Abstract
After fertilization, the genome of zygotes is transcriptionally silent. The timing of the initiation of transcription is species-specific and occurs at the mid-1-cell stage in mice. Recent analyses using high-throughput sequencing (HTS) have identified thousands of genes transcribed at the 1-cell stage, and the pattern of expression among these genes appears to be unique. In this article, we show the result of an additional analysis using HTS data from a previous study, and present the hypothesis that an extremely loose chromatin structure causes promiscuous gene expression in 1-cell embryos.
Keywords: 1-Cell embryos, Gene expression, Transcription, Zygote
For individual organisms, life begins as a zygote after fertilization. The zygotic genome is not transcribed immediately after fertilization; rather, the initiation of transcription occurs later, with species-specific timing. Transcription in the early stages post-fertilization has been called zygotic gene activation (ZGA). In the 1980s, transcription was thought to be initiated at the 2-cell stage in mice [1, 2]. However, Matsumoto et al. [3] found that a transgene derived from the genome of spermatozoa was expressed at the late 1-cell stage. Furthermore, a sensitive method for detecting transcription (viz., detecting the incorporation of BrU in macromolecules) revealed that a low level of transcription is initiated at the mid-1-cell stage [4]. Thus, gene activation at the 1-cell stage is known as minor ZGA, whereas an increased level of transcription at the 2-cell stage is called major ZGA. The genes to be expressed must be critically important for regulating subsequent gene expression during development. However, the mechanism that determines which genes are first transcribed has not yet been elucidated.
Transcripts in 1-cell embryos comprise those derived from oocytes and those newly transcribed from the zygotic genome. Oocyte-derived mRNA (i.e., maternal mRNA) is synthesized and accumulated during the days before fertilization, whereas mRNA transcribed from the zygotic genome is synthesized only several hours after fertilization [4, 5]. Thus, the amount of newly synthesized mRNA from the zygotic genome is much less than the accumulated maternal mRNA in 1-cell embryos, which makes it difficult to identify the genes transcribed at the 1-cell stage by comparing the transcriptomes of oocytes and 1-cell embryos. Previous studies using microarrays were unable to identify the genes transcribed in 1-cell embryos [6,7,8]. Recently, transcriptome analyses by RNA sequencing (RNA-seq) found hundreds of candidates for genes transcribed at the 1-cell stage [9, 10]. Although these studies help to characterize gene expression after fertilization, candidates were determined on the basis of the reads per kilobase per million (RPKM) of the transcripts being only 1.5-fold [10] or 2-fold [9] higher in 1-cell embryos than in MII-stage oocytes, a criterion unlikely to be robust in the face of experimental variation.
Recently, we found that splicing mechanisms do not function adequately in 1-cell embryos [11]. Taking advantage of this property, we identified 4,039 genes transcribed at the 1-cell stage, based on the RPKMs of intron regions being 4-fold higher in 1-cell embryos than of those whose transcription had been inhibited with 5,6-dichloro-1-β-d-ribofuranosyl-benzimidazole [11]. When we changed this criterion to 1.5-fold on the grounds that 4-fold might be too conservative, 11,470 genes were obtained as candidates transcribed at the 1-cell stage. Gene expression pattern analysis in 1-cell embryos has revealed that many genes are highly expressed only at the 1-cell stage, and that housekeeping genes, which are highly expressed in various cells, are not highly expressed at this stage [12]. Thus, we demonstrated a unique gene expression pattern in 1-cell embryos, but we did not clarify the mechanism by which this expression pattern is induced. Here, we discuss the mechanism for the regulation of gene expression in 1-cell embryos.
