Abstract
We have found that early in infection of the intracellular pathogen Listeria monocytogenes in Madin-Darby canine kidney epithelial cells expressing actin conjugated to green fluorescent protein, F-actin rapidly assembles (∼25 s) and disassembles (∼30 s) around the bacteria, a phenomenon we call flashing. L. monocytogenes strains unable to perform actin-based motility or unable to escape the phagosome were capable of flashing, suggesting that the actin assembly occurs on the phagosome membrane. Cycles of actin assembly and disassembly could occur repeatedly on the same phagosome. Indirect immunofluorescence showed that most bacteria were fully internalized when flashing occurred, suggesting that actin flashing does not represent phagocytosis. Escherichia coli expressing invA, a gene product from Yersinia pseudotuberculosis that mediates cellular invasion, also induced flashing. Furthermore, polystyrene beads coated with E-cadherin or transferrin also induced flashing after internalization. This suggests that flashing occurs downstream of several distinct molecular entry mechanisms and may be a general consequence of internalization of large objects by epithelial cells.
INTRODUCTION
Bacterial pathogens such as Salmonella, Yersinia, and Listeria have a variety of mechanisms that allow them to enter and survive in eukaryotic cells. Their ability to survive intracellularly can protect them from host defenses and allow them to cross epithelial barriers. To enter eukaryotic cells, these invasive bacteria actively induce their own uptake by phagocytosis in normally nonphagocytic cells. Internalized bacteria either survive within the phagosome, encapsulated by the host cell membrane, or they escape from the phagosome and disseminate throughout the cytoplasm and to neighboring cells (reviewed in Cossart and Sansonetti, 2004). Modulation of the host cell actin cytoskeleton by invasive bacteria allows them to control many of these steps, including attachment and entry into cells, survival in the phagosome, and movement in the cytoplasm.
One bacterium with an intracellular niche is Listeria monocytogenes, a food-borne pathogen responsible for meningitis, meningoencephalitis, septicemia, gastroenteritis, and abortions (reviewed in Cossart and Lecuit, 1998). Two L. monocytogenes proteins, internalin (InlA) and internalin B (InlB), can interact with the host cell surface proteins E-cadherin, gC1q-R, and Met to induce bacterial uptake into nonphagocytic cells (Mengaud et al., 1996; Braun et al., 2000; Shen et al., 2000). Uptake of bacteria is driven by actin polymerization and membrane extension (reviewed in Cossart and Sansonetti, 2004). Once inside the cell, L. monocytogenes escapes from the phagosome into the cytoplasm. In the cytoplasm, L. monocytogenes nucleates actin filaments that assemble into actin clouds around the bacteria and then into polarized comet tails. The actin comet tails propel the movement of L. monocytogenes within the cytoplasm of an infected cell and into neighboring cells (Tilney and Portnoy, 1989; Mounier et al., 1990). The recruitment of host cell proteins to the bacteria for actin-based motility is solely dependent on bacterial expression of the surface protein ActA (Kocks et al., 1992; Smith et al., 1995).
Proteomic analysis and immunofluorescent staining has shown that actin is associated with InlA-coated bead phagosomes, InlB-coated bead phagosomes, and L. monocytogenes phagosomes from epithelial cells (Bierne et al., 2001; Pizarro-Cerda et al., 2002). It is known that L. monocytogenes modulates the host cell actin cytoskeleton during entry and intracellular movement. We were interested in determining whether L. monocytogenes also manipulated the actin cytoskeleton while it is in the phagosome. Because epithelial cells are the primary route of L. monocytogenes infection in vivo (Lecuit et al., 2001), we were particularly interested in L. monocytogenes infection in epithelial cells. In addition, the dynamics of actin assembly on phagosomes have not been studied in live cells. Therefore, epithelial cells expressing green fluorescent protein (GFP)-actin were an ideal system to study changes in the host cell actin cytoskeleton in response to infection because it permitted us to image actin dynamics in live cells.
Here, we show that actin repeatedly assembles and disassembles on the membrane of L. monocytogenes phagosomes, in a process we call flashing. The actin dynamics on the phagosome membrane are not only specific to L. monocytogenes phagosomes but also occur on the phagosomes of Escherichia coli expressing invasin from Yersinia pseudotuberculosis and polystyrene beads coated with E-cadherin or transferrin. Thus, the cycles of actin accumulation on the phagosome membrane are independent of specific molecular features of the internalized object and may reflect host cell actin dynamics.
MATERIALS AND METHODS
Cell Lines, Bacterial Strains, and Infections
Madin-Darby canine kidney (MDCK) G cells and MDCK A10* cells (stable GFP-actin transfectants of MDCK G cells) were cultured as described previously (Robbins et al., 1999). Twenty-four hours before infection, cells were seeded onto glass coverslips in six-well tissue culture plates containing growth medium without antibiotic-antimycotic. Cells were 60–80% confluent on the day of infection. Infections were performed with L. monocytogenes strains 10403S (wild-type) (Bishop and Hinrichs, 1987), DP-L973 (Sun et al., 1990), DP-L3078 ΔactA (Skoble et al., 2000), DP-L2319 Δhly ΔplcA ΔplcB (Gedde et al., 2000; O'Riordan et al., 2002), and DP-L4032 GGG actA allele (Skoble et al., 2001); as well as E. coli (ATM379) expressing invasin from Y. pseudotuberculosis (Isberg and Falkow, 1985; Monack and Theriot, 2001). L. monocytogenes strains were provided by Dr. D. A. Portnoy (University of California, Berkeley, CA) and E. coli (invasin) was provided by Dr. D. M. Monack (Stanford University, Stanford, CA).
