Abstract
In vivo, CCN2 (connective tissue growth factor) promotes angiogenesis, osteogenesis, tissue repair, and fibrosis, through largely unknown mechanisms. In vitro, CCN2 promotes cell adhesion in a variety of systems via integrins and heparin sulfate proteoglycans (HSPGs). However, the physiological relevance of CCN2-mediated cell adhesion is unknown. Here, we find that HSPGs and the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase cascade are required for adult human dermal fibroblasts to adhere to CCN2. Endogenous CCN2 directly binds fibronectin and the fibronectin receptors integrins α4 β1 and α5 and syndecan 4. Using Ccn2-/- mouse embryonic fibroblasts, we show that loss of endogenous CCN2 results in impaired spreading on fibronectin, delayed α-smooth muscle actin stress fiber formation, and reduced ERK and focal adhesion kinase phosphorylation. These results suggest that a physiological role of CCN2 is to potentiate the ability of fibroblasts to spread on fibronectin, which may be important in modulating fibroblast adhesion to the provisional matrix during tissue development and wound healing. These results are consistent with the notion that a principal function of CCN2 is to modulate receptor/ligand interactions in vivo.
INTRODUCTION
Human skin, the largest organ in the body, acts as a protective barrier between the internal organs and the external environment (Chuong et al., 2002). The barrier function of skin is provided by both the epidermis, which consists of extracellular lipid-like substances and intracellular insoluble keratin (Fuchs and Byrne, 1994), and the dermis, which consists of fibroblasts that produce extracellular matrix (ECM; Kielty and Shuttleworth, 1997).
As a response to environmental insults or trauma, or as a consequence of local inflammatory processes, structural damage to skin can occur. The resultant wound-healing response consists of an integrated series of biochemical, immunological, and structural changes culminating in the de novo synthesis of new epithelia, blood vessels, and connective tissue (Werner and Grose, 2003). During this process, fibroblasts synthesize new ECM components, such as collagen and fibronectin (Badylak, 2002). In addition, fibroblasts proliferate and repopulate the wound. These fibroblasts attach to, remodel, and contract the newly synthesized ECM, providing the tensile strength required for the support function of the dermis (Clark, 1985). Thus, understanding the mechanism underlying the ability of fibroblasts to adhere to ECM components such as fibronectin is a necessary prerequisite to understanding how the new dermis and other connective tissues are being synthesized.
Connective tissue growth factor (CCN2), a member of the CCN family of matricellular proteins (Bork, 1993; Lau and Lam, 1999; Moussad and Brigstock, 2000; Perbal, 2001; Leask and Abraham, 2003), is a cysteine-rich protein that is secreted via a 37-amino acid signal sequence (Chen et al., 2001b). Secondary sequence structure predictions have suggested that CCN2 comprises four modules sharing identity with insulin-like growth factor binding proteins, Von Willebrand factor, thrombospondin domain, and a cysteine knot-containing family of growth regulators (Bork, 1993; Lau and Lam, 1999; Moussad and Brigstock, 2000; Perbal, 2001). CCN2 is expressed by mesenchymal cells during development (Ivkovic et al., 2003). In adult mammals, CCN2 is not normally expressed in skin fibroblasts unless induced, such as in response to wounding or injury (Igarashi et al., 1993; Leask and Abraham, 2003; Leask et al., 2004). One of the most potent inducers of CCN2 is transforming growth factor β (TGFβ), which promotes CCN2 expression in dermal fibroblasts, but not in epidermal keratinocytes, through a complex network of transcriptional interactions, requiring Smads, protein kinase C, and ras/mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK), and a consensus transcription enhancer factor binding element in the CCN2 promoter (Holmes et al., 2001; Chen et al., 2002; Stratton et al., 2002; Leask et al., 2003). In contrast to the situation in normal dermal fibroblasts, CCN2 is constitutively expressed by fibroblasts present in fibrotic lesions (Igarashi et al., 1995; Abraham et al., 2000; Holmes et al., 2003; Leask and Abraham, 2003; Leask et al., 2004). Because CCN2 has profibrotic activity in vivo (Mori et al., 1999), CCN2 has been proposed to be a key mediator of the profibrotic effects of TGFβ (Grotendorst, 1997; Mori et al., 1999; Leask et al., 2004; Leask and Abraham, 2004).
Although CCN2 was initially identified over a decade ago, the precise physiological role of CCN2 is not clear. The recent generation of Ccn2 -/- mice has been a key initial step in the identification of the function of CCN2 in vivo (Ivkovic et al., 2003). Ccn2 -/- mice die soon after birth primarily due to an inability of the rib cage to properly ossify (Ivkovic et al., 2003). Ccn2 -/- mice also display decreased expression of extracellular matrix components and matrix metalloproteinases, suggesting that matrix remodeling is also defective in these animals (Ivkovic et al., 2003). However, the precise effect(s) of removing CCN2 on connective tissue biology and in isolated fibroblasts has not yet been investigated.
As might be expected of a secreted protein, exogenous CCN2 possesses activity. One activity of CCN2 revolves around the ability to promote cell adhesion through the integrins (Babic et al., 1999) and cell surface heparin sulfate proteoglycans (HSPGs) (Babic et al., 1999; Chen et al., 2001a; Schober et al., 2002; Gao and Brigstock, 2004). Recently, it was shown that the 10-kDa heparin-binding carboxyl-terminal domain of human CCN2, previously shown to promote cell proliferation (Brigstock et al., 1997), was sufficient to promote fibroblast cell adhesion (Ball et al., 2003). However, two key issues remain. First, the signal transduction cascades required for CCN2-promoted adhesion are unknown. Second, and perhaps more important, the precise biological relevance of CCN2-mediated cell attachment is unclear.
In this report, we explore the biological function of CCN2-promoted fibroblast adhesion. We address two crucial outstanding questions concerning CCN-mediated fibroblast adhesion. First, we investigate the downstream signaling pathways required for the ability of CCN2 to promote fibroblast adhesion. Second, we use Ccn2-/- embryonic fibroblasts to evaluate the biological significance of CCN2-promoted fibroblast adhesion to ECM. Our results provide new insights into the mechanism of action and function of CCN2 action in fibroblast biology and tissue repair.
MATERIALS AND METHODS
Isolation of Recombinant CCN2
A 1073-base pair CCN2 cDNA fragment was amplified (from a plasmid provided by Dr. Gary Grotendorst, University of Miami, Miami, FL) by polymerase chain reaction (PCR) with Pfu polymerase (New England Biolabs, Beverley, MA). Primer sets were designed such that a 24-base pair sequence encoding the widely used FLAG peptide was inserted at the 3′ end of the CCN2 cDNA. Insertion of the FLAG tag enables the purification to homogeneity of a single fusion protein without generally interfering with protein function (Einhauer and Jungbauer, 2001). This blunt-ended PCR fragment was subcloned into the plasmid pCR4Blunt-TOPO (Invitrogen, Paisley, United Kingdom). The insert encoding of CCN2-FLAG was excised with EcoRI and subcloned into the EcoRI site of pcDNA3.1 (Invitrogen), yielding pcDNA3-CCN2-FLAG. The orientation of CCN2-FLAG within this plasmid was confirmed by sequencing and restriction enzyme digestion.
To generate recombinant CCN2, human embryonic kidney (HEK) 293 or NIH 3T3 cells (American Type Culture Collection, Manassas, VA) were transfected with pcDNA3-CCN2-FLAG by the calcium phosphate method (BD Biosciences Clontech, Palo Alto, CA). After 2 d of incubation, cells were harvested, washed once with phosphate-buffered saline (PBS), and suspended in PBS supplemented with 0.1% Triton X-100. Cell lysis was performed by repeated freezing and thawing. The crude lysates were centrifuged at 20,200 × g 4°C for 15 min. The FLAG-tagged proteins from the supernatants were precleared with Sepharose and bound overnight to anti-FLAG M2 affinity gel (Sigma-Aldrich, St. Louis, MO) by rotating at 4°C. Beads with immobilized protein were then washed four times with Tris-buffered saline (TBS), eluted overnight by competition with 1 ml of TBS containing 100 μg/ml FLAG peptides (Sigma-Aldrich), and dialyzed against 20 mM HEPES, pH 7.4. The purity was checked by SDS-PAGE followed by silver staining of the gel (our unpublished data).
