Abstract
The impact of deer overabundance is a worldwide problem. Along with habitat expansion and population increase, damage by sika deer to the forest ecosystem and agriculture has become a serious issue in Japan. Deer also transmit a number of diseases and parasites to humans and livestock. The overabundance of deer is a result of their strong fecundity, and therefore the present situation should, in theory, be tackled by experts in reproductive biology.
Keywords: Contraception, Overabundance, Sika deer, Vaccine
Impact of sika deer
The increase in the population of sika deer (Cervus nippon) has become an important issue in Japan. Sika deer inhabit large areas of East Asia and comprise 14 subspecies, six of which exist in Japan: C. n. yesoensis, C. n. centralis, C. n. nippon, C. n. mageshimae, C. n. yakushimae and C. n. keramae. Deer used to be a natural food resource for humans, and the processed meat, skin, antlers and other parts were important life necessities. At the same time, it was necessary for people in rural Japan to prevent deer damage to harvest crops, for example by building deer embankments. During the period of extreme national poverty in the time of and after World War II, overkilling of sika deer occurred, and a protection policy was implemented in 1947 to prevent deer from becoming extinct. Thereafter, the deer population began to increase, and at the beginning of the 1990s, agricultural and forestry damage by deer began to be a serious issue in several prefectures. At present, damage due to overabundant deer is a serious problem in many prefectures and municipalities, and local governments have taken preventive measures with financial support from the Japanese government. However, during the latest decade, the amount of agricultural damage caused by wildlife has been estimated to be approximately 20 billion yen per year, with deer accounting for more than one third of the total. Persistent damage due to deer discourages farmers, some of whom give up cultivation activity and emigrate from rural areas. This accelerated depopulation and abandonment of farmlands in turn favors increased feeding activity by deer, and this vicious cycle has become a serious problem, especially in the outlying mountainous areas of Japan. Expansion of the deer habitat causes diverse problems including deer-vehicle collisions and transmission of external parasites such as leeches and ticks into suburban areas.
The impact of deer abundance also seriously perturbs the forest ecosystem. According to a report from the Forestry Agency of Japan, approximately 8,800 ha of forest areas were affected by wildlife in 2014, about 80% being attributable to over-browsing by deer (http://www.rinya.maff.go.jp/j/hogo/higai/pdf/01mennseki_h26.pdf, in Japanese). Repetitive nipping of palatable plants by deer results in domination of the forest floor by unpalatable plant species. Loss of shrubs and seedlings and severe browsing on trees up to a height of about 1.5 m creates a ‘browsing line′ in forests. The resulting reduction in plant species diversity creates a risk to inhabiting invertebrate species, and loss of shrubs and seedlings also reduces forest regeneration activity. When mature trees die or are knocked down by strong winds, the lack of young growing trees creates gaps in the canopy that last for several decades and there is a loss of fertile soil in the surface layer. In serious cases, this diminishes erosion control in forest areas. Such damage attributable to deer can be avoided, at least in part, by surrounding forest areas with fences, enclosing seedlings to help them grow, or wrapping the trunks of trees with guard-nets to prevent debarking in winter (Fig. 1). These methods are all effective if affected areas are patrolled and any broken parts repaired, but this is laborious and perpetual work.
Fig. 1.
Trunk of a fir tree fitted with a guard net to prevent debarking by deer (left) and a damaged trunk (right). In the winter, sika deer strip off the tree bark and eat the nutritious tissue beneath it. Unless wrapped, the trunks of fir trees are often damaged. Damage to half the circumference or more will kill the tree.