Cells were infected with L. monocytogenes and E. coli (invasin) as described previously (Robbins et al., 1999; Monack and Theriot, 2001). After 45–60 min at 37°C, 5% CO2, infected cells were washed three times with DMEM supplemented with 10% fetal bovine serum (FBS) (DMEM/FBS) to remove unattached bacteria, and fresh DMEM/FBS was added. Cells infected with L. monocytogenes strains or E. coli (invasin) were imaged from 1 to 4 h from the start of infection.
Bead Experiments
The extracellular domain of E-cadherin conjugated to the human immunoglobulin hinge region and Fc domain (E-cadherin.Fc) (Chen and Nelson, 1996) was a generous gift from T. D. Perez (Stanford University). To coat polystyrene beads with E-cadherin.Fc, 1-μm polystyrene beads (Polysciences, Warrington, PA) were incubated in 1 mg/ml biotinylated protein A (Pierce Chemical, Rockford, IL) at room temperature with rotation for 3 h. Beads were washed once in PBS, resuspended in E-cadherin.Fc (0.2 mg/ml), and incubated for 1 h at room temperature and then overnight at 4°C with rotation. Beads were washed once in 137 mM NaCl, 3 mM KCl, 50 μM CaCl2, 5 mM HEPES, pH 7.4, and resuspended in this buffer to a final concentration of ∼2 × 106 beads/μl.
To coat polystyrene beads with transferrin, 1-μm polystyrene beads were incubated in 1 mg/ml canine apotransferrin (Sigma-Aldrich, St. Louis, MO) in phosphate-buffered saline (PBS) at room temperature for 2 h and then overnight at 4°C with rotation. The beads were washed twice in PBS. To load iron onto the apotransferrin, the beads were resuspended in 50 mM Tris-HCl, pH 8.0, 10 mM NaHCO3, 1 mM ferric citrate (Markelonis et al., 1982); incubated for 3 h at room temperature; and then overnight at 4°C with rotation. Beads were washed twice in PBS and resuspended in PBS to a final concentration of ∼2 × 106 beads/μl.
For bead uptake experiments, MDCK A10* cells were plated as for bacterial infections, except that cells were grown in the presence of antibiotic-antimycotic. The beads were briefly sonicated and 2–4 × 107 E-cadherin–coated beads or 4–6 × 107 transferrin-coated beads were added to cells per well of a six-well plate. The plates were centrifuged at 1000 rpm for 10 min; incubated at 37°C, 5% CO2 for 30–45 min; and then washed three times with growth media to remove unbound beads. Cells were imaged from 1 to 4 h after the addition of beads. In addition, for uptake of the transferrin-coated beads, cells were washed twice in PBS and then starved for 1 h in DMEM (without serum) before addition of beads to promote uptake of transferrin. All subsequent incubations and imaging were performed in the absence of serum.
Immunostaining
An immunofluorescence protection assay that distinguishes extracellular from intracellular bacteria was modified from Marquis et al. (1995) and Alrutz et al. (2001). Twenty minutes or 1.5 h after infection of MDCK A10* or MDCK G cells with L. monocytogenes Δhly ΔplcA ΔplcB, cells were fixed in 4% electron microscopy (EM)-grade formaldehyde (Ted Pella, Redding, CA) in cytoskeleton buffer (Symons and Mitchison, 1991) with 0.32 M sucrose (CBS) at room temperature for 20 min. Cells were rinsed three times in PBS and blocked in PBS + 3% bovine serum albumin (PBS-BSA). To detect extracellular bacteria, rabbit polyclonal serum raised against L. monocytogenes O antigen (Difco, Detroit, MI) was diluted in PBS-BSA and added for 30 min. Cells were washed four times with PBS-BSA and then incubated with the secondary antibody. In addition, for MDCK G cells, cells were then permeabilized with PBS containing 0.2% Triton X-100 and 4% formaldehyde for 5 min and blocked in PBS-BSA containing 0.1% Triton X-100. F-actin was then fluorescently labeled with 0.4 μM fluorescein isothiocyanate (FITC)-phalloidin (Molecular Probes, Eugene, OR) for 15 min.
Phalloidin staining of MDCK A10* cells was performed by fixing the cells in 4% EM-grade formaldehyde at room temperature for 15 min. Cells were washed and blocked, and incubated with 0.013 μM tetramethylrhodamine B isothiocyanate-phalloidin (Sigma-Aldrich) at room temperature for 15 min to label the F-actin.
Indirect immunofluorescent staining for Arp3 and p34 was performed 1 h after infection of MDCK A10* cells with L. monocytogenes Δhly ΔplcA ΔplcB or 1 h after addition of transferrin-coated beads to MDCK A10* cells. Arp3 and p34 were detected using affinity-purified rabbit polyclonal antibodies against Arp3 and p34 (a generous gift from M. D. Welch, University of California, Berkeley, CA). Immunostaining was performed as described previously (Welch et al., 1997a,b), except that cells were fixed in CBS with 4% formaldehyde, 0.1% Triton X-100, and 0.4 μM FITC phalloidin for 15 min and washed once in PBS, before being postfixed in–20°C methanol for 3 min.
Microscopy
Live cell imaging was performed at 37°C by mounting the coverslips on a temperature-controlled chamber. Cells were imaged in Leibovitz's L-15 medium without phenol red and supplemented with 10% FBS, and covered with a thin layer of silicone DC-200 fluid, 0.937 g/ml (Fluka Chemika, Sigma-Aldrich, Steinheim, Switzerland) to prevent evaporation. Time-lapse phase contrast and epifluorescent images of live cells were acquired using a Nikon Diaphot-300 inverted microscope with a 60× (numerical aperture [NA] 1.4) or 100× (NA 1.4) objective. Images of fixed cells were collected on the Nikon Diaphot-300 microscope with the 60× (NA 1.4) objective or an Axioplan 2 microscope (Carl Zeiss, Thornwood, NY) by using a 63× (NA 1.4) or 100× (NA 1.4) objective. All images were collected with a cooled back-thinned charge-coupled device camera (MicroMax 512BFT; Princeton Instruments, Princeton, NJ) with a 2× optivar attached, using MetaMorph version 4.x software (Universal Imaging, Downingtown, PA).
