | 38.93 ± 2.71 | 4.78 ± 2.53 | 0.08 | 37.60 ± 2.26 | 35.39 ± 2.21 | −2.21 ± 2.10 | 0.54 | −6.99 ± 3.28 | 0.10 |
| CD8, % | 19.24 ± 1.43 | 19.15 ± 1.37 | 0.18 ± 1.12 | 0.59 | 17.77 ± 1.06 | 16.93 ± 0.97 | −0.84 ± 0.73 | 0.18 | −1.02 ± 1.33 | 0.19 |
| CD8 CM, % | 20.06 ± 2.13 | 19.98 ± 2.13 | −0.18 ± 2.44 | 0.97 | 20.17 ± 2.03 | 21.32 ± 2.21 | 1.14 ± 2.16 | 0.63 | 1.32 ± 3.25 | 0.76 |
| CD8 EM, % | 19.63 ± 1.65 | 20.15 ± 1.80 | 0.74 ± 1.77 | 0.87 | 18.99 ± 1.93 | 20.58 ± 2.16 | 1.59 ± 2.52 | 0.58 | 0.86 ± 3.11 | 0.79 |
| CD8 TEM, % | 30.07 ± 2.83 | 29.46 ± 2.89 | −0.99 ± 2.65 | 0.80 | 31.02 ± 2.63 | 29.18 ± 2.85 | −1.84 ± 3.28 | 0.41 | −0.85 ± 4.24 | 0.70 |
| CD8 naive, % | 29.62 ± 2.02 | 30.20 ± 2.48 | 0.87 ± 2.15 | 0.89 | 29.81 ± 2.24 | 28.46 ± 1.95 | −1.36 ± 1.90 | 0.66 | −2.22 ± 2.87 | 0.69 |
| B cells, % | 10.28 ± 0.86 | 10.54 ± 1.06 | 0.26 ± 1.06 | 0.94 | 11.59 ± 0.69 | 11.87 ± 0.69 | 0.48 ± 0.54 | 0.32 | 0.22 ± 1.21 | 0.44 |
| NK cells, % | 6.43 ± 0.77 | 6.29 ± 0.63 | −0.14 ± 0.66 | 0.20 | 5.66 ± 0.67 | 5.93 ± 0.78 | 0.19 ± 0.55 | 0.47 | 0.33 ± 0.86 | 0.70 |
| NK T cells, % | 1.11 ± 0.92 | 0.38 ± 0.14 | −0.73 ± 0.92 | 0.77 | 0.38 ± 0.14 | 0.59 ± 0.36 | 0.21 ± 0.39 | 0.51 | 0.94 ± 1.00 | 0.79 |
| CD4/CD25+, % | 14.49 ± 0.69 | 12.50 ± 0.69 | −1.99 ± 0.82 | 0.004 | 14.58 ± 0.85 | 13.94 ± 0.60 | −0.65 ± 0.83 | 0.37 | 1.34 ± 1.23 | 0.11 |
All values are means ± SEMs. n = 38–40 in the RG group, and n = 41 in the WG group. There were no differences between groups at baseline. P values were obtained with the use of an ANCOVA and controlling for the baseline measure, BMI, age, and sex. CM, central memory; EM, effector memory; NK, natural killer; RG, refined grain; TEM, terminal effector memory; WG, whole grain.
There was no within- or between-group difference in T cell proliferation in response to the mitogens Con A, phytohemagglutinin, or anti-CD3 and anti-CD28 at any of the concentrations used (Supplemental Table 5).
Plasma cytokines and LBP, ex vivo production of cytokines, and NK cell activity
WG intake had no effect on plasma concentrations of cytokines or LBP (Supplemental Table 6). Although several inflammatory markers are used to define chronic inflammation, it is increasingly appreciated that the measurement of an integrated index, such as an IS, that takes into account multiple cytokines may be a better representation of systemic inflammation status. Thus, we computed an IS in this study. There were no between-group differences in the IS; however, there was a moderate inverse correlation between the IS and total alkylresorcinol in the WG group (Pearson’s r = −0.40, P = 0.02).
There was a difference in the change from baseline to follow-up between the 2 groups in LPS-stimulated TNF-α production (ΔWG compared with ΔRG mean ± SEM: 2131 ± 1399 pg/mL; P = 0.04) (Table 10), which was mainly due to a decrease in the RG group. A partial correlation analysis showed a modest positive correlation between TNF-α and 19:0 (r = 0.23, P = 0.03) or total alkylresorcinols (r = 0.23, P = 0.04), which suggested that the change in LPS-induced production of TNF-α was associated with an increase in WG intake.