Cell Culture and Adhesion Assay
Normal adult human dermal fibroblasts were isolated and cultured as described previously (Abraham et al., 2000). Mouse embryonic fibroblasts were isolated and cultured as described previously (Ivkovic et al., 2003). Adhesion assays were performed by coating wells of 96-well plates overnight, at 4°C, with 10 μg/ml CCN2 (Babic et al., 1999) or 10 μg/ml fibronectin (Sigma-Aldrich), 1× PBS or 10 μg/ml bovine serum albumin in 1× PBS. Nonspecific binding sites on substrates were blocked for 1 h in 1% bovine serum albumin (BSA) in PBS at room temperature before addition of cells. Fibroblasts were cultured in DMEM 0.5% fetal calf serum for 24 h, and then were harvested with 2 mM EDTA in PBS (20 min, room temperature), washed twice with DMEM medium containing 0.5% BSA (Sigma-Aldrich), and resuspended in the same medium at 2.5 × 105 cells/ml. Where indicated, heparinase III (0.2 U/ml); chondrotinase (0.2 U/ml); heparin sulfate; heparin; chrondroitins A, B, and C sulfate (50 μg/ml; Sigma-Aldrich); the MEK/ERK inhibitor U0126 (Promega, Southampton, United Kingdom; 10 μM; Favata et al., 1998) were preincubated with cells for 45 min before plating. Cells (100 μl/well) were added to wells of the 96-well plate and incubated at 37°C for 60 min. Wells were then washed with PBS to remove nonadherent cells. The remaining adherent cells were fixed and stained with methylene blue. Fibroblast adhesion was quantified by dye extraction and measurement of absorbance at 620 nm. In some experiments, an acid phosphatase assay was used, in which adherent cells were quantified by incubation with 100 μl of substrate solution (0.1 M sodium acetate, pH 5.5, 10 mM p-nitophenylphosphate, and 0.1% Triton X-100) for 2 h at 37°C. The reaction was stopped by the addition of 15 μl of 1 N NaOH/well, and A450 was measured (Babic et al., 1999).
Immunofluorescence Analysis
Cells were permitted to adhere for defined periods of time to glass chamber slides (Nalge Nunc International, Naperville, IL) that had been coated for 18 h at 4°C by 0 μg/ml CCN2 or 10 μg/ml fibronectin or 10 μg/ml bovine serum albumin (Sigma-Aldrich) in PBS. When appropriate, cells were preincubated in the presence or absence of 50 μg/ml FLAG-CTGF in PBS for 1 h, at 4°C. Before adding cells, nonspecific binding sites on proteins were blocked with a 1-h incubation with 0.5% BSA, PBS, at 37°C. To detect phospho-ERK residues, cells were fixed in methanol (-20°C; 10 min) and subjected to staining with rabbit anti-phospho-ERK antibody (Cell Signaling Technology, Beverly, MA) and Texas Red-conjugated anti-rabbit (Jackson Immuno-Research Laboratories, West Grove, PA). To detect phosphorylated-focal adhesion kinase (FAK) or α-smooth muscle actin (SMA), cells were fixed for 10 min in 3% paraformaldehyde/PBS, blocked in 10% normal goat serum (Jackson ImmunoResearch Laboratories), and subjected to staining with either anti-α-SMA antibody (1:100; Sigma-Aldrich) or anti-phospho-FAK (1:1000; Cell Signaling Technology) antibody followed by fluorescein isothiocyanate (FITC)-conjugated anti-mouse (1:100; Jackson ImmunoResearch Laboratories) and Texas Red-conjugated anti-rabbit antibody (1:100; Jackson ImmunoResearch Laboratories), respectively. To detect F-actin, cells were similarly treated but were stained with rhodamine-phalliodin (Sigma-Aldrich). Nuclei were detected by a 10-min incubation with 4,6-diamidino-2-phenylindole (DAPI) (1 μg/ml; Molecular Probes, Eugene, OR). Cells were photographed using an Axiphot microscope and Axiovision software (Carl Zeiss, Welwyn Garden City, United Kingdom), and images were subsequently exported into Adobe Photoshop version 7.0 (Adobe Systems, San Jose, CA).
Western Blot Analysis
Fibroblasts were grown in monolayer until 80% confluent, serum starved cells for 24 h, and then removed by treatment with versene. Cells were then allowed to adhere to fibronectin as described above. After various lengths of time, cell layers were washed in PBS and harvested in 2% SDS. The resultant protein extracts were quantified (Bradford; Bio-Rad, Hercules, CA), and equal amounts of protein (20 μg) were subjected to SDS-PAGE. When indicated, recombinant CCN2 (10 ng/lane) was used as a control. Gels were electro-phoretically transferred to nitrocellulose (Invitrogen). Membrane was blocked with BLOTTO (5% nonfat dry milk in Tris-buffered saline, 0.1% Tween 20; Sigma-Aldrich), and immunoblotting was performed using anti-phospho-ERK, anti-ERK anti-FAK, or anti-phospho-FAK antibodies (Cell Signaling Technology) or anti CCN2 (Abcam, Cambridge, United Kingdom) antibody as described by the manufacturer. Blots were then developed by incubation with biotinylated anti-rabbit or mouse antibodies (1:1000; Vector Laboratories, Burlingame, CA) as secondary antibodies, followed by incubation with ABC regent (Vector Laboratories). Signal was detected using a luminescence kit (ECL kit; Amersham Biosciences UK, Little Chalfont, Buckinghamshire, United Kingdom) and x-ray film. Densitometry was performed using Gel Base/Gel-Blot Pro (Synoptics, Cambridge, United Kingdom).
Immunoprecipitation Assay
Cells were cultured for 24 h in DMEM, 0.5% fetal bovine serum (FBS), and membrane preparations were isolated (Chen et al., 2001b). Equal amounts of protein were subjected to immunoprecipitation analysis with rabbit anti-CCN2 antibody (Abcam) or mouse IgG (Sigma-Aldrich) by using protein G-Sepharose (Pharmacia, Amersham, United Kingdom) as described previously (Holmes et al., 2001). Protein was detected using anti-CCN2 (Abcam), anti-α5 integrin, anti-β1 integrin, anti-α4 integrin (Zymed Laboratories, South San Francisco, CA) anti-α6 integrin anti-endoglin (all from BD Biosciences), anti-syndecan 4, and anti-syndecan 2 antibodies (Zymed Laboratories). Blots were then developed by incubation with biotinylated anti-rabbit or mouse antibodies (1:1000; Vector Laboratories) as secondary antibodies, followed by incubation with ABC regent (Vector Laboratories). Signal was detected using a luminescence kit (ECL kit; Amersham Biosciences UK) and x-ray film.
125I-CCN2 Binding Assay
Recombinant CCN2 was iodinated with 125I (Amersham Biosciences UK) as described previously (Segarini et al., 2001). Wells of a 96-well plate were coated overnight at 4°C with PBS, 50 μg/ml BSA suspended in PBS, or 50 μg/ml fibronectin suspended in PBS. Wells were blocked in 1% BSA/PBS for 1 h at room temperature and then 125I-CCN2 (500,000 cpm/ml) was added for 1 h at room temperature. Unbound 125I-CCN2 was then removed by washing three times with 1% BSA/PBS. Remaining bound 125I-CCN2 was measured using a gamma counter (N = 6).
RESULTS
The Ability of CCN2 to Bind to the Cell Surface of Adult Human Dermal Fibroblasts Is HSPG Dependent
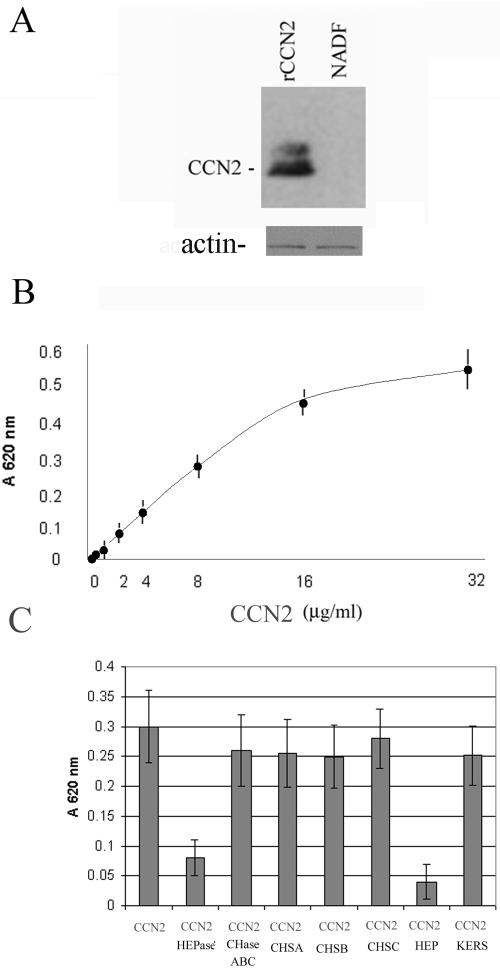
Normal dermal fibroblasts cultured in 0.5% serum express little appreciable CCN2 expression (Abraham et al., 2000; Leask et al., 2003). Thus, these cells provide a useful model system to assess the contribution of exogenously added CCN2 to fibroblast biology without the complication of having to consider the effects of endogenous CCN2. When adsorbed to tissue culture plates, CCN2 promotes adhesion of a variety of cell types (Babic et al., 1999; Chen et al., 2001a). However, the signal transduction mechanisms required for CCN2-mediated cell adhesion are not known. To begin to explore the signaling requirements involved with the ability of CCN2 to promote adhesion of primary adult dermal fibroblasts, we bound varying amounts of CCN2 to tissue culture plates. After blocking nonspecific binding sites on CCN2 with BSA, we added fibroblasts that had been cultured in 0.5% serum that had been detached from tissue culture plates with EDTA. After cells had been allowed to adhere to CCN2 for 40 min, the tissue culture plates were washed to remove unbound cells. Western blot analysis of cell lysates prepared from fibroblasts adhered to CCN2 showed that normal dermal fibroblasts cultured in 0.5% serum and allowed to adhere to CCN2 for 40 min did not show appreciable CCN2 expression (Figure 1A). In addition, we found that CCN2, when coated on 96-well plates, dose dependently promoted adhesion of normal adult dermal fibroblasts (Figure 1B). The ability of CCN2 to promote fibroblast adhesion was dependent on cell surface HSPGs as a 45-min preincubation of cells with heparinase or heparin sulfate, but not keratin sulfate, chondroitinase, or chondrotin A, B, or C, blocked the ability of cells to adhere to CCN2 (Figure 1C). Thus, adhesion of adult human dermal fibroblasts to CCN2 was HSPG dependent.