Pharmacological Treatments
All pharmacological agents were added to cells while in the temperature-controlled chamber on the microscope. The same cells with flashing bacteria or beads were imaged before and after the addition of the pharmacological agent. Chloroquine (Sigma-Aldrich) was added to the cells at a final concentration of 100 μM, and at least 30 min elapsed before reimaging the cells. Latrunculin A (Molecular Probes) was added to the cells at a final concentration of 1 μM, and at least 5 min elapsed before reimaging the cells. LY 294002 (Calbiochem, San Diego, CA) was added to the cells at a final concentration of 50 or 100 μM, and at least 5 min elapsed before reimaging the cells.
Quantitative Image Analysis
Boxes (3 × 3 pixels; 0.34 × 0.34 μm for the 60× objective) were placed at the circumference of the object where the flashing actin was present. The average fluorescence intensity of the GFP-actin signal was measured in each box for each time point of the movies. Average fluorescence intensity from a nearby area of the cell was designated the background level of fluorescence. The GFP-actin fluorescence intensity (after background subtraction) was plotted versus time. These fluorescence traces had a variety of peak shapes, ranging from single isolated peaks to multiple overlapping peaks.
To detect periodicity in the changes in GFP-actin fluorescence intensity associated with flashing, fast Fourier transforms were performed using MatLab on fluorescence traces that had three or more peaks, and the power spectrum was calculated. Ten traces from six wild-type L. monocytogenes and 12 traces from seven E-cadherin beads were analyzed in this way.
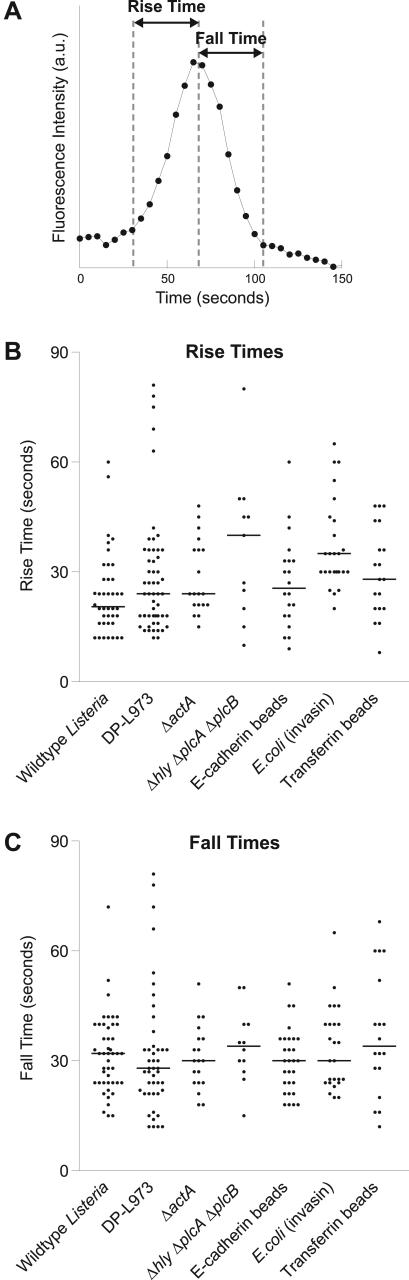
Approximately 50% of the peaks were single isolated peaks, and these peaks were analyzed to determine the rise time and fall time for each flash. Where there was more than one shoulder, the peak was not included in the data analysis. Analysis of single peaks was performed on 17 wild-type L. monocytogenes, 21 DP-L973 L. monocytogenes, 10 ΔactA L. monocytogenes, six Δhly ΔplcA ΔplcB L. monocytogenes, nine E. coli (invasin), 10 E-cadherin–coated beads, and nine transferrin-coated beads. The rise times and fall times for each flash were measured (Figure 6A). The duration of the rise and fall time was judged by eye, taking into account fluctuations in the fluorescence intensity of the background with time. The beginning and end of each flash were identified as the points where the slope of the fluorescence trace changed significantly, with the flash defined as occurring during the steepest part of the fluorescence trace. This resulted in a total of 189 data points for the rise times, and 194 data points for the fall times. To compare the rise times and fall times of different populations, one-way analysis of variance (ANOVA) with Tukey's posttest and the Kruskal-Wallis test with Dunn's posttest was performed using GraphPad Prism version 3.03 for Windows (GraphPad Software, San Diego, CA). Both tests gave the same outcome.
Figure 6.
Various objects have similar flashing kinetics. (A) Trace of fluorescence intensity (GFP-actin) versus time illustrating the definition of the rise time and the fall time of a flash. (B) Rise times for flashing points on L. monocytogenes wild-type (n = 40), DP-L973 (n = 55), ΔactA (n = 19), and Δhly ΔplcA ΔplcB (n = 11), and E-cadherin–coated beads (n = 20), E. coli (invasin) (n = 25), and transferrin-coated beads (n = 19). Horizontal lines depict the median value for each group. (C) Fall times for flashing points on L. monocytogenes wild-type (n = 46), DP-L973 (n = 45), ΔactA (n = 19), and Δhly ΔplcA ΔplcB (n = 12), and E-cadherin–coated beads (n = 29), E. coli (invasin) (n = 25), and transferrin-coated beads (n = 18). Horizontal lines depict the median values for each group.