A loosened chromatin structure is involved in transcriptional regulation at the 1-cell stage
Our previous analysis for cis-regulatory elements was unable to elucidate the mechanism regulating the gene expression pattern in 1-cell embryos, except for the GC-rich nature of regions upstream of active genes [11, 12]. Since there is no cis-regulatory element specific to 1-cell embryos, it is possible that the chromatin structure is involved in regulating gene expression in 1-cell embryos. Generally, genes require enhancers for their active expression. The chromatin structure is essentially repressive for transcription, necessitating the presence of enhancers to help transcription factors access the gene promoters [13]. However, it has been shown by reporter gene assays that enhancers do not increase transcriptional activity in 1-cell embryos, suggesting that transcription is regulated independently of enhancers at this stage [2, 14, 15]. Furthermore, we demonstrated that transcription occurs only via the core promoter in 1-cell embryos, but not in 2-cell embryos [16]. Majumder et al. [15] suggested that enhancer-independent transcription is caused by the loosened chromatin structure in 1-cell embryos, since this type of transcription was still observed at the 2-cell stage when the embryos were treated with butyrate (an inhibitor of histone deacetylase), which increases histone acetylation to loosen the chromatin structure. Using fluorescence recovery after photobleaching analysis, we have recently shown that preimplantation embryos at the 1-cell stage form the loosest chromatin structure [17]. A recent analysis of the genome-wide landscape of chromatin accessibility to DNase I demonstrated that the number of DNase I-hypersensitive sites (DHSs) is the fewest at the 1-cell stage and increases during preimplantation development [18], which appears to contradict the hypothesis made by the above-mentioned reports [2, 14,15,16,17]. However, in the analysis using DNase I, the DHSs do not necessarily correspond to the sites of the genome with a loosened chromatin structure. In sites with a loosened chromatin structure, transcription factors can easily access the DNA and bind to it, thereby obstructing the DNase I access to the DNA. In this case, the sites of chromatin with a loosened structure are insensitive, rather than sensitive, to DNase I. Furthermore, since the chromatin structure is extremely loose in 1-cell embryos, DNase I might have cleaved an enormously large number of sites to make small fragments of DNA that were removed by size selection (> 50 bp) for high-throughput sequencing, which would underestimate the number of DHSs. Taken together, we propose that 1-cell embryos have a loose chromatin structure that does not require enhancers for gene expression.