Figure 1.
Adhesion of normal adult human dermal fibroblasts to CCN2 is HSPG dependent. (A) Normal adult human dermal fibroblasts (NADF) show little appreciable CCN2 expression. Primary normal adult dermal fibroblasts were cultured in DMEM/0.5% FBS for 24 h, detached with EDTA, and allowed to adhere to CCN2 that had been coated on a tissue culture plate. After 40 min, attached cells were lysed, and the resultant protein extracts were subjected to Western blot analysis with an anti-CCN2 antibody. NADF, normal adult dermal fibroblasts (20 μg/lane). rCCN2, 10 ng of recombinant CCN2 (10 ng/lane). (B) CCN2 promotes fibroblast adhesion in a dose-dependent manner. Wells of a 96-well plate were incubated overnight with BSA (10 μg/ml) or different concentrations of CCN2, as indicated. After a blocking step, primary adult dermal fibroblasts were allowed to adhere to CCN2 for 40 min, and cell adhesion was assessed as described in Materials and Methods (6 wells/data point; average [closed black dot] ± SEM [error bar] is shown). The values obtained from the BSA control experiment, which were not significantly above background, were subtracted from the experimental values. (C) Adhesion of fibroblasts to CCN2 is dependent on cell surface heparin-containing proteoglycans. Adhesion assays were repeated, but cells were incubated with or without heparinase (HEPase) or chondrotinase ABC (CHaseABC), or soluble chondrotinase A, B, or C, keratin sulfate, or heparin (50 μg/ml; 6 wells/data point; average [gray box] ± SEM [error bar] are shown).
Attachment of Adult Human Dermal Fibroblasts to CCN2 Results in Cell Adhesion and ERK Phosphorylation, but Not the Presence of Filopodia or the Induction of Focal Adhesions
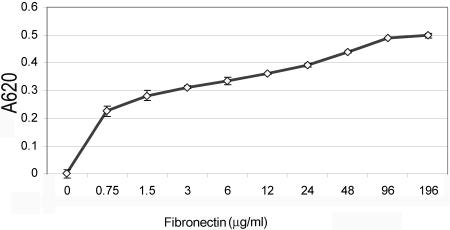
To begin to probe the physiological relevance of CCN2-promoted adhesion, we compared the adhesion of adult dermal fibroblasts to CCN2 and fibronectin. To confirm that adult human dermal fibroblasts were capable of binding fibronectin, we performed adhesion assays, identical to those described above, except that cells were allowed to adhere for 40 min to increasing amounts of fibronectin. We showed that fibronectin promoted fibroblast attachment in a dose-dependent manner (Figure 2). To compare the abilities of CCN2 and fibronectin to promote fibroblast attachment, we coated tissue culture plates with 10 μg/ml CCN2 or 10 μg/ml fibronectin, and allowed suspended normal adult dermal fibroblasts to adhere to substrata for either 40 or 120 min. After removing nonadherent cells, attached cells were fixed in 4% paraformaldehyde and examined cells under a light microscope. Cells also were stained with phalliodin to detect F-actin (Figure 3, actin), anti-phospho-focal adhesion kinase (Figure 3, p-FAK), and anti-phospho-p42/p44 (Figure 3, p-ERK) antibodies. Fibroblasts adhered to CCN2 for 40 min were attached but filopodia did not form (Figure 3A, CCN2). By 120 min after attachment, fibroblasts adhered to CCN2 displayed lamellopodia (Figure 3B, CCN2, actin, arrow with l). Conversely, normal adult human dermal fibroblasts adhered to fibronectin for either 40 or 120 min displayed filipodial extensions (Figure 3A, FN, actin, arrow with f). In addition, cells adhered to fibronectin, but not to CCN2, showed formation of actin stress fibers (denoted by an arrow in Figure 3A, FN, actin), and induction of focal adhesions as visualized by punctuate cell surface anti-phospho-FAK staining (denoted by an arrow in Figure 3A, FN p-FAK). These results are consistent with the known involvement of integrin-mediated FAK phosphorylation in fibronectin-mediated fibroblast adhesion (Zamir and Geiger, 2001).
Figure 2.
Fibronectin promotes adhesion of normal adult dermal fibroblasts in a dose-dependent manner. Wells of a 96-well plate were incubated overnight with different concentrations of fibronectin, as indicated. After a blocking step, primary adult dermal fibroblasts were allowed to adhere to fibronectin for 40 min, and cell adhesion was assessed as described in Materials and Methods (3 wells/data point; average [open diamond] ± SEM [error bar] are shown). The values obtained from a control adhesion experiment with BSA, which were not statistically significantly above background, were subtracted from the experimental values.
Figure 3.
Comparison of adult dermal fibroblast adhesion to CCN2 and fibronectin. Staining of cells adhered to CCN2 and fibronectin with phalloidin (ACTIN), anti-phospho-FAK (p-FAK), and anti-phospho-ERK (p-ERK) antibodies are shown. Fibroblasts were allowed to adhere to substrates, as described in Materials and Methods, for (A) 40 or (B) 120 min before fixation of cells in paraformaldehyde. (A) Fibroblasts adhered to fibronectin for 40 min showed extension of filopodia (arrow with f, actin), actin stress fibers, and punctuate phospho-FAK (arrow, p-FAK, FN) and phospho-ERK staining, consistent with the induction of focal adhesions. Conversely, fibroblasts adhered to CCN2 did not show extended filopodia, displayed disorganized actin staining, and did not show clustered, localized phospho-FAK staining (400×). Fibroblasts adhered to CCN2 and fibronectin showed punctuate foci of phospho-ERK staining (arrow, CCN2, FN, p-ERK), consistent with an induction of cellular signaling in fibroblasts adhered to both substrates. Conversely, cells adhered to BSA showed no phospho-ERK induction. Nuclei were detected by DAPI staining (blue).
Fibroblasts adhered to both fibronectin and CCN2 for 40 min showed induction of cellular signaling as visualized by punctuate phospho-ERK staining (denoted by arrows, Figure 3 FN and CCN2, p-ERK). Western blot analysis of lysates prepared from fibroblasts adhered to CCN2 verified that adhesion of fibroblasts to CCN2 resulted in ERK phosphorylation (Figure 4). The very small number of cells (>100-fold fewer than those adhering to CCN2 or FN) nonspecifically binding BSA showed no induction of phospho-ERK staining (Figure 3A, BSA, p-ERK). Overall, our data suggest that normal adult human dermal fibroblast adhesion to CCN2 and fibronectin seemed to be fundamentally distinct, in that adhesion of normal adult human dermal fibroblasts onto CCN2 does not result in the extension of filopodia, the induction of focal adhesions, or the formation of actin stress fibers.
Figure 4.

Adhesion of adult dermal fibroblasts to fibronectin results in ERK phosphorylation. Normal adult dermal fibroblasts were cultured and detached as described in Materials and Methods and in the legend for Figure 1. Fibroblasts were allowed to adhere to six-well plates that had been coated with CCN2 (10 μg/ml) as described above. Protein extracts were prepared after adhesion at the times indicated. The resultant protein extracts (20 μg/lane) were subjected to SDS-PAGE and Western blot analysis with anti-ERK and anti-phospho-ERK antibodies. Densitometry was performed on the resultant autoradiograms, and ratio of phosphorylated/non-phosphorylated ERK is shown.