RESULTS
Flashing: Cyclic Assembly and Disassembly of Actin around Bacteria
We used epithelial cells expressing GFP-actin (MDCK A10*) infected with wild-type L. monocytogenes (10403S) to study the interaction of actin with L. monocytogenes at early stages of infection. Time-lapse fluorescence microscopy of live infected cells revealed that actin rapidly assembled and disassembled around bacteria, resulting in a cyclic appearance and disappearance of an actin cloud (Figure 1, A and B; Vid1Fig1 and Vid2Fig1 in Supplementary Materials). To reflect the rapid dynamics observed, we called this phenomenon flashing. Actin was rapidly recruited to the bacterium and just as rapidly disassembled, such that an entire flash usually lasted <80 s. Flashing occurred 1–4 h after infection of cells with L. monocytogenes and occurred before the onset of comet tail formation and intracellular motility. We observed that flashing seemed to occur more frequently on bacteria that were closer to the cell periphery and that not all internalized bacteria exhibited flashing.
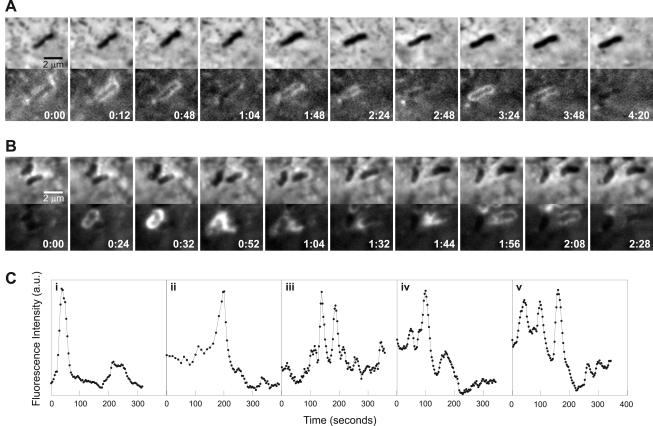
Figure 1.
Flashing. Cyclic assembly and disassembly of actin around L. monocytogenes. (A and B) MDCK A10* cells infected with wild-type L. monocytogenes. Paired phase contrast and fluorescence (GFP-actin) time-lapse images. Time in minutes:seconds. (A) Three cycles of transient actin accumulation occur on this bacterium. (B) Flashing actin is passed from the bacterium on the left to the bacterium on the right. (C) Traces of fluorescence intensity (GFP-actin) versus time for various points on flashing bacteria. Peaks in the fluorescence intensity represent transient accumulation of GFP-actin around bacteria. i corresponds to a point on the bacterium on the left in B. i–iv represent points from different bacteria. iv and v represent different points from the same bacterium. Full videos Vid1Fig1 (A) and Vid2Fig1 (B) are available as supplementary material.
A single bacterium could flash multiple times. Figure 1A shows a bacterium flashing three times in <5 min. The intensity and spatial distribution of GFP-actin was not always the same for each flash. Flashing also could propagate along and between bacteria. Figure 1B illustrates propagation of an actin flash from the bacterium on the left to its neighbor on the right. The bacterium on the right gains the flash that then propagates along the entire bacterium.
To quantitate flashing actin dynamics, we measured the GFP-actin fluorescence intensity at various points on the bacterial surface for each frame of the movies and plotted the fluorescence intensity versus time (Figure 1C). The peaks in fluorescence intensity represent the transient accumulation of GFP-actin we observe. Figure 1C, i, represents a point from the bacterium on the left in Figure 1B. A variety of peak shapes were observed, varying from single peaks (Figure 1C, i) to multiple overlapping peaks (Figure 1C, v). Figure 1C, i–iv, are traces from different bacteria, illustrating the variability in flashing among bacteria. In addition, not all points on a bacterium always behaved in the same way. For example, Figure 1C, iv and v, are different points on the same bacterium. The peak intensities of flashes differed within one bacterium and among different bacteria. Differences in peak intensity might be due to a different number of actin filaments being involved in each flash as a result of variation in the initial conditions of each event. Differences in GFP-actin expression of host cells and intensity of fluorescence illumination also contributed to differences in peak intensity of flashes. Power spectrum analysis was performed on fluorescence traces which contained three or more peaks to determine whether different flashing bacteria shared a common flashing frequency. Nine of 10 traces analyzed showed a dominant peak frequency corresponding to a period of 72–120 s.
Flashing Occurs on the Phagosome Membrane
ActA is the only L. monocytogenes protein required for actin-based motility of the bacteria in host cells (Kocks et al., 1992; Smith et al., 1995). ActA recruits all additional proteins required for actin-based motility from the host cell cytoplasm. As actin is nucleated on the bacterial surface, it initially forms a cloud around the whole bacterium. This then transforms into a polarized comet tail that propels movement of the bacterium (Tilney and Portnoy, 1989). We initially hypothesized that flashing depended on ActA and represented a stage in actin nucleation that preceded stable cloud formation. To test this, we used L. monocytogenes mutants expressing different amounts of ActA.