Changes in the human social structure are fundamentally attributable to deer overabundance. In 2007, the estimated deer population in Japan (except for Hokkaido) was 1,800,000–1,900,000, and this had increased to 3.05 million by 2014 (http://www.env.go.jp/press/102196.html, in Japanese). On the other hand, the total number of deer harvested by hunting or culling was more than 200,000 in 2007, and by 2011 this had increased continuously, reaching more than 350,000 (approximately 11.5% of the population). Since the annual rate of increase in the deer population is estimated to be 15–20%, it seems necessary to reduce the population further and control the density of deer to curb the detrimental effects on the forest ecosystem. At present, lethal methods (hunting and culling) are the only measure available in Japan. The Ministry of the Environment and the Ministry of Agriculture, Forestry and Fisheries have decided to halve the deer population in the current decade through accelerated culling. The policy is outlined in guidelines published in 2015 to establish a system that can work to achieve this objective (https://www.env.go.jp/nature/choju/effort/effort9/kyouka.pdf, in Japanese). Many Japanese local governments have adopted their own management plans based on assessment of impact, recording the numbers of deer captured in the past and monitoring and estimating their current activity. Capture technology has been improved through introduction of various types of trap, effective baiting methods and sensor technology. On the other hand, most of the hunters currently operating are now 60 years of age or older. The decrease in the number of gun license holders is a serious obstacle to halving the deer population and maintaining the decreased level thereafter, as the high fecundity of deer means that the population recovers in a few years in the absence of culling or control pressure.
Lethal methods of control are not applicable in areas that are close to residential locations, tourist areas or historical deer sanctuaries due to public concern. Within such areas, there is no choice but to protect ornamental plants and food crops from over-browsing using deer exclosures.
Strong fecundity of sika deer
The sika deer is short-day seasonal breeder. The breeding season is from mid-September to late October in Honshu and late October to mid-November in Hokkaido. During these periods, females have multiple estrous cycles (at approximately 3-week intervals) until they become pregnant. Investigation of does captured in February and March in Hokkaido and prefectures in Kyushu and Honshu has revealed that approximately 80% of yearlings and more than 90% of older females are pregnant, the pregnancy rate being high in does 10 years old and older [1, 2] ([2] Koizumi, personal communication). Each pregnant doe bears a single fawn in May–June and nurses it in the female herd. Delivery at the proper time is important for ensuring that yearlings have grown by the winter. Female offspring live in the herd where they were born, while male offspring usually leave the herd when they become two years old in early summer.
The breeding season of deer, known as the “rut”, is from August to December, when the rutting calls and courtship behavior of bucks are characteristic signs of libido. Recrudescence of spermatogenesis and active sperm productions occur in the breeding season [3, 4]. Initiation of spermatogenesis is observed in yearling in the breeding season [4]. Dominant bucks have a privileged mating position, and try to monopolize female herds to have many opportunities to copulate. Visual displays and actual contests with antlers are important for establishing dominancy and dissuading other suitors. After the end of the breeding season, the antlers drop, the velvet is formed, and hardening occurs as the next breeding season approaches.
Female and male deer have a maximum life expectancy of 12–15 years and 10–13 years, respectively. The mortality rates of adults and fawns are estimated to be 10–15% and 30–50%, respectively (http://www.env.go.jp/nature/choju/plan/plan3-2e/nihonjika.pdf, in Japanese). These rates are influenced by nutrition. It appears that recent warm winters with lower snowfall have favored the survival of deer, resulting in overabundance. However, the deer population has also been increasing in snow-free areas such as the southern islands of Japan. There are indications that alterations to the environment by humans related to changes in industrial structure and land uses over the last few decades have provided a more favorable habitat for deer, thus explaining the population explosion that has occurred in most regions of Japan [5].
Use of immunocontraception in the United States of America
In the USA, non-lethal methods have been developed and applied for control of the wildlife population especially in urban and suburban areas where there is opposition to the use of lethal methods. Attempts to develop immunocontraceptives for management of white-tailed deer (Odocoileus virginianus) started in the 1990s, when overabundance led to deer-vehicle collisions. Researchers focused on porcine zona pellucida (PZP) as the antigen to render females infertile, because the protein is expressed specifically in females. An early study demonstrated significant reduction of fawning rates in treated does [6], and a decrease in the deer population was observed in the field following multiple injections of PZP over several years [7]. PZP is prepared by extraction from porcine ovaries. Comparison of several factors led to the establishment of suitable conditions for extraction and modification to produce a controlled-release, single-shot injection of PZP emulsified with AdjuVac, a mineral oil-based adjuvant [8], and this achieved successful contraception for seven years (≓ life span) in free-roaming while-tailed deer [9]. Recent reports have indicated that a single injection of PZP vaccine can diminish deer density [10, 11]. The contraceptive effect of the PZP vaccine in deer is considered to be due to production of antibodies that interfere with sperm-egg interaction, rather than inhibition of follicular growth/maturation. In fact, treated does showed an extended estrous season because of non-pregnancy with a cyclic increase of the serum progesterone concentration [6, 12]. When the antibody titer dropped in the extended breeding season, the treated does bred, resulting in late birth. Such late-birth fawns are much less likely to survive the winter as they have a shorter period in which to grow.