Adult Human Dermal Fibroblast Attachment to CCN2 Requires MEK/ERK Activity
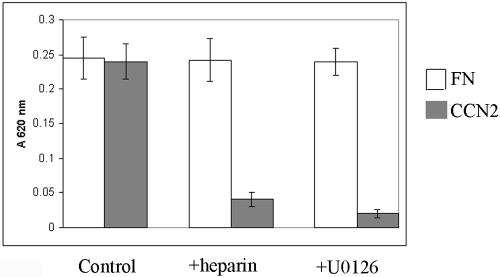
Because fibroblast attachment to both CCN2 and fibronectin resulted in the induction of the MEK/ERK pathway, we decided to compare the requirements for the MEK/ERK pathway in CCN2- and fibronectin-promoted fibroblast adhesion. Thus, we treated fibroblasts for 45 min with or without the MEK1/2 inhibitor U1026 (10 μM; Favata et al., 1998) before performing adhesion assays on fibronectin or CCN2. We found that preincubation with U0126 completely blocked the ability of CCN2 to promote attachment of adult fibroblasts (Figure 5). Conversely, U0126 did not appreciably affect the ability of adult human dermal fibroblasts to adhere to fibronectin (Figure 5). Because we had previously shown that the ability of CCN2 to promote attachment of adult dermal fibroblasts was HSPG dependent, we determined whether soluble heparin affected fibroblast attachment to fibronectin. We found that whereas, as shown above, soluble heparin blocked the ability of adult fibroblasts to adhere to CCN2, soluble heparin did not appreciably impact the number of fibroblasts that adhered to fibronectin (Figure 5). These data support the notion that attachment of adult fibroblasts to CCN2 and fibronectin possess different requirements, in that attachment of adult fibroblasts to CCN2, but not fibronectin, required both MEK/ERK activity and HSPGs.
Figure 5.
Adhesion of adult dermal fibroblasts to CCN2, but not fibronectin, requires the MEK/ERK pathway. Fibroblasts were allowed to adhere for 40 min onto fibronectin (FN), CCN2, or BSA. Before performing the adhesion assay, suspended fibroblasts were preincubated, as indicated, in the presence or absence of soluble heparin (50 μg/ml) or the MEK1/2 inhibitor U0126 (10 μM). After a washing step, adherent cells were quantified as described in Materials and Methods. Assays were performed in 96-well plates, each condition, in triplicate, two independent times (N = 6). Average optical density values ± SEM are shown.
CCN2 Binds Fibronectin, α4 α5, and β1 Integrins and Syndecan-4
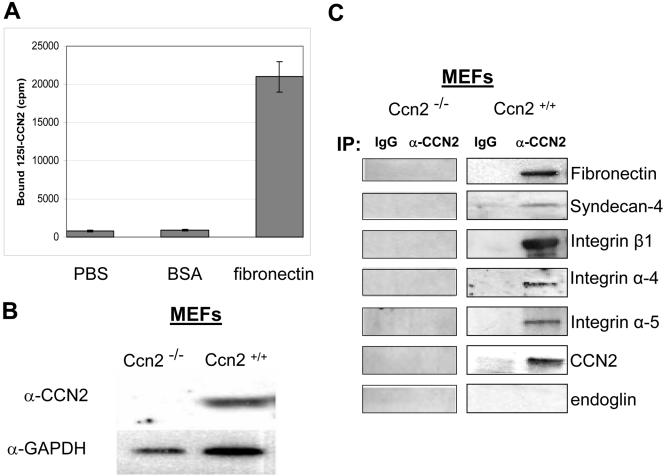
Although it has been previously shown that CCN2 promotes fibroblast adhesion, the functional relevance of this activity has not yet been clarified. CCN2 has been proposed to modulate the ability of receptors to bind ligands (Abreu at al., 2002; Leask and Abraham, 2003). Thus, we hypothesized that the biological significance of CCN2-promoted fibroblast adhesion might be to enhance the ability of fibroblasts to interact with extracellular matrix components through cell surface receptors. To begin to test this hypothesis, we reasoned that CCN2 might bind the extracellular matrix component fibronectin. We coated wells of a 96-well tissue culture plate with BSA or fibronectin overnight, and, after a blocking step, added 125I-labeled CCN2 for 1 h. After unbound 125I-CCN2 was removed by washing, 125I-CCN2 bound to fibronectin was detected using a gamma counter. We found that 125I-CCN2 bound fibronectin, but not BSA (Figure 6A). These results were consistent with the notion that a physiological role of CCN2 might to enhance the ability of fibroblasts to interact with fibronectin.
Figure 6.
CCN2 binds fibronectin and the fibronectin receptors integrin α4, integrin α5, integrin β1, and syndecan-4. (A) 125I-CCN2 binds fibronectin. Wells of a 96 well plate were coated overnight at 4°C with 50 μg/ml fibronectin or 1% BSA in PBS or PBS and blocked for 1 h at room temperature with 1% BSA in PBS. 125I-CCN2 (500,000 cpm/well) was added for 1 h, at room temperature. Wells were washed three times with 1% BSA in PBS and bound 125I-CCN2 was measured with a scintillation counter. Values represent average ± SEM (N = 6). (B) Protein extracts (20 μg) from Ccn2+/+ and Ccn2-/- mouse embryonic fibroblasts (MEF) were subjected to Western blot analysis with an anti-CCN2 antibody as described in Materials and Methods. (C) Membrane preparations from Ccn2+/+ and Ccn2-/- mouse embryonic fibroblasts were immunoprecipated using protein A-Sepharose with rabbit anti-CCN2 antibody or rabbit IgG. Immunoprecipitates were subjected to Western blot analysis with anti-CCN2, anti-syndecan 4, anti-fibronectin, anti-endoglin, and anti-integrin α4, α5, and β1 antibodies, as indicated.
To continue our investigation into the role of CCN2-mediated adhesion to fibroblast biology, we cultured mouse embryonic fibroblasts, which constitutively express CCN2 (Holmes et al., 2001; Figure 6B) and thus can be used as a basis to probe the function of endogenous CCN2. We reasoned that if a function of CCN2 is to potentiate the ability of fibroblasts to adhere to fibronectin, endogenous cell-surface associated CCN2 might be in a complex with fibronectin and cell-surface fibronectin receptors. We cultured mouse embryonic fibroblasts to confluence and isolated membrane preparations from these cells. The resultant protein preparations were subjected to immunoprecipitation analysis with an anti-CCN2 antibody. The immunoprecipitated protein was then subjected to Western blot analysis with antibodies to fibronectin and the fibronectin receptors integrin α4, integrin β1, integrin α5, and syndecan 4. The latter protein is an HSPG that acts with integrin α5β1 to promote cell spreading on fibronectin (Saoncella et al., 1999). We found that endogenous membrane-bound CCN2 was present in a complex with fibronectin, and the fibronectin receptors syndecan 4 and integrin α4, integrin β1, and integrin α5 (Figure 6C). CCN2 did not interact with the protein endoglin (Figure 6C), an accessory TGFβ receptor (Leask et al., 2002). This latter suggests that CCN2 is not linked to TGFβ signaling via endoglin. CCN2 also was present in a complex containing syndecan 2 and integrin α6 (our unpublished data), consistent with the notion that CCN2 can interact with a variety of integrins and HSPGs (Gao and Brigstock, 2003; Leu et al., 2003; Leask and Abraham, 2003; Gao et al., 2004).
Ccn2-/- Fibroblasts Show Impaired Spreading on Fibronectin
Our immunoprecipitation results were consistent with the hypothesis that a physiological role of CCN2 might be to potentiate the ability of cell surface membrane proteins to adhere to fibronectin. Consequently, we wished to test the effect of the loss of CCN2 on fibroblast attachment and spreading on fibronectin, by comparing the ability of Ccn2+/+ and Ccn2-/- mouse embryonic fibroblasts to adhere to fibronectin. That Ccn2-/- mouse embryonic fibroblasts lacked CCN2 expression was confirmed by Western blot analysis (Figure 6, B and C). Due to culture conditions, fibroblasts can often become spontaneously differentiated into myofibroblasts, as visualized by α-SMA expression (Masur et al., 1996). Myofibroblasts mediate matrix contraction during wound healing and fibrosis (Gabbiani, 2003; Shi-wen et al., 2004). Indeed, immunofluorescence analysis showed that ∼90% of Ccn2+/+ and Ccn2-/- fibroblasts had differentiated into myofibroblasts, as visualized by expression of α-SMA (our unpublished data). Because we were interested in exploring the contribution CCN2 may play in wound healing and fibrosis, we wanted to evaluate the impact of loss of CCN2 on the induction of α-SMA stress fibers upon cell attachment and spreading on fibronectin.