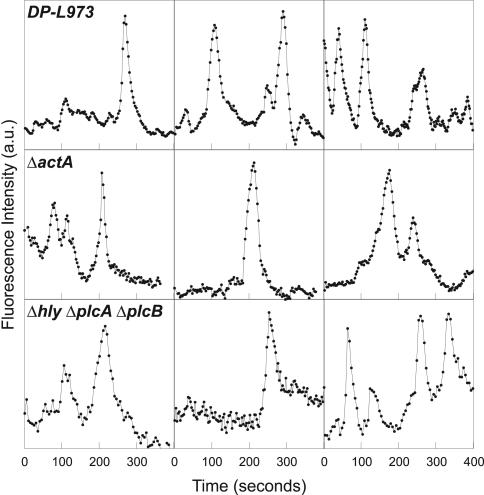
L. monocytogenes strain DP-L973 expresses decreased levels of gene products regulated by the transcriptional activator PrfA, including ActA, and DP-L973 is deficient in actin-based motility (Freitag et al., 1993; Smith et al., 1996). DP-L973 invaded MDCK A10* cells and was able to induce flashing (Figure 2), suggesting that decreased ActA levels had no effect on flashing. To determine whether ActA had any involvement in flashing, we infected cells with L. monocytogenes ΔactA. L. monocytogenes ΔactA were able to flash (Figure 2). Therefore, flashing is not dependent on ActA-mediated nucleation of actin.
Figure 2.
Flashing is independent of ActA and occurs on the phagosome membrane. Traces of fluorescence intensity (GFP-actin) versus time for various points on flashing L. monocytogenes strains DP-L973, ΔactA, and Δhly ΔplcA ΔplcB. L. monocytogenes Δhly ΔplcA ΔplcB is unable to escape the phagosome but still flashes.
We had noticed that the proportion of flashing bacteria for DP-L973 was approximately twice that of wild-type L. monocytogenes. PrfA also regulates genes required for escape of L. monocytogenes from the phagosome (reviewed in Cossart and Lecuit, 1998). Because DP-L973 remains in the phagosome for a longer period (Sun et al., 1990) and flashed more frequently compared with wild-type L. monocytogenes, we hypothesized that flashing might be associated with the phagosome rather than the bacterial surface.
Infection of MDCK A10* cells with L. monocytogenes Δhly ΔplcA ΔplcB, a mutant that is unable to escape the phagosome (O'Riordan et al., 2002), demonstrated that these bacteria were able to flash (Figure 2). Thus, the actin dynamics we observe corresponds to actin assembly and disassembly on the phagosome membrane, and direct contact between the bacterial surface and the cytosol is not required. That the actin accumulates close to the bacterial surface is consistent with electron micrographs of L. monocytogenes in epithelial cells which show a close apposition of the phagosome membrane with the bacteria (Gaillard et al., 1987; Mengaud et al., 1996; Lecuit et al., 2000).
Flashing Occurs after Internalization of Bacteria
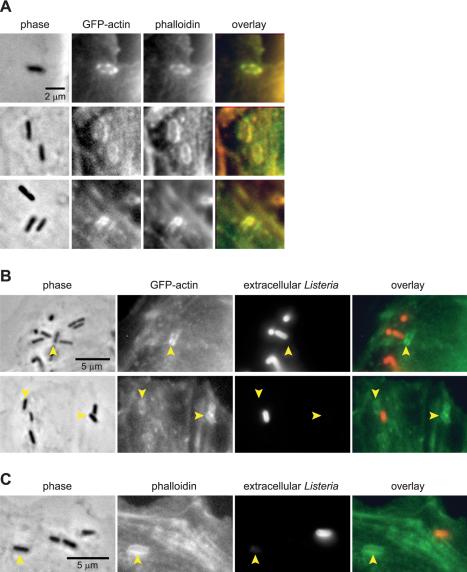
To determine whether the GFP-actin accumulation during flashing represented F-actin, we fixed MDCK A10* cells infected with L. monocytogenes Δhly ΔplcA ΔplcB and labeled them with phalloidin. GFP-actin accumulation on the phagosome colocalized with phalloidin (Figure 3A). Hence, the actin accumulated on the phagosome is polymerized actin.
Figure 3.
Flashing occurs after internalization of bacteria. (A) MDCK A10* cells infected with L. monocytogenes Δhly ΔplcA ΔplcB fixed and labeled with phalloidin. Phalloidin labeling colocalizes with GFP-actin accumulation around the bacteria. (B) MDCK A10* cells or (C) MDCK G cells infected with L. monocytogenes Δhly ΔplcA ΔplcB fixed and stained for extracellular L. monocytogenes. MDCK G cells also were stained with phalloidin. Arrowheads indicate bacteria associated with actin. Actin localization around bacteria occurs on bacteria that are fully internalized and not accessible to the polyclonal serum raised against L. monocytogenes O antigen.
To determine whether flashing occurred during or after internalization of bacteria, cells infected with L. monocytogenes Δhly ΔplcA ΔplcB were fixed and stained such that intracellular and extracellular bacteria could be distinguished from each other. We fixed cells without permeabilization, and added polyclonal serum raised against L. monocytogenes O antigen such that only extracellular bacteria were stained. Intracellular bacteria were protected from the antibody and were nonfluorescent. When actin was present on a bacterium, the bacterium was protected from the anti-serum, indicating that the bacterium was intracellular (Figure 3B). Also, a nonuniform distribution of actin was not necessarily associated with a bacterium that was partially inside and partially outside the cell (Figure 3B, top), suggesting that flashing actin and entry are not necessarily correlated. In a minority (∼35%) of instances where bacteria were associated with actin, the bacteria seemed to be partially inside the cell. These cases were presumed to be bacteria in the process of entering the cell and were often characterized by fainter actin staining compared with those bacteria that were fully internalized. Extracellular bacteria and extracellular portions of bacteria did not costain for actin. Therefore, flashing most likely does not represent abortive attempts at phagocytosis and represents actin accumulation on a fully formed phagosome.
To test whether actin accumulation on fully formed phagosomes also occurred on MDCK cells that do not express GFP-actin, we used MDCK G cells. Phalloidin staining showed that F-actin also colocalized around the phagosome of L. monocytogenes Δhly ΔplcA ΔplcB in MDCK G cells (Figure 3C). Similar to MDCK A10* cells, actin accumulation was observed on bacteria that were intracellular. Therefore, flashing is not an artifact of GFP-actin expression.