TABLE 10.
LPS-induced cytokine production of participants at baseline (week 2) and follow-up (week 8)1
| RG group |
WG group |
|||||||||
| Measure | Baseline | Follow-up | ΔRG | P | Baseline | Follow-up | ΔWG | P | ΔWG − ΔRG | P |
| IFN-γ, pg/mL | 3015 ± 473 | 1888 ± 290 | −1127 ± 449 | 0.0004 | 3006 ± 619 | 2461 ± 567 | −545 ± 243 | 0.07 | 582 ± 507 | 0.17 |
| IL-10, pg/mL | 743 ± 68 | 809 ± 74 | 66 ± 60 | 0.07 | 724 ± 56 | 747 ± 55 | 23 ± 36 | 0.43 | −42 ± 69 | 0.46 |
| TNF-α, pg/mL | 15,319 ± 1305 | 12,915 ± 1109 | −2404 ± 1103 | 0.005 | 16,276 ± 1307 | 16,003 ± 1408 | −273 ± 868 | 0.97 | 2131 ± 1399 | 0.04 |
| IL-1β, pg/mL | 1392 ± 106 | 1244 ± 99 | −148 ± 91 | 0.02 | 1662 ± 147 | 1521 ± 131 | −141 ± 105 | 0.44 | 6 ± 139 | 0.26 |
| IL-6, pg/mL | 3711 ± 318 | 3339 ± 264 | −372 ± 202 | 0.05 | 3530 ± 255 | 3367 ± 240 | −164 ± 191 | 0.45 | 209 ± 278 | 0.38 |
| IL-8, pg/mL | 54,247 ± 5298 | 66,556 ± 8977 | 12,310 ± 5603 | 0.04 | 72,232 ± 9921 | 76,619 ± 10,901 | 4386 ± 6115 | 0.27 | −7923 ± 8304 | 0.46 |
All values are means ± SEMs. n = 40 in the RG group, and n = 41 in the WG group. There were no differences between groups at baseline. P values were obtained with the use of an ANCOVA and controlling for the baseline measure, BMI, age, and sex. IFN-γ, interferon γ RG, refined grain; WG, whole grain.
There was no difference in the ex vivo production of cytokines after stimulation with anti-CD3 and anti-CD28 at any of the concentrations used (Supplemental Table 7). The WG intervention had no effect on NK cell activity (Supplemental Table 8).
DISCUSSION
This study was designed to determine the effects of the consumption of diets that were matched for overall energy contents and macronutrient compositions but that differed in amounts of WGs on gut microbiota, their metabolites, and immune and inflammatory responses in metabolically healthy adults. As intended, body weight was maintained fairly constant within 1 kg of baseline weights throughout the duration of the study, and furthermore, the increase of WGs in the diet was well tolerated because self-reported gastrointestinal side effects did not differ between groups. In addition to increasing plasma alkylresorcinol, the frequency of bowel movements, and stool weight, the consumption of WGs had modest effects on the gut microbiota composition, stool SCFA concentrations, and a few variables of immune cell phenotype and function.
Plasma concentrations of alkylresorcinol as a biomarker of WG intake can serve to monitor adherence because alkylresorcinol are mainly found in the outer parts of WG wheat and rye, are not destroyed during food processing (61), and are well absorbed in humans (62). Significantly increased plasma alkylresorcinol in the WG group compared with in the RG group indicated good adherence to the assigned diets, which was further supported by the similar changes in stool output and frequency because fiber is known to increase stool frequency and output (63). The fiber from wheat is mostly insoluble, and insoluble fibers are known to increase stool bulk, reduce transit time, and make fecal elimination easier and quicker. In this way, insoluble fiber can regulate bowel functions to promote the wellbeing of healthy people and can function as a remedy to alleviate several gastrointestinal diseases such as peptic ulcers and cancer (64, 65).