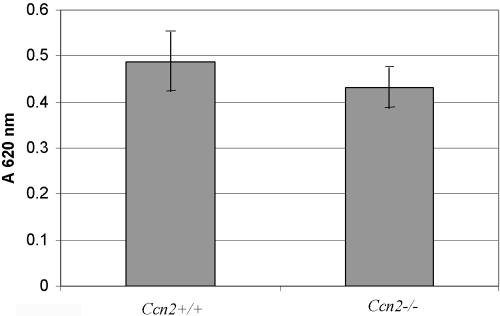
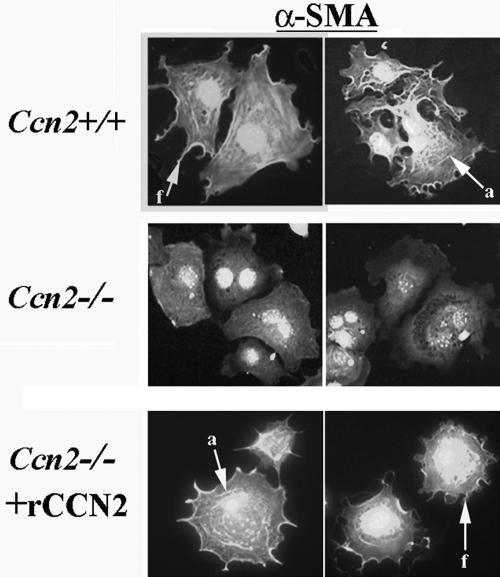
First, to detect whether both Ccn2+/+ and Ccn2-/- mouse embryonic fibroblasts showed similar levels of CCN2 receptors, we compared the ability of Ccn2+/+ and Ccn2-/- mouse embryonic fibroblasts to adhere to CCN2. We found that Ccn2+/+ and Ccn2-/- mouse myofibroblasts showed similar abilities to bind to CCN2 (Figure 7). To compare the abilities of Ccn2+/+ and Ccn2-/- mouse embryonic fibroblasts to induce α-SMA stress fibers, Ccn2+/+ and Ccn2-/- mouse embryonic fibroblasts were allowed to adhere to fibronectin for 40 min. Cells were then fixed with paraformaldehyde and subjected to indirect immunofluorescence with an anti-α-SMA antibody. After 40 min of attachment, Ccn2+/+ fibroblasts showed extensive spreading, extension of filopodia, and α-SMA localization at the cell periphery (denoted by an arrow with f, Figure 8, Ccn2+/+). A few stress fibers were observed (denoted by an arrow with a, Figure 8, Ccn2+/+). Ccn2+/+ fibroblasts showed a few α-SMA stress fibers after 40 min of attachment on fibronectin. Conversely, 40 min after attachment on fibronectin, Ccn2-/- fibroblasts showed neither extensive filopodia nor organization of α-SMA along the cell periphery or into stress fibers (Figure 8, Ccn2-/-). To assess whether the spreading defect displayed by Ccn2-/- fibroblasts could be rescued by recombinant CCN2, we subjected Ccn2-/- fibroblasts to a 1-h preincubation of Ccn2-/- fibroblasts at 4°C prior with recombinant CCN2. We then placed cells on fibronectin and examined the appearance of Ccn2-/- fibroblasts 40 min after attachment. We found that preincubating Ccn2-/- fibroblasts with recombinant CCN2 at least partially reversed the spreading defect of Ccn2-/- fibroblasts, because Ccn2-/- fibroblasts pretreated with CCN2 showed both α-SMA localization along the cell periphery and filipodial extensions (denoted by arrow with f, Figure 8, Ccn2-/- + CCN2), and a few stress fibers (denoted by an arrow with a, Figure 8, Ccn2-/- + CCN2).
Figure 7.
Comparison of the adhesive abilities of Ccn2+/+ and Ccn2-/- mouse embryonic fibroblasts on CCN2. Ccn2+/+ and Ccn2-/- mouse embryonic fibroblasts were cultured in DMEM, 0.5% FBS for 24 h; detached in EDTA; and allowed to adhere to CCN2 that had been adsorbed to 96-well plates as described in Materials and Methods and the legend to Figure 1. Cell adhesion after 40 min was determined (N = 6; average ± SEM is shown) as described in Materials and Methods and in the legend to Figure 1B.
Figure 8.
Ccn2-/- mouse embryonic fibroblasts show impaired spreading on fibronectin. Ccn2+/+ and Ccn2-/- mouse embryonic fibroblasts (MEFs) were allowed to adhere to fibronectin for 40 min as described in Materials and Methods. In addition, Ccn2-/- fibroblasts were preincubated with rCCN2 for 1 h before adhesion to fibronectin. Cells were fixed in paraformaldehyde and subjected to indirect immunofluorescence analysis with anti-α-SMA antibody and detected with an appropriate FITC-conjugated antibody as described in Materials and Methods and in the legend to Figure 1C. Forty minutes postadhesion, Ccn2+/+ MEFs adhered to fibronectin show extension of filopodia and α-SMA localization to the cell periphery (arrow with f, Ccn2+/+) and display a few stress fibers (arrow with a, Ccn2+/+). Conversely, Ccn2-/- MEFs do not display filopodia, α-SMA localization to the cell periphery, or stress fibers (Ccn2-/-). Ccn2-/- fibroblasts that were preincubated with rCCN2 for 1 h before adhesion to fibronectin showed extension of filopodia and α-SMA localization to the cell periphery (arrow with s, Ccn2-/-) and display a few stress fibers (arrow with a, Ccn2-/- + rCCN2). Nuclei were detected by DAPI staining (blue).
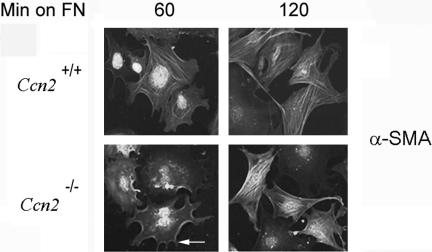
To investigate whether spreading of Ctgf+/+ mouse embryonic fibroblasts on fibronectin absolutely required CCN2, we monitored the ability of Ctgf+/+ and Ctgf-/- mouse embryonic fibroblasts for up to 120 min. After 60 min of attachment, Ccn2+/+ fibroblasts began to show extensive α-SMA stress fiber networks (Figure 9, Ccn2+/+, 60). Intriguingly, after 60 min of attachment, Ccn2-/- fibroblasts showed a phenotype similar to that displayed by Ccn2+/+ fibroblasts attached to fibronectin for 40 min, in that Ccn2-/- fibroblasts attached to fibronectin for 60 min possessed both filopodia, and localization of α-SMA along the cell periphery (denoted by an arrow, Figure 9, Ccn2-/-, 60). Intriguingly, after 2 h of adhesion to fibronectin, Ccn2+/+ and Ccn2-/- fibroblasts showed a similar phenotype; both cell types possessed similar filopodia extensions and α-SMA staining patterns (Figure 9, FN, Ccn2+/+ and Ccn2-/-). Collectively, our results are consistent with the hypothesis that CCN2 potentiates, but is not required for, the ability of embryonic fibroblasts to spread on fibronectin.
Figure 9.
Ccn2-/- mouse embryonic fibroblasts show delayed induction of α-SMA stress fibers. Ccn2+/+ and Ccn2-/- mouse embryonic fibroblasts (MEFs) were allowed to adhere to fibronectin for 60 and 120 min (min on FN) as described in Materials and Methods. Sixty minutes postadhesion, Ccn2+/+ MEFs adhered to fibronectin show stress fiber formation (Ccn+/+, 60). Conversely, 60 minutes postadhesion, Ccn2-/- MEFs adhered to fibronectin show extension of filopodia and α-SMA localization to the cell periphery not display abundant stress fiber formation (arrow, Ccn2-/-, 60). Two hours postadhesion, Ccn2+/+ and Ccn2+/+ MEFs show similar phenotypes, as visualized by extension of filopodia and formation of α-SMA stress fiber networks. (Ccn2+/+, and Ccn2-/-, 120). Nuclei were detected by DAPI staining (blue).