Previous studies of macrophages and epithelial cells infected with wild-type L. monocytogenes show that approximately one-third of the bacteria remain within the phagosome 2 h after infection (Tilney and Portnoy, 1989; Marquis et al., 1995). We observed flashing 1–4 h after infection of MDCK cells with L. monocytogenes, consistent with the period during which the bacteria reside in the phagosome.
Flashing Actin Is Associated with Arp2/3 Complex
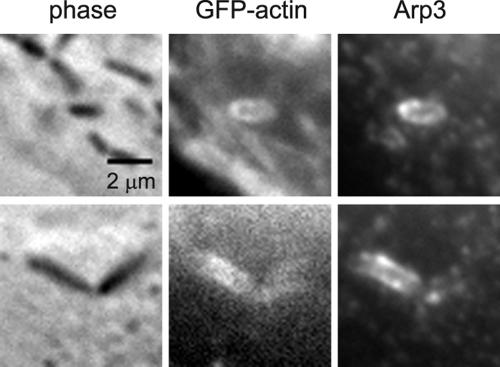
ActA mediates actin polymerization through activation of the Arp2/3 complex. Arp2/3 complex has nucleating and branching activity that enables it to form dendritic networks of actin (Mullins et al., 1998). Even though flashing is not dependent on ActA, Arp2/3 complex may still be involved in flashing. We immunostained MDCK A10* cells infected with L. monocytogenes Δhly ΔplcA ΔplcB for Arp3 and p34, two members of the Arp2/3 complex. Flashing actin colocalized with Arp3 (Figure 4). Similar results were obtained for p34 staining. Therefore, Arp2/3 complex is probably involved in mediating actin filament network growth in flashing.
Figure 4.
Flashing actin is associated with Arp3. MDCK A10* cells infected with L. monocytogenes Δhly ΔplcA ΔplcB, fixed and stained for Arp3. Arp3 staining colocalizes with GFP-actin around the bacterial phagosome.
In experiments performed with L. monocytogenes and E-cadherin–coated beads (see below), sequestration of actin monomers by treating cells with 1 μM latrunculin A abolished flashing. Flashing is not dependent on the activity of phosphatidylinositol phosphate 3-kinase (PI 3-kinase), because addition of 50 or 100 μM LY 294002, a PI-3 kinase inhibitor, to the cells failed to inhibit flashing or perturb flashing dynamics. In addition, flashing is not dependent on acidification of the phagosome because treatment of the cells with chloroquine, a membrane-permeant weak base that neutralizes the endosomal compartment, had no effect on flashing.
Flashing Occurs Downstream of Several Distinct Molecular Entry Mechanisms
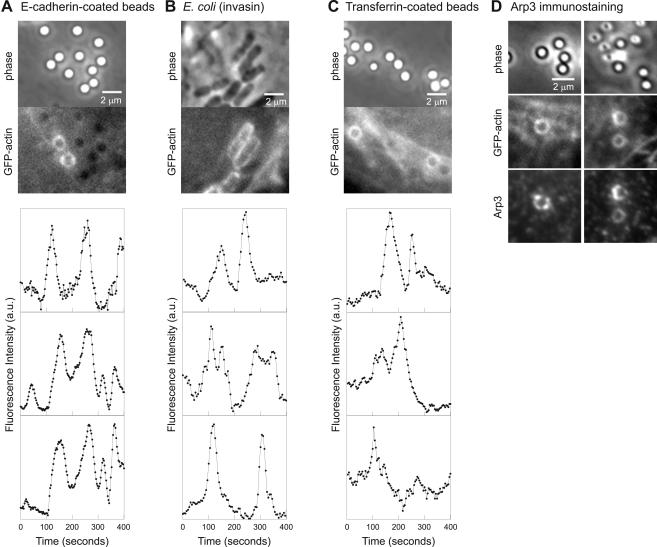
We have shown that flashing occurs on L. monocytogenes phagosomes in MDCK epithelial cells. Entry of L. monocytogenes into epithelial cells is primarily mediated by internalin (InlA) (Mengaud et al., 1996; Lecuit et al., 2001). The receptor for InlA is E-cadherin, a calcium-dependent cell-cell adhesion molecule normally involved in homophilic interactions at adherens junctions of polarized epithelial cells. The interaction of InlA with E-cadherin promotes binding and entry of L. monocytogenes into epithelial cells (Mengaud et al., 1996). Polystyrene beads coated with the extracellular domain of E-cadherin also are internalized when added to MDCK cells, via interactions with E-cadherin on the cell surface (Perez and Nelson, personal communication). Internalized E-cadherin–coated beads flashed (Figure 5A; Vid3Fig5 in Supplementary Materials), demonstrating that E-cadherin–mediated entry into epithelial cells is sufficient for flashing to occur. E-cadherin–coated beads flashed with similar spatial and temporal characteristics to L. monocytogenes flashing (Figure 5A). The power spectrum of 8 of 12 fluorescence traces of flashing E-cadherin beads shared a peak corresponding to a period of 82–118 s, similar to that of wild-type L. monocytogenes. Furthermore, because the beads are inert, the ability of E-cadherin–coated beads to trigger flashing demonstrates that flashing is not dependent on expression of bacterial proteins, signaling from the bacteria across the phagosome membrane, or escape from the phagosome.
Figure 5.
Flashing occurs downstream of several distinct molecular entry mechanisms. MDCK A10* cells with internalized E-cadherin–coated beads (A), E. coli (invasin) (B), or transferrin-coated beads (C) all flash. Top, matched phase contrast and GFP-actin image. Bottom, traces of fluorescence intensity (GFP-actin) versus time for various points on the flashing objects. (D) GFP-actin on transferring-coated beads is associated with Arp3. Transferrin-coated beads were added to MDCK A10* cells, and cells were fixed and stained for Arp3. Full video Vid3Fig5 (A) is available as supplementary material.