To determine the impact of WGs on immune and inflammatory responses and gut microbiota, our study assessed the following: 1) systemic and stool inflammatory cytokine concentrations and plasma LBP concentrations, 2) phenotypic and functional immune variables, and 3) gut microbiota and microbial products. We showed no effect of WGs on plasma or stool inflammatory cytokine concentrations or on LBP concentration in plasma. Our findings are consistent with 4 (32–34, 66) of 5 [including Sofi et al. (29)] published WG studies that only looked at inflammatory status but not at the impact on the cell mediated immune response or gut microbiota composition. The study of Sofi et al. (29) used a unique type of Italian WG wheat with a special processing method, and this WG flour is known to contain higher amounts of polyphenols and flavonoids, which have anti-inflammatory effects (31).
We showed no effect of WGs on the majority of phenotypic or functional immune variables. The increased DTH response in both groups may have suggested a boosting effect of a repeated exposure to the antigen, which may have masked the additional effect of WGs even if the effect existed. In relation to this, we showed a significant difference in the percentage of terminal effector memory T cells between WG and RG groups, which may have implied that there was an enhanced potential in the adaptive immune response to a recall antigen. A similar finding was reported by Ampatzoglou et al. (35) who showed a trend toward increased CD4+ terminal central memory cells in a WG group than in a control group. In addition, we showed that the LPS-stimulated TNF-α production was significantly better maintained in the WG group that in the RG group. Because TNF-α serves as an ex vivo surrogate marker for the inflammatory response after a pathogen challenge, these data may have suggested that there was a more-robust response to antigens. However, with consideration of the lack of differences in other variables of immune and inflammatory responses, the overall impact of an increase in the consumption of WGs, in the absence of weight loss, on the resistance to pathogens remains to be determined.
In this study, we showed a modest effect of WGs on the composition of microbiota and stool SCFA concentrations. These observations were consistent with 2 (30, 31) of 3 (30, 31, 35) previous studies that investigated the effects of WGs on gut microbiota. We did not observe a difference in the bacterial diversity or phyla between groups, which was in agreement with the other intervention trials that used WG wheat as the main source of WGs (31, 67). In contrast, Martínez et al. (30) used WG barley and brown rice as the main sources of WGs and reported increases in gut microbial diversity and in the Firmicutes:Bacteroidetes ratio. Similar to the results that were reported by Martínez et al. (30) and Vitaglione et al. (31), we observed differences at the genus level whereby there was an increase in the SCFA producer Lachnospira and a decrease in proinflammatory Enterobacteriaceae. In variance to Martínez et al. (30), we observed a difference in stool acetate between groups, which was mainly driven by a decrease in the RG group. Note that the amount of SCFAs that is present in the stool only accounts for ∼5–10% of SCFAs produced (68); acetate is mainly produced in the colon (69); and a lower pH favors the production of SCFA (70). In our study, the RG group showed an increase in stool pH, whereas the WG group showed a decrease in stool pH. Therefore, increased SCFA production after WG consumption may be related to a favorable environment such as lower pH in the colon. Of 3 studies (30, 31, 35), Martínez et al. (30) and Vitaglione et al. (31) reported a decrease in systemic inflammatory markers and changes in the gut microbiota composition. The reason for the inconsistent findings in these clinical trials is not completely understood; however, factors such as differences in the composition of WG diets, not having completely controlled for other components of the diets, the extent of adherence to the diets, and the population studied may have contributed to the divergent findings.
The strength of the current study is that, to our knowledge, this is the first WG intervention report that completely controlled the diet, maintained weight, and kept other dietary components except fiber constant. Furthermore, we included an analysis of plasma alkylresorcinol to verify adherence. In doing so, we were able to assess the effect of WGs on gut microbiota and immune and inflammatory markers without the influence of confounding factors. Intervention studies that have not included biomarkers of adherence have consistently report mixed results (29, 32–34, 66) on inflammatory markers, whereas studies that have included markers of WG adherence and observed significant changes in gut microbiota composition have reported beneficial effects on inflammatory markers (30, 31, 35). Thus, the modest effects that we observed on immune and inflammatory markers and gut microbiota suggest that more-pronounced changes in the gut microbiota, which may require a prolonged intervention period, are needed to observe more-dramatic effects on immune and inflammatory responses. Furthermore, the current study was conducted in healthy individuals who were not likely to be immune compromised or to have high inflammatory status. It is possible that more-pronounced changes would have been observed in participants who were preselected for having high inflammatory status or chronic disease. Note that WG foods contain more micronutrients and phenolic compounds that are known to have various health benefits, including those on immune and inflammatory responses, and we could not determine the contribution of these components, as well as their interactions with fiber, to the final effects in our clinical trial. Therefore, future intervention studies should also consider the inclusion of a variety of grains because grains vary in types of fiber and compositions of phytochemicals and micronutrients. In particular, the WG in the current study was predominantly from wheat, whereas oats contributed <5%; however, oats are more prominent sources of soluble fiber, which are known to beneficially alter risk factors for diseases (71). Finally, genomic and epigenetic variations should be determined for the varied responses to WG intake in individuals in terms of changes in gut microbiota, inflammation status, and the immune response.