Gonadotropin-releasing hormone (GnRH) is another target molecule for immunocontraception. Immunization with GnRH blocks gonadotropin secretion from the pituitary and consequently disrupts gonadal function. Since GnRH is common to both male and female, the effect of the vaccine can appear in both sexes. In fact, active immunization using keyhole limpet hemocyanin (KLH)-conjugated GnRH renders both male and female white-tailed deer infertile. The treated does show diminished or precluded libido in the breeding season, and the decreased mating activity results in a reduction of the number of fawns per doe [6]. The treated bucks exhibit an immunocastration phenotype: small testes, lack of a masculine appearance, no interest in estrus females and disordered antler formation [12]. A single shot of GnRH-KLH conjugate emulsified with AdjuVac induced female infertility for five years after the treatment in white-tailed deer [13] and for at least three years in elk (Cervus elaphus) [14]. Although safety of the vaccination while pregnant had been concerned, the treatment of pregnant does did not effect upon continuation of pregnancy and delivery [15]. In 2009, a GnRH contraceptive, GonaConTM-Cervid, formulated from an emulsified mixture of GnRH-KLH conjugate and AdjuVac, was registered with the Environmental Protection Agency as a ‘restricted use′ product. Its use is restricted to adult female white-tailed deer inhabiting urban/suburban areas. Federal/state wildlife agencies certify the applicators to make sure the humane treatment of animals, to avoid human hazard while handling and to minimize non-target use [16]. Effectiveness of the GnRH vaccine for elimination of reproductive behavior is desirable to reduce the incidence of deer-vehicle collisions.
Future of immunocontraceptives in Japan
Development of a remotely deliverable single-shot vaccine has potential for immunocontraception of sika deer to control their population [17]. As mentioned above, deer overabundance is considered attributable to the high pregnancy rate. If these contraceptives inhibit pregnancy and reduce the fawning rate, the rate of population increase (about 15% annually) could be suppressed. However, there are still challenges for the application of vaccines that have been developed abroad.
The effectiveness of a vaccine is largely dependent on the adjuvant, which is administered with the antigen(s) to stimulate an immune response. Both the GnRH and PZP vaccines achieve long-term effectiveness by combination with AdjuVac, a powerful adjuvant containing killed bacteria (Mycobacteriumavium). This adjuvant was developed to replace Freund′s complete adjuvant (FCA), a strong adjuvant generally used for experimental purposes, because FCA was shown to induce inflammation at the injection site [8]. In Japan, the domestic Policy for Control of Infectious Diseases in Domestic Animals does not permit the use of AdjuVac. Therefore, a new adjuvant that is both effective and permissible for field use is needed. Recently the author′s laboratory has derived a formula that evokes immune responses comparable to that by FCA [18]. Since it is based on a veterinary vaccine adjuvant and includes a non-pathogenic bacteria-derived molecule, there is a possibility that it could be registered as a vaccine adjuvant for field use.
Another challenge is to develop a vaccine that is effective in both males and females. It is difficult to distinguish male yearlings in a female herd because the former have not yet developed antlers. The vaccine should be delivered easily and effectively to all the target animals in the field. Although GnRH contraceptives are effective in both sexes, treatment of males would eliminate mating behavior because of lowered testosterone secretion. The effect in males might not help to decrease the fawning rate; since affected males lacking mating behavior are excluded from the breeding population, normal males (possibly immigrating into the female herd) could gain mating privileges. In addition, the adverse effect on antler maturation/growth [12] due to inhibition of testosterone secretion could render the treated bucks undesirable in terms of appearance. Follicle stimulating hormone (FSH) has crucial roles in both spermatogenesis and folliculogenesis. In males, immunoneutralization of FSH is expected to cause aspermatogenesis without influence in testosterone secretion (i.e., without inhibition of the harem formation). For these reasons, FSH has an advantage over GnRH as an immunogen for a hormonally based unisex vaccine. Previous studies of primates have revealed that immunization with FSHβ, which is not bioactive, could cause oligospermia without altering testosterone secretion [19]. Similar results could be expected by immunization with FSH receptor-related proteins.