To determine whether flashing was specific to E-cadherin–mediated entry into cells, we tested two other uptake mechanisms: that of Y. pseudotuberculosis and transferrin. Invasin, a bacterial outer membrane protein from Y. pseudotuberculosis, binds β1-integrins on the host cell surface and is sufficient for entry of bacteria into host cells (Isberg and Falkow, 1985; Isberg and Leong, 1990). E. coli expressing invasin also induced flashing (Figure 5B). Therefore, although E-cadherin binding is sufficient for flashing, it is not necessary.
Using E. coli (invasin), we were able to compare entry of a bacterium into a cell with flashing. During one movie (Video4 in Supplementary Materials), a bacterium was taken up by a cell and subsequently began to flash. Entry of the bacterium was associated with weak actin accumulation, consistent with previous fixed cell studies of invasin-mediated entry into cells (Finlay et al., 1991; Mengaud et al., 1996). Weak actin accumulation began ∼1 min after first contact between the bacterium and the cell, and faint actin outlining the presumptive phagocytic cup enveloped the bacterium for a duration of 3–4 min. We observed more intense actin accumulation during flashing, which began ∼6 min later.
Both E-cadherin and β-integrin normally have a close, albeit indirect, interaction with actin as part of their adhesive function. To investigate a mechanism of entry that involves receptors that do not consistently interact with the actin cytoskeleton, we added transferrin-coated beads to epithelial cells. Transferrin binds to transferrin receptors on the cell membrane and is internalized by a clathrin-dependent pathway. Neither filamentous actin nor actin assembly play an obligatory role for clathrin-mediated endocytosis of transferrin (Fujimoto et al., 2000). Transferrin-coated beads also flashed (Figure 5C). Arp3 and p34 immunostaining of transferrin-coated beads added to MDCK A10* cells demonstrated that the actin accumulated on the bead phagosomes also associated with Arp3 (Figure 5D) and p34, as was the case for the L. monocytogenes phagosomes.
Flashing seemed similar among the different L. monocytogenes strains, E. coli (invasin), E-cadherin–coated beads, and transferrin-coated beads. The traces of GFP-actin fluorescence intensity versus time (Figures 1C, 2 and 5) allowed us to quantitate flashing behavior and to compare the flashing kinetics between the different objects. We analyzed the peaks in GFP-actin fluorescence associated with flashing. The analysis was restricted to single isolated peaks. Multiple overlapping peaks were assumed to be composed of overlapping single events and excluded from the analysis. Plateaus were extremely rare (<2% of points examined).
The rise time and fall time (Figure 6A) were defined as the time it took for the actin to assemble and disassemble, respectively, during the steepest part of the flash. Rise times clustered around 15–40 s (Figure 6B), and fall times clustered around 20–45 s (Figure 6C). The rise and fall times were independent of peak intensity. Comparison of the rise times (Figure 6B) for all the L. monocytogenes strains, E-cadherin–coated beads and transferrin-coated beads showed that none of populations were significantly different from each other (p >0.05). The E. coli (invasin) rise time might be slightly longer than that of wild-type L. monocytogenes and DP-L973 (p < 0.01). Comparison of the fall times (Figure 6C) for the L. monocytogenes strains, E-cadherin–coated beads, E. coli (invasin), and transferrin-coated beads showed that none of the fall times were significantly different from each other (p > 0.05). The similarity in the rise and fall times for all the populations suggests that the actin dynamics in all cases are the same. Thus, flashing seems to be independent of the molecular entry mechanism of the object.
Flashing May Be Associated with Membrane Deformation
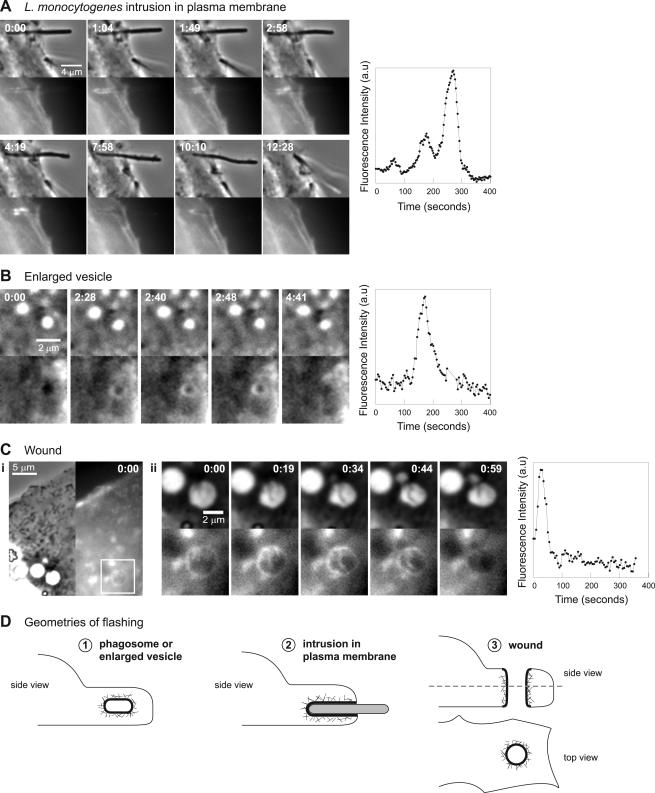
Surprisingly, flashing can occur on membrane domains other than completed phagosomes. Actin dynamics with kinetics similar to flashing were observed as a result of perturbation of the cell membrane by objects that were not fully phagocytosed, on the surface of induced vesicles in the cells, and at the edge of a wound in the cell (Figure 7). In these cases, flashing occurs on the deformed membrane long after the initial change has occurred. Transient or shallow membrane deformations are probably insufficient to induce flashing.