In conclusion, our study shows that 6 wk of WG consumption of an isocaloric diet that does not result in weight loss is well tolerated by healthy, middle-aged individuals; in addition to a significant increase in stool frequency and weight, we also show a very modest effect on the gut microbiota composition, SCFAs, and certain indicators of the immune response. Additional studies that use other WGs with higher soluble fiber and/or phenolic compounds are needed to determine the health benefits of WGs on healthy individuals. In addition, studies that use WGs with and without weight loss are needed for a better understanding of the impacts of WGs on the gut microbiota and their associated health benefits.
Acknowledgments
We thank Stephanie Marco for her assistance in the preparation of the manuscript and Weimin Guo for his help in the preparation of the figures and tables.
The authors’ responsibilities were as follows—SMV, MM, JBB, BG, AK, HR, SJ, KK, JPK, ES, DW, and SNM: designed the research; SMV, CB, PV, and DK: performed the statistical analysis; SMV, BG, AK, PV, DK, DW, and SNM: contributed to interpretation of the results; SMV, AK, HR, JPK, MT, GD, and LL: conducted the research; SMV, DW, and SNM: wrote the manuscript and had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript. Kerry Ingredients did not contribute financially or intellectually to this research study. SJ was employed by the General Mills Bell Institute of Health and Nutrition during the study conception and conduct; and KK is an employee of the General Mills Bell Institute of Health and Nutrition. The remaining authors reported no conflicts of interest related to the study.
Footnotes
Abbreviations used: Con A, concanavalin A; CRP, C-reactive protein; DTH, delayed-type hypersensitivity; FDR, false-discovery rate; HNRCA, Human Nutrition Research Center on Aging; IFN-γ, interferon-γ IS, inflammatory score; LBP, LPS-binding protein; NK, natural killer; PBMC, peripheral blood mononuclear cell; RG, refined grain; RPMI, Roswell Park Memorial Institute; SCFA, short-chain fatty acid; vol:vol, volume:volume; WG, whole grain.
REFERENCES
- 1.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993;259:87–91. [DOI] [PubMed] [Google Scholar]
- 2.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 2005;115:1111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egger G. In search of a germ theory equivalent for chronic disease. Prev Chronic Dis 2012;9:E95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobs DR Jr, Meyer HE, Solvoll K. Reduced mortality among whole grain bread eaters in men and women in the Norwegian County Study. Eur J Clin Nutr 2001;55:137–43. [DOI] [PubMed] [Google Scholar]
- 5.Jensen MK, Koh-Banerjee P, Franz M, Sampson L, Gronbaek M, Rimm EB. Whole grains, bran, and germ in relation to homocysteine and markers of glycemic control, lipids, and inflammation 1. Am J Clin Nutr 2006;83:275–83. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs DR Jr, Andersen LF, Blomhoff R. Whole-grain consumption is associated with a reduced risk of noncardiovascular, noncancer death attributed to inflammatory diseases in the Iowa Women’s Health Study. Am J Clin Nutr 2007;85:1606–14. [DOI] [PubMed] [Google Scholar]
- 7.Hamer DH, Sempertegui F, Estrella B, Tucker KL, Rodriguez A, Egas J, Dallal GE, Selhub J, Griffiths JK, Meydani SN. Micronutrient deficiencies are associated with impaired immune response and higher burden of respiratory infections in elderly Ecuadorians. J Nutr 2009;139:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nettleton JA, Schulze MB, Jiang R, Jenny NS, Burke GL, Jacobs DR Jr. A priori-defined dietary patterns and markers of cardiovascular disease risk in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2008;88:185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Q, Spiegelman D, van Dam RM, Holmes MD, Malik VS, Willett WC, Hu FB. White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch Intern Med 2010;170:961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slavin J, Jacobs D, Marquart L. Whole-grain consumption and chronic disease: protective mechanisms. Nutr Cancer 1997;27:14–21. [DOI] [PubMed] [Google Scholar]