Ccn2-/- Fibroblasts Show Impaired Phosphorylation of FAK and ERK
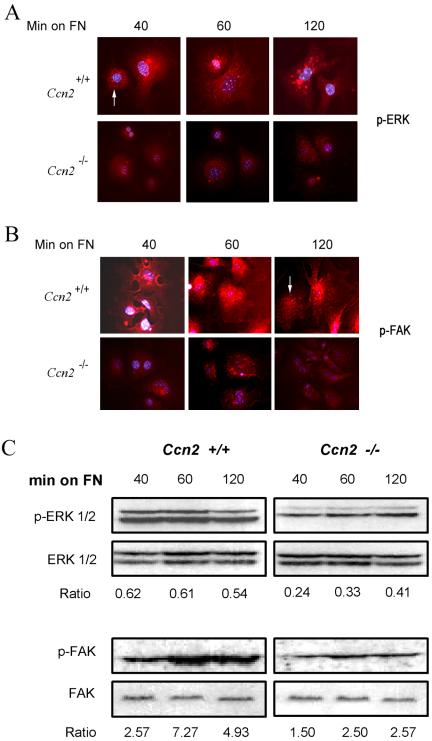
To assess the potential contribution of CCN2 to mediate cell signaling resulting from spreading on fibronectin, we allowed Ccn2+/+ and Ccn2-/- myofibroblasts to adhere to fibronectin for up to 2 h. Cells were fixed and stained with anti-phospho-FAK and anti-phospho-ERK antibodies. By immunofluorescence analysis, Ccn2-/- fibroblasts showed substantially reduced levels of FAK and ERK phosphorylation (Figure 10, A and B). To confirm these results, we incubated Ccn2+/+ and Ccn2-/- embryonic fibroblasts for up to 2 h on fibronectin and subjected cell lysates to Western blot analysis with anti-phospho-FAK and anti-phospho-ERK antibodies. Giving support to our immunofluorescence data, we found that although Ccn2 -/- fibroblasts displayed some induction of FAK and ERK phosphorylation, we showed that Ccn2-/- fibroblasts showed greatly impaired FAK and ERK phosphorylation relative to Ccn2+/+ fibroblasts (Figure 10C). Thus, CCN2 was required for maximal induction of FAK and ERK phosphorylation upon the adhesion of Ccn2+/+ mouse embryonic fibroblasts to fibronectin.
Figure 10.
CCN2-/- mouse embryonic fibroblasts show impaired phosphorylation of FAK and ERK. Ccn2+/+ and Ccn2-/- mouse embryonic fibroblasts were allowed to adhere to fibronectin (FN), as described in Materials and Methods, for up to 120 min (min on FN). (A and B) Indirect immunofluorescence analysis. Cells were fixed as described in Materials and Methods and in the legend to Figure 7 and were stained with anti-phospho-ERK (A) and anti-phospho-FAK (B) antibodies. Whereas Ccn2+/+ MEFs showed abundant phospho-ERK (arrow, A, Ccn2+/+ 40) and phospho-FAK (arrow, Ccn2+/+ 120) staining, Ccn2-/- MEFs showed markedly reduced phospho-ERK and phospho-FAK staining (A and B, Ccn2-/-). Nuclei were detected by DAPI staining (blue). (C) Western blot analysis. Experiments were performed as described above, except cell layers were harvested at different time points postadhesion (min on FN), and equal amounts of protein were subjected to Western blot analysis with anti-phospho-FAK and anti-FAK antibodies or anti-phospho-ERK and ERK antibodies, as indicated. Densitomery values showing ratio between phosphorylated epitopes and total protein, are indicated.
Soluble Heparin and U0126 Impede Fibroblast Spreading on Fibronectin
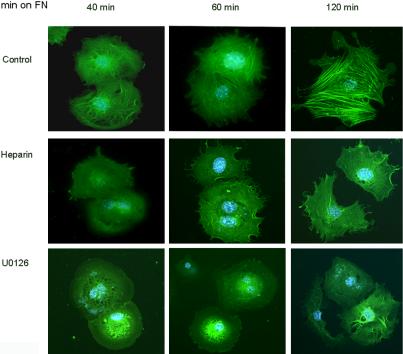
Because we had shown that fibroblast adhesion to CCN2 was blocked by soluble heparin and U0126, we tested the impact of adding soluble heparin or U1026 to Ccn2+/+ mouse embryonic fibroblasts before placing cells on fibronectin. We found that heparin or U0126 potently impeded the ability of Ccn2+/+ fibroblasts to spread cells on fibronectin. Even 120 min after adding Ccn2+/+ fibroblasts to fibronectin, cells pretreated with U1026 or soluble heparin showed markedly reduced numbers of both filopodia and α-SMA stress fibers (Figure 11). Intriguingly, heparin or U0126 did not affect the ability of cells to attach to fibronectin (our unpublished data). Our results showed that the effect on cell spreading of adding soluble heparin or U0126 to Ccn2+/+ fibroblasts was more severe than the spreading phenotype displayed by Ccn2-/- fibroblasts. Collectively, these results are consistent with the notion that the ability of CCN2 to interact with HSPGs and induce the MEK/ERK cascade is not absolutely required for fibroblast spreading or actin stress fiber induction. Rather, these results strongly suggest that signaling through CCN2 results in the enhancement of spreading of Ccn2+/+ mouse embryonic fibroblasts on fibronectin. Our results support the hypothesis that the biological significance of CCN2-promoted fibroblast adhesion is to enhance the ability of fibroblasts to interact with the matrix component fibronectin through cell surface receptors.
Figure 11.
Soluble heparin and U0126 impede the ability of Ccn2+/+ mouse embryonic fibroblasts to spread on fibronectin. Experiments were performed essentially as described in the legend to Figure 9. Ccn2+/+ mouse embryonic fibroblasts were allowed to adhere to fibronectin for up to 120 min (min on FN) as described in Materials and Methods, in the presence of soluble heparin (50 μg/ml) or U0126 (10 μM). Cells were fixed after 45 min and subjected to indirect immunofluorescence analysis with an anti-α-SMA antibody and an appropriate FITC-conjugated secondary antibody. Nuclei were detected by DAPI staining (blue).
DISCUSSION
Although CCN2 was discovered over a decade ago, the precise biological function of CCN2 remains elusive. When coated on glass or plastic, CCN2 is to promote cell attachment. However, the physiological significance of this activity has not been previously elucidated. In this report, we uncover several novel findings. First, we show that inhibition of the MEK/ERK cascade prevents CCN2-promoted fibroblast adhesion. Second, we show that CCN2 binds fibronectin and fibronectin receptors. Third, we show that endogenous CCN2 potentiates the cell adhesive properties of fibronectin. A novel implication of our results is that a biological role of CCN2 might be to promote cell attachment to fibronectin. In development, CCN2 is expressed during tissue remodeling (Ivkovic et al., 2003). Similarly, in adults, although CCN2 is not normally expressed by adult dermal fibroblasts, CCN2 is potently induced in this cell type during normal tissue repair and fibrotic disease (Igarashi et al., 1993, 1995; Shi-wen et al., 2000; Leask and Abraham, 2003; Leask et al., 2004). Accordingly, we hypothesize that CCN2 may promote tissue remodeling and repair in vivo by facilitating the adhesion of cells to the provisional matrix. This activity may be crucial for the enhanced ability of fibrotic fibroblasts to contract extracellular matrix (Shi-wen et al., 2004).
Direct adhesion of adult dermal fibroblasts to CCN2 did not resulting appreciable filopodia, FAK phosphorylation, or induction of stress fibers. However, adhesion of human foreskin fibroblasts to CCN2 was recently shown to result in extension of filopodia, FAK phosphorylation, and stress fiber formation (Chen et al., 2001a). The differences in our results may have arisen due to the different cell types used for our respective studies, because fibroblasts taken from different parts of the body show different expression profiles (Chang et al., 2002). Supporting this latter notion, Chen et al. (2001a) also showed that the CCN2-related protein CCN1 (Cyr61) promoted adhesion of human foreskin fibroblasts, resulting in the extension of filopodia and FAK phosphorylation. However, CCN1 (Cyr61) prepared by the same laboratory has been shown to promote the adhesion of cells isolated from Xenopus blastulae, without resulting in the extension of filopodia (Latinkic et al., 2003). Instead, in these series of experiments CCN1 (Cyr61)-promoted adhesion of Xenopus blastulae cells resulted in the formation of lamellopodia (Latinkic et al., 2003), similar to our results obtained when we examined CCN2 (CTGF)-promoted attachment of normal adult human dermal fibroblasts. These cell type-dependent variations in responses may ultimately arise due to differences in the receptors engaged by CCN2 (and CCN1) on these particular cell types.
That CCN family members may engage different receptors depending on the cell type is supported by a series of observations suggesting that although recombinant CCN2 adsorbed to plastic promotes cell adhesion through integrins and HSPGs (Babic et al., 1999; Chen et al., 2001a; Ball et al., 2003), the actual integrins through which CCN2 mediates adhesion vary depending on the cell type (Babic et al., 1999; Chen et al., 2001a; Schober et al., 2002; Leu et al., 2003). Similarly, the HSPGs that CCN2 interacts with may vary depending on the system (this report; Gao and Brigstock, 2003; Nishida et al., 2003). The notion that CCN2 can interact with a variety of integrins and HSPGs is supported by our observations that CCN2 is present in a complex in the cell surface of fibroblasts with several different integrins and HSPGs (Figure 6). Although CCN1 has not been as extensively studied as CCN2, CCN1 may also bind a variety of integrins and HSPGs (Grzeszkiewicz et al., 2002; Schober et al., 2002). Adding further complexity to this issue, CCN2 promotes adhesion of hepatic stellate cells through integrin αvβ3 HSPGs and low-density lipoprotein-receptor related protein (Gao and Brigstock, 2003, 2004), which had previously been shown to be involved with internalization and degradation of CCN2 (Segarini et al., 2001). We hypothesize that CCN2 (or CCN1) might not promote adhesion through a specific receptor but exert its function through a variety of proadhesive cell surface receptors, albeit through specific domains in the CCN2 molecule (Brigstock et al., 1997; Ball et al., 2003; Leu et al., 2003; Gao and Brigstock, 2004).