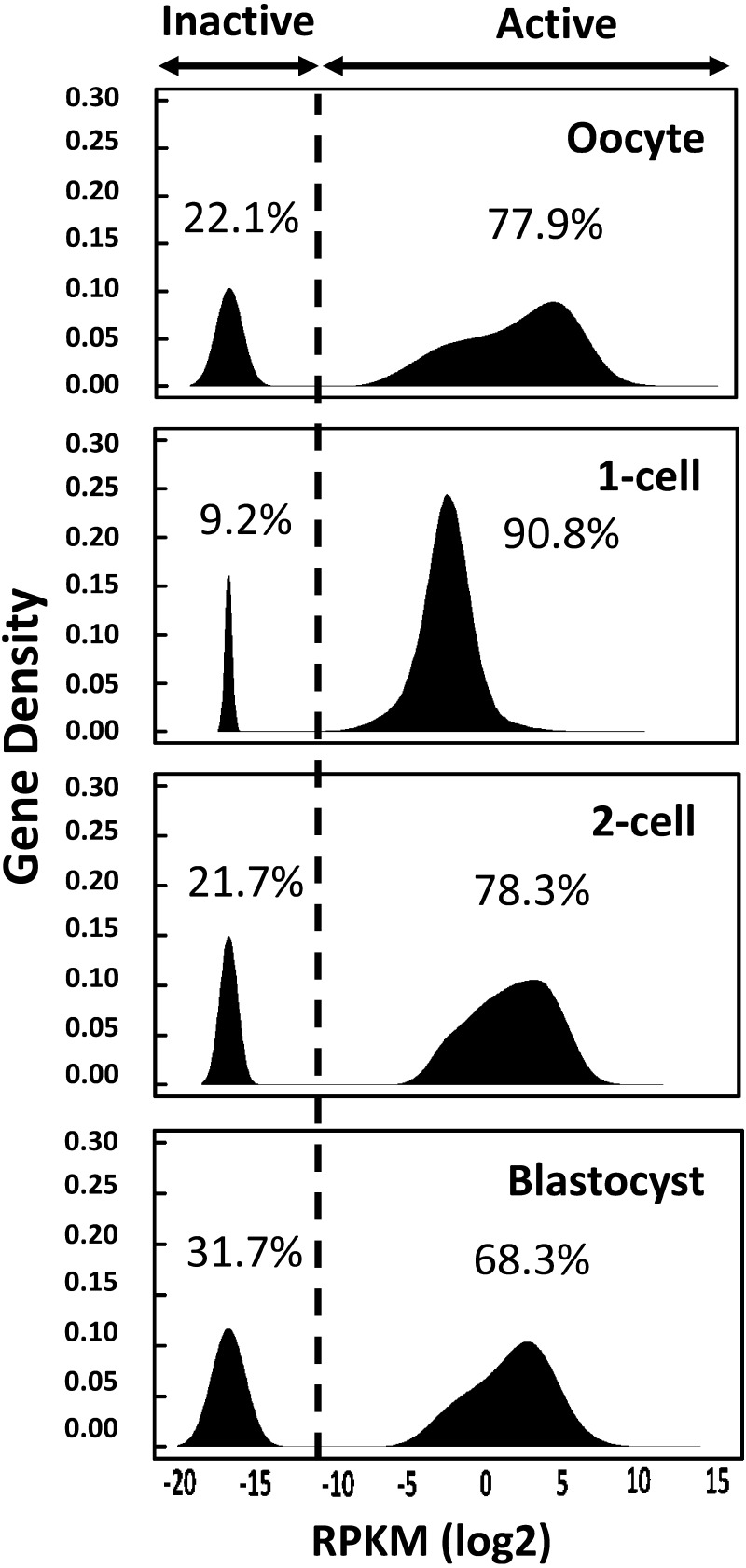
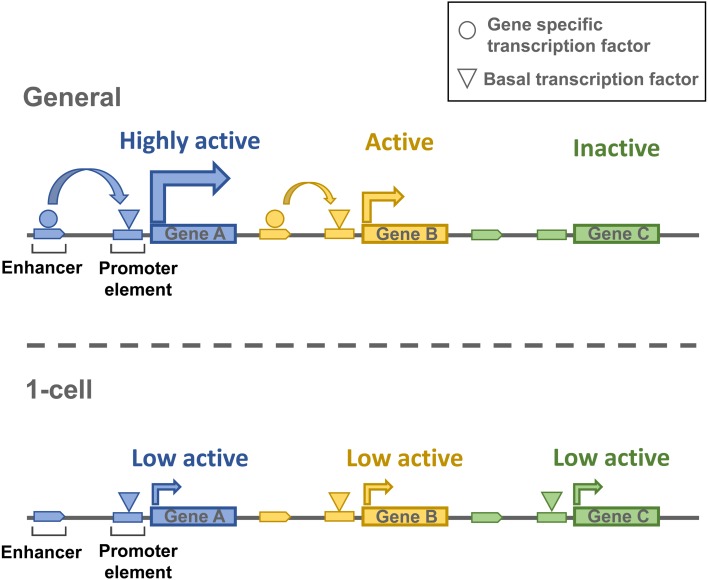
If this is the case, we should expect a large number of genes to be promiscuously expressed, and the variation in their expression level should be relatively low. Since many transcription factors would be able to easily access the promoters, enhancers should not have a strong effect on transcription. Indeed, the analysis of our RNA-seq data bears out this hypothesis. When genes are classified as active or inactive based on kernel density estimation, more than 90% of genes are active in 1-cell embryos, whereas only around 70% of genes are active in embryos at other stages and in oocytes (Fig. 1). This analysis also reveals that the variation in expression level is much lower in 1-cell embryos than in cells of other stages; the slope of the histogram is steeper for 1-cell embryos. Therefore, we suggest that a low level of enhancer-independent transcription occurs promiscuously in a large proportion of genes, which is probably caused by a loosened chromatin structure in 1-cell embryos (Fig. 2).
Fig. 1.
Histogram of gene expression levels in the preimplantation embryos. The kernel density estimation was calculated to classify all known genes as active or inactive in oocytes, 1-cell and 2-cell embryos, and blastocysts. Horizontal and vertical axes are shown as the log2 of reads per kilobase per million (RPKM) and gene density, respectively. Reads in exons were used to calculate the RPKM values in oocytes, 2-cell embryos, and blastocysts, whereas reads in introns were used for 1-cell embryos. The broken line represents the border between active and inactive genes.
Fig. 2.
Diagram of transcriptional regulation in 1-cell embryos. The upper section illustrates the general mechanism of transcription regulation. Gene expression is facilitated by the enhancers, which also regulate the gene expression levels. The lower section depicts enhancer-independent transcription in 1-cell embryos. The chromatin structure is loosened genome-wide, which allows a low level of transcription in most genes independently of enhancers.