- 11.Adom KK, Sorrells ME, Liu RH. Phytochemicals and antioxidant activity of milled fractions of different wheat varieties. J Agric Food Chem 2005;53:2297–306. [DOI] [PubMed] [Google Scholar]
- 12.Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev 2010;23:65–134. [DOI] [PubMed] [Google Scholar]
- 13.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 2010;107:14691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011;334:105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ou J, Carbonero F, Zoetendal EG, DeLany JP, Wang M, Newton K, Gaskins HR, O’Keefe SJ. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr 2013;98:111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benus RF, van der Werf TS, Welling GW, Judd PA, Taylor MA, Harmsen HJ, Whelan K. Association between Faecalibacterium prausnitzii and dietary fibre in colonic fermentation in healthy human subjects. Br J Nutr 2010;104:693–700. [DOI] [PubMed] [Google Scholar]
- 17.Bengmark S. Gut microbiota, immune development and function. Pharmacol Res 2013;69:87–113. [DOI] [PubMed] [Google Scholar]
- 18.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009;139:485–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 2008;4:337–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostman S, Rask C, Wold AE, Hultkrantz S, Telemo E. Impaired regulatory T cell function in germ-free mice. Eur J Immunol 2006;36:2336–46. [DOI] [PubMed] [Google Scholar]
- 21.Segain JP, Raingeard de la Bletiere D, Bourreille A, Leray V, Gervois N, Rosales C, Ferrier L, Bonnet C, Blottiere HM, Galmiche JP. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut 2000;47:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009;461:1282–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qi L, van Dam RM, Liu S, Franz M, Mantzoros C, Hu FB. Whole-grain, bran, and cereal fiber intakes and markers of systemic inflammation in diabetic women. Diabetes Care 2006;29:207–11. [DOI] [PubMed] [Google Scholar]
- 24.Gaskins AJ, Mumford SL, Rovner AJ, Zhang C, Chen L, Wactawski-Wende J, Perkins NJ, Schisterman EF. Whole grains are associated with serum concentrations of high sensitivity C-reactive protein among premenopausal women. J Nutr 2010;140:1669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masters RC, Liese AD, Haffner SM, Wagenknecht LE, Hanley AJ. Whole and refined grain intakes are related to inflammatory protein concentrations in human plasma. J Nutr 2010;140:587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montonen J, Boeing H, Fritsche A, Schleicher E, Joost HG, Schulze MB, Steffen A, Pischon T. Consumption of red meat and whole-grain bread in relation to biomarkers of obesity, inflammation, glucose metabolism and oxidative stress. Eur J Nutr 2013;52:337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen MK, Koh-Banerjee P, Hu FB, Franz M, Sampson L, Gronbaek M, Rimm EB. Intakes of whole grains, bran, and germ and the risk of coronary heart disease in men. Am J Clin Nutr 2004;80:1492–9. [DOI] [PubMed] [Google Scholar]
- 28.Lutsey PL, Jacobs DR Jr, Kori S, Mayer-Davis E, Shea S, Steffen LM, Szklo M, Tracy R. Whole grain intake and its cross-sectional association with obesity, insulin resistance, inflammation, diabetes and subclinical CVD: the MESA Study. Br J Nutr 2007;98:397–405. [DOI] [PubMed] [Google Scholar]
- 29.Sofi F, Ghiselli L, Cesari F, Gori AM, Mannini L, Casini A, Vazzana C, Vecchio V, Gensini GF, Abbate R, et al. Effects of short-term consumption of bread obtained by an old Italian grain variety on lipid, inflammatory, and hemorheological variables: an intervention study. J Med Food 2010;13:615–20. [DOI] [PubMed] [Google Scholar]
- 30.Martínez I, Lattimer JM, Hubach KL, Case JA, Yang J, Weber CG, Louk JA, Rose DJ, Kyureghian G, Peterson DA, et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J 2013;7:269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vitaglione P, Mennella I, Ferracane R, Rivellese AA, Giacco R, Ercolini D, Gibbons SM, La Storia A, Gilbert JA, Jonnalagadda S, et al. Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: role of polyphenols bound to cereal dietary fiber. Am J Clin Nutr 2015;101:251–61. [DOI] [PubMed] [Google Scholar]