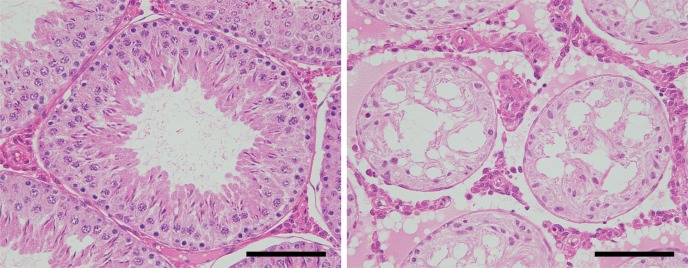
Recently, the author′s laboratory has succeeded in reducing sperm production in rats by injection of sperm emulsified with FCA. The rats showed normal reproductive behavior and serum testosterone levels comparable with those of control males (administered FCA only). Histologically, the testicular tissue showed a prevalence of autoimmune orchitis, accompanied by shedding of germ cells into the seminiferous tubule lumen without considerable infiltration of immune cells into the interstitial tissue (Fig. 2). These results indicate that oligo-/azoospermia could be induced by induction of autoimmune orchitis without any influence on mating behavior. Investigation of sperm-derived molecules with high antigenicity for orchitis is currently underway in our laboratory. Immunization with these molecules might be applicable for female contraception by influencing, for example, migration of sperm in the female genital tract or sperm-egg interactions.
Fig. 2.
Autoimmune orchitis in rats. Animals were administered endogenous sperm emulsified with Freund′s complete adjuvant (FCA) twice with a 2-week interval in the immature period. Testes were examined histologically at 20 weeks of age. Control testis (after administration of FCA only, left) shows normal spermatogenesis, whereas sperm inoculation has caused autoimmune orchitis (right), resulting in complete aspermatogenesis. Scale bar = 100 μm.
Non-lethal management of deer density must meet following conditions: effective for population control, safe for animals as well as for food chain, and public acceptance. An application of steroids or other chemical contraceptives, for example, has safety concerns especially with regard to side effects in the treated animals and consumption by non-target animals (including humans). At present, an immunocontraceptive vaccine is considered to be the most feasible approach. However, development of an effective delivery method will be an important issue to solve. Recent progress in capture technology and automatic control systems might enable the remote delivery to free-roaming animals with limited manpower. Various challenges to achieving a good vaccine formulation remain, and future efforts directed at practical use of non-lethal methods will require the collaboration of specialists in broad fields, including animal and plant ecology, ethology, veterinary medicine, sensor and automatic control technology and public psychology.
Acknowledgments
The author is deeply grateful to Drs T Koizumi (Forestry and Forest Products Research Institute) and H Kaneko (Institute of Agrobiological Sciences, NARO) for their helpful comments. The introduced experimental results are based on the research undertaken with the support by the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry (25026A) of the Ministry of Agriculture, Forestry and Fishries of Japan.
References
- 1.Suzuki M, Ohtaishi N. Reproduction of female sika deer (Cervus nippon yesoensis Heude, 1884) in Ashoro district, Hokkaido. J Vet Med Sci 1993; 55: 833–836. [DOI] [PubMed] [Google Scholar]
- 2.Koizumi T, Hamasaki S, Kishimoto M, Yokoyama M, Kobayashi M, Yasutake A. Reproduction of female sika deer in western Japan. In: McCullough DR, Takatsuki S, Kaji K (eds.), Sika Deer: Biology and Management of Native and Introduced Populations. Springer; 2009: 327-344.