We hope that this hypothesis sheds light on the mechanism that regulates the initiation of the gene expression program in embryos.
References
- 1.Aoki F. Regulation of zygotic gene activation in mammalian embryo. J. Mamm. Ova Res 1997; 14: 125–131. [Google Scholar]
- 2.Schultz RM. Regulation of zygotic gene activation in the mouse. BioEssays 1993; 15: 531–538. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto K, Anzai M, Nakagata N, Takahashi A, Takahashi Y, Miyata K. Onset of paternal gene activation in early mouse embryos fertilized with transgenic mouse sperm. Mol Reprod Dev 1994; 39: 136–140. [DOI] [PubMed] [Google Scholar]
- 4.Aoki F, Worrad DM, Schultz RM. Regulation of transcriptional activity during the first and second cell cycles in the preimplantation mouse embryo. Dev Biol 1997; 181: 296–307. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Zheng P, Dean J. Maternal control of early mouse development. Development 2010; 137: 859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamatani T, Carter MG, Sharov AA, Ko MS. Dynamics of global gene expression changes during mouse preimplantation development. Dev Cell 2004; 6: 117–131. [DOI] [PubMed] [Google Scholar]
- 7.Wang QT, Piotrowska K, Ciemerych MA, Milenkovic L, Scott MP, Davis RW, Zernicka-Goetz M. A genome-wide study of gene activity reveals developmental signaling pathways in the preimplantation mouse embryo. Dev Cell 2004; 6: 133–144. [DOI] [PubMed] [Google Scholar]
- 8.Zeng F, Schultz RM. RNA transcript profiling during zygotic gene activation in the preimplantation mouse embryo. Dev Biol 2005; 283: 40–57. [DOI] [PubMed] [Google Scholar]
- 9.Xue Z, Huang K, Cai C, Cai L, Jiang CY, Feng Y, Liu Z, Zeng Q, Cheng L, Sun YE, Liu JY, Horvath S, Fan G. Genetic programs in human and mouse early embryos revealed by single-cell RNA sequencing. Nature 2013; 500: 593–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SJ, Komata M, Inoue F, Yamada K, Nakai K, Ohsugi M, Shirahige K. Inferring the choreography of parental genomes during fertilization from ultralarge-scale whole-transcriptome analysis. Genes Dev 2013; 27: 2736–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abe K, Yamamoto R, Franke V, Cao M, Suzuki Y, Suzuki MG, Vlahovicek K, Svoboda P, Schultz RM, Aoki F. The first murine zygotic transcription is promiscuous and uncoupled from splicing and 3 processing. EMBO J 2015; 34: 1523–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto R, Abe K, Suzuki Y, Suzuki MG, Aoki F. Characterization of gene expression in mouse embryos at the 1-cell stage. J Reprod Dev 2016; 62: 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smale ST, Kadonaga JT. The RNA polymerase II core promoter. Annu Rev Biochem 2003; 72: 449–479. [DOI] [PubMed] [Google Scholar]
- 14.Wiekowski M, Miranda M, DePamphilis ML. Regulation of gene expression in preimplantation mouse embryos: effects of the zygotic clock and the first mitosis on promoter and enhancer activities. Dev Biol 1991; 147: 403–414. [DOI] [PubMed] [Google Scholar]
- 15.Majumder S, Miranda M, DePamphilis ML. Analysis of gene expression in mouse preimplantation embryos demonstrates that the primary role of enhancers is to relieve repression of promoters. EMBO J 1993; 12: 1131–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamamoto G, Suzuki T, Suzuki MG, Aoki F. Regulation of transketolase like 1 gene expression in the murine one-cell stage embryos. PLoS ONE 2014; 9: e82087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ooga M, Fulka H, Hashimoto S, Suzuki MG, Aoki F. Analysis of chromatin structure in mouse preimplantation embryos by fluorescent recovery after photobleaching. Epigenetics 2016; 11: 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu F, Liu Y, Inoue A, Suzuki T, Zhao K, Zhang Y. Establishing chromatin regulatory landscape during mouse preimplantation development. Cell 2016; 165: 1375–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]