- 32.Andersson A, Tengblad S, Karlstrom B, Kamal-Eldin A, Landberg R, Basu S, Aman P, Vessby B. Whole-grain foods do not affect insulin sensitivity or markers of lipid peroxidation and inflammation in healthy, moderately overweight subjects. J Nutr 2007;137:1401–7. [DOI] [PubMed] [Google Scholar]
- 33.Tighe P, Duthie G, Vaughan N, Brittenden J, Simpson WG, Duthie S, Mutch W, Wahle K, Horgan G, Thies F. Effect of increased consumption of whole-grain foods on blood pressure and other cardiovascular risk markers in healthy middle-aged persons: a randomized controlled trial. Am J Clin Nutr 2010;92:733–40. [DOI] [PubMed] [Google Scholar]
- 34.Brownlee IA, Moore C, Chatfield M, Richardson DP, Ashby P, Kuznesof SA, Jebb SA, Seal CJ. Markers of cardiovascular risk are not changed by increased whole-grain intake: the WHOLEheart study, a randomised, controlled dietary intervention. Br J Nutr 2010;104:125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ampatzoglou A, Williams CL, Atwal KK, Maidens CM, Ross AB, Thielecke F, Jonnalagadda SS, Kennedy OB, Yaqoob P. Effects of increased wholegrain consumption on immune and inflammatory markers in healthy low habitual wholegrain consumers. Eur J Nutr 2016;55:183–95. [DOI] [PubMed] [Google Scholar]
- 36.Meydani SN, Meydani M, Blumberg JB, Leka LS, Siber G, Loszewski R, Thompson C, Pedrosa MC, Diamond RD, Stollar BD. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial. JAMA 1997;277:1380–6. [DOI] [PubMed] [Google Scholar]
- 37.Cossack ZT. T-lymphocyte dysfunction in the elderly associated with zinc deficiency and subnormal nucleoside phosphorylase activity: effect of zinc supplementation. Eur J Cancer Clin Oncol 1989;25:973–6. [DOI] [PubMed] [Google Scholar]
- 38.Vulevic J, Drakoularakou A, Yaqoob P, Tzortzis G, Gibson GR. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am J Clin Nutr 2008;88:1438–46. [DOI] [PubMed] [Google Scholar]
- 39.US Department of Agriculture, US Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th ed. Washington (DC): US Government Posting Office; 2010. [Google Scholar]
- 40.McKeown NM, Marklund M, Ma J, Ross AB, Lichtenstein AH, Livingston KA, Jacques PF, Rasmussen HM, Blumberg JB, Chen CY. Comparison of plasma alkylresorcinols (AR) and urinary AR metabolites as biomarkers of compliance in a short-term, whole-grain intervention study. Eur J Nutr 2016;55:1235–44. [DOI] [PubMed] [Google Scholar]
- 41.Miletic ID, Schiffman SS, Miletic VD, Sattely-Miller EA. Salivary IgA secretion rate in young and elderly persons. Physiol Behav 1996;60:243–8. [DOI] [PubMed] [Google Scholar]
- 42.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 2012;6:1621–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010;7:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 2012;6:610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010;26:2460–1. [DOI] [PubMed] [Google Scholar]
- 46.Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R. UniFrac: an effective distance metric for microbial community comparison. ISME J 2011;5:169–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomcik K, Ibarra RA, Sadhukhan S, Han Y, Tochtrop GP, Zhang GF. Isotopomer enrichment assay for very short chain fatty acids and its metabolic applications. Anal Biochem 2011;410:110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao G, Nyman M, Jonsson JA. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed Chromatogr 2006;20:674–82. [DOI] [PubMed] [Google Scholar]
- 49.Saraji M, Farajmand B. Application of single-drop microextraction combined with in-microvial derivatization for determination of acidic herbicides in water samples by gas chromatography-mass spectrometry. J Chromatogr A 2008;1178:17–23. [DOI] [PubMed] [Google Scholar]
- 50.Nicholls S, Stephens S, Braegger CP, Walker-Smith JA, MacDonald TT. Cytokines in stools of children with inflammatory bowel disease or infective diarrhoea. J Clin Pathol 1993;46:757–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davis C, Wu X, Li W, Fan H, Reddy M. Stability of immunophenotypic markers in fixed peripheral blood for extended analysis using flow cytometry. J Immunol Methods 2011;363:158–65. [DOI] [PubMed] [Google Scholar]