- 3.Suzuki M, Kaji K, Nigi H. Annual changes of testis size, seminiferous tubules and plasma testosterone concentration of wild Sika deer (Cervus nippon yesoensis Heude, 1884) in Hokkaido. J Vet Med Sci 1992; 54: 551–556. [DOI] [PubMed] [Google Scholar]
- 4.Hayakawa D, Sasaki M, Suzuki M, Tsubota T, Igota H, Kaji K, Kitamura N. Immunohistochemical localization of steroidogenic enzymes in the testis of the sika deer (Cervus nippon) during developmental and seasonal changes. J Reprod Dev 2010; 56: 117–123. [DOI] [PubMed] [Google Scholar]
- 5.Agetsuma N. Are deer populations increasing abnormally? Biol Sci 2013; 65: 108–116. (In Japanese). [Google Scholar]
- 6.Miller LA, Killian GJ. Seven years of white-tailed deer immunocontraceptive research at Penn State University: A comparison of two vaccines. In: The Ninth Wildlife Damage Management Conference Proceedings; 2000; State College, USA. 60-69.
- 7.Rutberg AT, Naugle RE. Population-level effects of immunocontraception in white-tailed deer (Odocoileus virginianus). Wildl Res 2008; 35: 494–501. [Google Scholar]
- 8.Powers JG, Nash PB, Rhyan JC, Yoder CA, Miller LA. Comparison of immune and adverse effects induced by AdjuVac and Freunds complete adjuvant in New Zealand white rabbits (Oryctolagus cuniculus). Lab Anim (NY) 2007; 36: 51–58. [DOI] [PubMed] [Google Scholar]
- 9.Miller LA, Fagerstone KA, Wagner DC, Killian GJ. Factors contributing to the success of a single-shot, multiyear PZP immunocontraceptive vaccine for white-tailed deer. Hum-Wildl Confl 2009; 3: 103–115. [Google Scholar]
- 10.Rutberg AT, Naugle RE, Verret F. Single-treatment porcine zona pellucida immunocontraception associated with reduction of a population of white-tailed deer (Odocoileus virginianus). J Zoo Wildl Med 2013; 44(Suppl): S75–S83. [DOI] [PubMed] [Google Scholar]
- 11.Rutberg AT, Naugle RE, Turner JW, Fraker MA, Flanagan DR. Field testing of single-administration porcine zona pellucida contraceptive vaccines in white-tailed deer (Odocoileus virginianus). Wildl Res 2013; 40: 281–288. [Google Scholar]
- 12.Killian GJ, Miller LA. Behavioral observations and physiological implications for white-tailed deer treated with two different immunocontraceptives. In: The Ninth Wildlife Damage Management Conference Proceedings; 2000; State College, USA. 283-291.
- 13.Miller LA, Gionfriddo JP, Fagerstone KA, Rhyan JC, Killian GJ. The single-shot GnRH immunocontraceptive vaccine (GonaCon) in white-tailed deer: comparison of several GnRH preparations. Am J Reprod Immunol 2008; 60: 214–223. [DOI] [PubMed] [Google Scholar]
- 14.Killian G, Kreeger TJ, Rhyan J, Fagerstone K, Miller L. Observations on the use of GonaCon in captive female elk (Cervus elaphus). J Wildl Dis 2009; 45: 184–188. [DOI] [PubMed] [Google Scholar]
- 15.Miller LA, Fagerstone KA, Eckery DC. Twenty years of immunocontraceptive research: lessons learned. J Zoo Wildl Med 2013; 44(Suppl): S84–S96. [DOI] [PubMed] [Google Scholar]
- 16.Fagerstone KA, Miller LA, Killian G, Yoder CA. Review of issues concerning the use of reproductive inhibitors, with particular emphasis on resolving human-wildlife conflicts in North America. Integr Zool 2010; 5: 15–30. [DOI] [PubMed] [Google Scholar]
- 17.Kirkpatrick JF, Lyda RO, Frank KM. Contraceptive vaccines for wildlife: a review. Am J Reprod Immunol 2011; 66: 40–50. [DOI] [PubMed] [Google Scholar]
- 18.Noguchi J, Watanabe S, Dang-Nguyen T, Kikuchi K, Kaneko H. Development of a lipopolysaccharide (LPS)-supplemented adjuvant and its effects on cell-mediated and humoral immune responses in male rats immunized against sperm. J Reprod Dev 2017; 63: 111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moudgal NR, Jeyakumar M, Krishnamurthy HN, Sridhar S, Krishnamurthy H, Martin F. Development of male contraceptive vaccinea perspective. Hum Reprod Update 1997; 3: 335–346. [DOI] [PubMed] [Google Scholar]