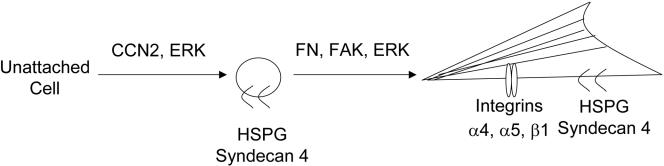
Fibronectin promotes adhesion, spreading, and attachment via integrins α5β1 and α4β1 and the HSPG coreceptor syndecan 4 (Saoncella et al., 1999; Zamir and Geiger, 2001; Wilcox-Adelman et al., 2002). Whereas the ability of fibroblasts to attach on fibronectin depends on integrins, the ability of fibroblasts to spread on fibronectin requires the heparin binding domain of fibronectin and syndecan 4 (Saoncella et al., 1999; Zamir and Geiger, 2001; Wilcox-Adelman et al., 2002). However, in this report, for the first time we show, that ERK activation is required for spreading of fibroblasts on fibronectin, and for the induction of α-SMA stress fibers. That spreading on fibronectin requires HSPGs and ERK is consistent with our hypothesis that, although CCN2 is not absolutely required for the spreading of fibroblasts and induction of α-SMA stress fibers, CCN2 potentiates the ability of fibroblasts to spread on fibronectin through ERK activation and by modulating the interaction between integrins α4, α 5, β1, syndecan 4, and fibronectin (Figure 12). We would anticipate that CCN2 might generally act by potentiating the ability of cells to adhere to other components of the extracellular matrix. Intriguingly, CCN2 has been recently shown to promote the ability of hepatic stellate cells to proliferate through ERK, suggesting that CCN2 may generally function through MEK/ERK activation (Gao et al., 2004). We have found that ERK activation may be a key component of fibrogenesis (Stratton et al., 2002). Thus, the CCN2-mediated activation of ERK may play a role in this process.
Figure 12.
Schematic diagram summarizing CCN2-promoted fibroblast adhesion. CCN2 supports cell adhesion by binding to fibronectin, cell surface proteoglycans, and integrins; potentiates the phosphorylation of FAK and ERK; and thus enhances focal adhesion formation, cell spreading, and myofibroblast formation.
It is possible that the defects in cell spreading results observed with our Ccn2-/- fibroblasts could be explained due to the fact that Ccn2-/- fibroblasts display reduced levels of receptors for CCN2 or fibronectin. However, we believe that this interpretation is unlikely because 1) Ccn2+/+ and Ccn2-/- fibroblasts show similar abilities to adhere to CCN2 and 2) a 1-h preincubation at 4°C of Ccn2-/- fibroblasts with recombinant CCN2 before allowing spreading on fibronectin at least partially rescued the spreading defect of the Ccn2-/- fibroblasts (Figure 8). Although beyond the scope of this current study, we are currently in the process of comparing the gene expression profile of Ccn2+/+ and Ccn2-/- fibroblasts. Preliminary data suggest that Ccn2-/- fibroblasts do not show reduced levels of fibronectin receptor transcription (A. Holmes, D.J.A, X. Shi-wen, C.M.B., K.L., A.L., unpublished data). However, “rescued” Ccn2-/- cells did not quite show the same degree of spreading as Ccn2+/+ fibroblasts. The full repertoire of CCN2 binding proteins has not yet been identified; Ccn2-/- cells may possess some qualitative aberrations in integrin/HSPG organization or may show altered expression of signal transduction components required for CCN2 action. Addressing this issue requires further investigations.
Collectively, our results are consistent with the recently proposed hypothesis that CCN2 may generally function as an adaptor molecule that promotes binding of extracellular proteins to their cell surface receptors (Leask and Abraham, 2003). For example, a recent report showed that CCN2 bound TGFβ through a binding site in the amino-terminal Von-Willebrand factor domain of CCN2 and assisted in presenting TGFβ to the high-affinity TGFβ type II receptor (Abreu et al., 2002). We hypothesize CCN2 may generally act by modulating the activity of specific receptor/ligand interactions and thus may influence a range of signaling events by binding cell surface HSPGs via the heparin-binding carboxyl-terminal domain and by interacting with other receptors or ligands, whose identity may be specified depending on the context, through other domains in the CCN2 protein (Leask and Abraham, 2003). In this manner, CCN2 may play a broad role in matrix organization and play diverse roles in signal transduction through promoting cell adhesion and/or potentiating the signaling mediated by other receptors and by acting as a molecular bridge aiding in the integration between extracellular and intracellular signaling networks.
Acknowledgments
We thank Maryam Hosseini for technical assistance. This work was supported in part by The Scleroderma Foundation, The Arthritis Research Campaign (United Kingdom), The Raynaud's and Scleroderma Association Trust, the Welton Foundation, the Scleroderma Society (United Kingdom), and the National Institutes of Health (grant AR44528).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04–06–0490. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–06–0490.
References
- Abraham, D.J., Shiwen, X., Black, C.M., Sa, S., Xu, Y., and Leask, A. (2000). Tumor necrosis factor alpha suppresses the induction of connective tissue growth factor by transforming growth factor-beta in normal and scleroderma fibroblasts. J. Biol. Chem. 275, 15220-15225. [DOI] [PubMed] [Google Scholar]
- Abreu, J.G., Ketpura, N.I., Reversade, B., and De Robertis, E.M. (2002). Connective-tissue growth factor (CCN2) modulates cell signalling by BMP and TGF-beta. Nat. Cell Biol. 4, 599-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babic, A.M., Chen, C.C., and Lau, L.F. (1999). Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin alphavbeta3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol. Cell. Biol. 19, 2958-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badylak, S.F. (2002). The extracellular matrix as a scaffold for tissue reconstruction. Semin. Cell Dev. Biol. 13, 377-383. [DOI] [PubMed] [Google Scholar]
- Ball, D.K., Rachfal, A.W., Kemper, S.A., and Brigstock, D.R. (2003). The heparin-binding 10 kDa fragment of connective tissue growth factor (CCN2) containing module 4 alone stimulates cell adhesion. J. Endocrinol. 176, R1-R7. [DOI] [PubMed] [Google Scholar]
- Bork, P. (1993). The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett. 327, 125-130. [DOI] [PubMed] [Google Scholar]
- Brigstock, D.R., Steffen, C.L., Kim, G.Y., Vegunta, R.K., Diehl, J.R., and Harding, P.A. (1997). Purification and characterization of novel heparin-binding growth factors in uterine secretory fluids. Identification as heparin-regulated Mr 10,000 forms of connective tissue growth factor. J. Biol. Chem. 272, 20275-20282. [DOI] [PubMed] [Google Scholar]
- Chang, H.Y., Chi, J.T., Dudoit, S., Bondre, C., van de Rijn, M., Botstein, D., and Brown, P.O. (2002). Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc. Natl. Acad. Sci. USA 99, 12877-12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong, C.M., et al. (2002). What is the `true' function of skin? Exp. Dermatol. 11, 159-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C.C., Chen, N., and Lau, L.F. (2001a). The angiogenic factors Cyr61 and connective tissue growth factor induce adhesive signaling in primary human skin fibroblasts. J. Biol. Chem. 276, 10443-10452. [DOI] [PubMed] [Google Scholar]
- Chen, Y., Blom, I.E., Sa, S., Goldschmeding, R., Abraham, D.J., and Leask, A. (2002). CCN2 expression in mesangial cells: involvement of SMADs, MAP kinase, and PKC. Kidney Int. 62, 1149-1159. [DOI] [PubMed] [Google Scholar]
- Chen, Y., Segarini, P., Raoufi, F., Bradham, D., and Leask, A. (2001b). Connective tissue growth factor is secreted through the Golgi and is degraded in the endosome. Exp. Cell Res. 271, 109-117. [DOI] [PubMed] [Google Scholar]
- Clark, R.A. (1985). Cutaneous tissue repair: basic biologic considerations. I. J. Am. Acad. Dermatol. 13, 701-725. [DOI] [PubMed] [Google Scholar]
- Einhauer, A., and Jungbauer, A. (2001). The FLAG peptide, a versatile fusion tag for the purification of recombinant proteins. J. Biochem. Biophys. Methods 49, 455-465. [DOI] [PubMed] [Google Scholar]