- 52.Marklund M, McKeown NM, Blumberg JB, Chen CY. Hepatic biotransformation of alkylresorcinols is mediated via cytochrome P450 and beta-oxidation: a proof of concept study. Food Chem 2013;139:925–30. [DOI] [PubMed] [Google Scholar]
- 53.Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S, Noronha A, Manoharan M, Akira S, de Fougerolles A, et al. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med 2005;11:263–70. [DOI] [PubMed] [Google Scholar]
- 54.Cassidy A, Rogers G, Peterson JJ, Dwyer JT, Lin H, Jacques PF. Higher dietary anthocyanin and flavonol intakes are associated with anti-inflammatory effects in a population of US adults. Am J Clin Nutr 2015;102:172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bloemena E, Roos MT, Van Heijst JL, Vossen JM, Schellekens PT. Whole-blood lymphocyte cultures. J Immunol Methods 1989;122:161–7. [DOI] [PubMed] [Google Scholar]
- 56.De Groote D, Zangerle PF, Gevaert Y, Fassotte MF, Beguin Y, Noizat-Pirenne F, Pirenne J, Gathy R, Lopez M, Dehart I, et al. Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation. Cytokine 1992;4:239–48. [DOI] [PubMed] [Google Scholar]
- 57.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 1990;5:46–51. [Google Scholar]
- 58.Kim GG, Donnenberg VS, Donnenberg AD, Gooding W, Whiteside TL. A novel multiparametric flow cytometry-based cytotoxicity assay simultaneously immunophenotypes effector cells: comparisons to a 4 h 51Cr-release assay. J Immunol Methods 2007;325:51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 60.Hornik K. The comprehensive R archive network. WIREs Comp Stat 2012;4:394–8. [Google Scholar]
- 61.Ross AB, Shepherd MJ, Schupphaus M, Sinclair V, Alfaro B, Kamal-Eldin A, Aman P. Alkylresorcinols in cereals and cereal products. J Agric Food Chem 2003;51:4111–8. [DOI] [PubMed] [Google Scholar]
- 62.Ross AB, Kamal-Eldin A, Lundin EA, Zhang JX, Hallmans G, Aman P. Cereal alkylresorcinols are absorbed by humans. J Nutr 2003;133:2222–4. [DOI] [PubMed] [Google Scholar]
- 63.Wrick KL, Robertson JB, Van Soest PJ, Lewis BA, Rivers JM, Roe DA, Hackler LR. The influence of dietary fiber source on human intestinal transit and stool output. J Nutr 1983;113:1464–79. [DOI] [PubMed] [Google Scholar]
- 64.Anand P, Kunnumakkara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, Sung B, Aggarwal BB. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res 2008;25:2097–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vomero ND, Colpo E. Nutritional care in peptic ulcer. Arq Bras Cir Dig 2014;27:298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kristensen M, Toubro S, Jensen MG, Ross AB, Riboldi G, Petronio M, Bugel S, Tetens I, Astrup A. Whole grain compared with refined wheat decreases the percentage of body fat following a 12-week, energy-restricted dietary intervention in postmenopausal women. J Nutr 2012;142:710–6. [DOI] [PubMed] [Google Scholar]
- 67.Ampatzoglou A, Atwal KK, Maidens CM, Williams CL, Ross AB, Thielecke F, Jonnalagadda SS, Kennedy OB, Yaqoob P. Increased whole grain consumption does not affect blood biochemistry, body composition, or gut microbiology in healthy, low-habitual whole grain consumers. J Nutr 2015;145:215–21. [DOI] [PubMed] [Google Scholar]
- 68.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 2001;81:1031–64. [DOI] [PubMed] [Google Scholar]
- 69.Louis P, Scott KP, Duncan SH, Flint HJ. Understanding the effects of diet on bacterial metabolism in the large intestine. J Appl Microbiol 2007;102:1197–208. [DOI] [PubMed] [Google Scholar]
- 70.Lu ZX, Gibson PR, Muir JG, Fielding M, O’Dea K. Arabinoxylan fiber from a by-product of wheat flour processing behaves physiologically like a soluble, fermentable fiber in the large bowel of rats. J Nutr 2000;130:1984–90. [DOI] [PubMed] [Google Scholar]
- 71.Davy BM, Davy KP, Ho RC, Beske SD, Davrath LR, Melby CL. High-fiber oat cereal compared with wheat cereal consumption favorably alters LDL-cholesterol subclass and particle numbers in middle-aged and older men. Am J Clin Nutr 2002;76:351–8. [DOI] [PubMed] [Google Scholar]