- Favata, M.F., et al. (1998). Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273, 18623-18632. [DOI] [PubMed] [Google Scholar]
- Fuchs, E., and Byrne, C. (1994). The epidermis: rising to the surface. Curr. Opin. Genet. Dev. 4, 725-736. [DOI] [PubMed] [Google Scholar]
- Gabbiani, G. (2003). The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 200, 500-503. [DOI] [PubMed] [Google Scholar]
- Gao, R., Ball, D.K., Perbal, B., and Brigstock, D.R. (2004). Connective tissue growth factor induces c-fos gene activation and cell proliferation through p44/42 MAP kinase in primary rat hepatic stellate cells. J. Hepatol. 40, 431-438. [DOI] [PubMed] [Google Scholar]
- Gao, R., and Brigstock, D.R. (2003). Connective tissue growth factor (CTGF) in rat activated hepatic stellate cells. Hepatol. Res. 27, 214-220. [DOI] [PubMed] [Google Scholar]
- Gao, R., and Brigstock, D.R. (2004). Connective tissue growth factor (CCN2) induces adhesion of rat activated hepatic stellate cells by binding of its C-terminal domain to integrin alpha(v)beta(3) and heparan sulfate proteoglycan. J. Biol. Chem. 279, 8848-8855. [DOI] [PubMed] [Google Scholar]
- Grotendorst, G.R. (1997). Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev. 8, 171-179. [DOI] [PubMed] [Google Scholar]
- Grzeszkiewicz, T.M., Lindner, V., Chen, N., Lam, S.C., and Lau, L.F. (2002). The angiogenic factor cysteine-rich 61 (CYR61, CCN1) supports vascular smooth muscle cell adhesion and stimulates chemotaxis through integrin alpha(6)beta(1) and cell surface heparan sulfate proteoglycans. Endocrinology 143, 1441-1450. [DOI] [PubMed] [Google Scholar]
- Holmes, A., Abraham, D.J., Chen, Y., Denton, C., Shi-Wen, X., Black, C.M., and Leask, A. (2003). Constitutive connective tissue growth factor expression in scleroderma fibroblasts is dependent on Sp1. J. Biol. Chem. 278, 13008-13015. [DOI] [PubMed] [Google Scholar]
- Holmes, A., Abraham, D.J., Sa, S., Shiwen, X., Black, C.M., and Leask, A. (2001). CCN2 and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J. Biol. Chem. 276, 10594-10601. [DOI] [PubMed] [Google Scholar]
- Igarashi, A., Okochi, H., Bradham, D.M., and Grotendorst, G.R. (1993). Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol. Biol. Cell 4, 637-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi, A., Nashiro, K., Kikuchi, K., Sato, S., Ihn, H., Grotendorst, G.R., and Takehara, K. (1995). Significant correlation between connective tissue growth factor gene expression and skin sclerosis in tissue sections from patients with systemic sclerosis. J. Investig. Dermatol. 105, 280-284. [DOI] [PubMed] [Google Scholar]
- Ivkovic, S., Yoon, B.S., Popoff, S.N., Safadi, F.F., Libuda, D.E., Stephenson, R.C., Daluiski, A., and Lyons, K.M. (2003). Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development 130, 2779-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielty, C.M., and Shuttleworth, C.A. (1997). Microfibrillar elements of the dermal matrix. Microsc. Res. Tech. 38, 413-427. [DOI] [PubMed] [Google Scholar]
- Latinkic, B.V., Mercurio, S., Bennett, B., Hirst, E.M., Xu, Q., Lau, L.F., Mohun, T.J., and Smith, J.C. (2003). Xenopus Cyr61 regulates gastrulation movements and modulates Wnt signalling. Development 130, 2429-2441. [DOI] [PubMed] [Google Scholar]
- Lau, L.F., and Lam, S.C. (1999). The CCN family of angiogenic regulators: the integrin connection. Exp. Cell Res. 248, 44-57. [DOI] [PubMed] [Google Scholar]
- Leask, A, et al. (2002). Dysregulation of TGFbeta signaling in scleroderma: overexpression of endoglin in cutaneous scleroderma fibroblasts. Arthritis Rheum. 46, 1857-1865. [DOI] [PubMed] [Google Scholar]
- Leask, A., Holmes, A., Black, C.M., and Abraham, D.J. (2003). Connective tissue growth factor gene regulation. Requirements for its induction by transforming growth factor-beta 2 in fibroblasts. J. Biol. Chem. 278, 13008-13015. [DOI] [PubMed] [Google Scholar]
- Leask, A., and Abraham, D.J. (2003). The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. Biochem. Cell Biol. 81, 355-363. [DOI] [PubMed] [Google Scholar]
- Leask, A., Denton, C.P., and Abraham, D.J. (2004). Insights into the molecular mechanism of sustained fibrosis: the role of connective tissue growth factor in scleroderma. J. Investig. Dermatol. 122, 1-6. [DOI] [PubMed] [Google Scholar]
- Leask, A., and Abraham, D.J. (2004). The control of TGFβ signaling in wound healing and fibrosis. FASEB J. 18, 816-827. [DOI] [PubMed] [Google Scholar]
- Leu, S.J., Liu, Y., Chen, N., Chen, C.C., Lam, S.C., and Lau, L.F. (2003). Identification of a novel integrin alpha 6 beta 1 binding site in the angiogenic inducer CCN1 (CYR61). J. Biol. Chem. 278, 33801-33808. [DOI] [PubMed] [Google Scholar]
- Masur, S.K., Dewal, H.S., Dinh, T.T., Erenburg, I., and Petridou, S. (1996). Myofibroblasts differentiate from fibroblasts when plated at low density Proc. Natl. Acad. Sci. USA 93, 4219-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, T., Kawara, S., Shinozaki, M., Hayashi, N., Kakinuma, T., Igarashi, A., Takigawa, M., Nakanishi, T., and Takehara, K. (1999). Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: a mouse fibrosis model. J. Cell. Physiol. 181, 153-159. [DOI] [PubMed] [Google Scholar]
- Moussad, E.E., and Brigstock, D.R. (2000). Connective tissue growth factor: what's in a name? Mol. Genet. Metab. 71, 276-292. [DOI] [PubMed] [Google Scholar]
- Nishida, T., Kubota, S., Fukunaga, T., Kondo, S., Yosimichi, G., Nakanishi, T., Takano-Yamamoto, T., and Takigawa, M. (2003). CTGF/Hcs24, hypertrophic chondrocyte-specific gene product, interacts with perlecan in regulating the proliferation and differentiation of chondrocytes J. Cell. Physiol. 196, 265-275. [DOI] [PubMed] [Google Scholar]
- Perbal, B. (2001). NOV (nephroblastoma overexpressed) and the CCN family of genes: structural and functional issues. Mol. Pathol. 54, 57-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saoncella, S., Echtermeyer, F., Denhez, F., Nowlen, J.K., Mosher, D.F., Robinson, S.D., Hynes, R.O., and Goetinck, P.F. (1999). Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers Proc. Natl. Acad. Sci. USA 96, 2805-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober, J.M., Lau, L.F., Ugarova, T.P., and Lam, S.C. (2002). Identification of integrin alpha(M)beta(2) as an adhesion receptor on peripheral blood monocytes for Cyr61 (CCN1) and connective tissue growth factor (CCN2): immediate-early gene products expressed in atherosclerotic lesions. Blood 99, 4457-4465. [DOI] [PubMed] [Google Scholar]
- Segarini, P.R., Nesbitt, J.E., Li, D., Hays, L.G., Yates JR 3rd, and Carmichael, D.F. (2001). The low density lipoprotein receptor-related protein/alpha2-macroglobulin receptor is a receptor for connective tissue growth factor. J. Biol. Chem. 276, 40659-40667. [DOI] [PubMed] [Google Scholar]
- Shi-wen, X., et al. (2000). Autocrine overexpression of CTGF maintains fibrosis: RDA analysis of fibrosis genes in systemic sclerosis. Exp. Cell Res. 259, 213-224. [DOI] [PubMed] [Google Scholar]
- Shi-Wen, X., et al. (2004). Endothelin-1 promotes myofibroblast induction through the ETA receptor via a rac/phosphoinositide 3-kinase/Akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol. Biol. Cell 15, 2707-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton, R., Rajkumar, V., Ponticos, M., Nichols, B., Shiwen, X., Black, C.M., Abraham, D.J., and Leask, A. (2002). Prostacyclin derivatives prevent the fibrotic response to TGF-beta by inhibiting the Ras/MEK/ERK pathway. FASEB J. 16, 1949-1951. [DOI] [PubMed] [Google Scholar]
- Werner, S., and Grose, R. (2003). Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 83, 835-870. [DOI] [PubMed] [Google Scholar]
- Wilcox-Adelman, S.A., Denhez, F., and Goetinck, P.F. (2002). Syndecan-4 modulates focal adhesion kinase phosphorylation. J. Biol. Chem. 277, 32970-32977. [DOI] [PubMed] [Google Scholar]
- Zamir, E., and Geiger, B. (2001). Components of cell-matrix adhesions. J. Cell Sci. 114, 3577-3579. [DOI] [PubMed] [Google Scholar]