Figure 7.
Flashing may be associated with deformed membranes. (A) MDCK A10* cell infected with L. monocytogenes DP-L4032. Left, paired phase contrast and fluorescence (GFP-actin) time-lapse images. Time in minutes:seconds. Flashing actin is associated with an unusually long bacterium unable to enter the cell, which deforms the plasma membrane and is eventually thrust out of the cell. Right, trace of fluorescence intensity (GFP-actin) verus time for a point from the flashing bacterium. (B) MDCK A10* cell treated with 50 μM LY 294002. Left, paired phase contrast and fluorescence (GFP-actin) time-lapse images. Time in minutes:seconds. Enlarged vesicles flash. Right, trace of fluorescence intensity (GFP-actin) versus time for a point from the flashing vesicle. (C) MDCK A10* cell with microinjection needle wounds. (i) Phase contrast and fluorescence (GFP-actin) images of the wound in the cell at time 0:00. (ii) Left, paired phase contrast and fluorescence (GFP-actin) time-lapse images of the area of the cell containing the wound denoted by the box in i. Time in minutes:seconds. The edge of the wound has flashing actin dynamics. Right, trace of fluorescence intensity (GFP-actin) versus time for a point on the edge of the wound. (D) Various membrane geometries where we have observed flashing actin dynamics. Full videos Vid5Fig7 (A) and Vid7Fig7 (C) are available as supplementary material.
Where unusually long bacteria were partially engulfed by the host cell (Figure 7A; Vid5Fig7 in Supplementary Materials), actin assembled and disassembled around the membrane-bound portion of the bacterium. These bacteria could not be fully phagocytosed and deformed the plasma membrane for a prolonged period. Like flashing, the actin assembly was transient and propagated along the membrane. The rise times and fall times of the actin dynamics were consistent with those of flashing bacteria and beads. Flashing actin on the engulfed portion of a bacterium was accompanied by the bacterium being thrust away from the cell, suggesting that the force generated by actin polymerization on the membrane contributed to the ejection of the bacterium from the cell.
As stated earlier, addition of the PI 3-kinase inhibitor LY 294002 to the cells had no effect on flashing kinetics. It did, however, induce the accumulation of enlarged intracellular vesicles, which have previously been identified as having characteristics of endosomes (Chen and Wang, 2001). These enlarged vesicles also flashed, with rise times and fall times that were consistent with those of the flashing bacteria and beads (Figure 7B; Video6 in Supplementary Materials). Furthermore, when a cell was accidentally wounded with a microinjection needle, piercing through the apical and basal plasma membrane (diagrammed in Figure 7D) actin transiently accumulated at the edge of the wound. The rise and fall time of the actin flash around the wound edge was consistent with that of flashing (Figure 7C; Vid7Fig7 in Supplementary Materials).
These various observations argue that actin flashing is not only limited to fully internalized objects, but also occurs in situations where there is a perturbation in the shape of the plasma membrane such that the plasma membrane (or membrane derived from the plasma membrane) is in contact with cytoplasm that is not normally adjacent to the plasma membrane. We have observed three geometries of deformed membranes that trigger this dynamic actin assembly and disassembly (Figure 7D).
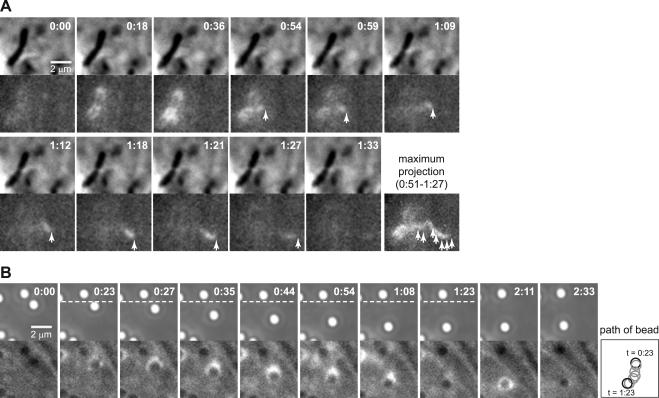
In some cases, small actin comet tails emerged from a flashing bacterium or bead and moved away from the flashing object (Figure 8A; Vid8Fig8 in Supplementary Materials). These comets are reminiscent of those observed on small vesicles and endosomes (Rozelle et al., 2000; Taunton et al., 2000). Actin tails also could be directly formed on a flashing phagosome (Figure 8B). This polarized accumulation of actin on phagosomes of bacteria or beads was usually accompanied by transient directional movement. The same phagosome could exhibit both polarized and symmetrical actin flashing (Figure 8B). Therefore, polarized flashing accompanied by an actin comet tail and symmetrical flashing are likely to be different expressions of the same phenomenon.
Figure 8.
Flashing can initiate directed movement. Paired phase and fluorescence (GFP-actin) time-lapse images. Time in minutes:seconds. (A) MDCK A10* cell infected with L. monocytogenes ΔactA. The bacterium flashes (0:18–0:59) and an actin comet tail emerges from the flashing actin. Arrowheads indicate the position of the comet tail. The maximum projection of fluorescence intensity across the images illustrates the path of the comet tail. (B) MDCK A10* cell with internalized E-cadherin–coated beads. The same bead can nucleate a short actin comet tail (0:23–1:23) propelling directional movement, and a nonpolar flash (2:11). Right, diagram depicting the position of the bead during movement from 0:23–1:23. Full video Vid8Fig8 (A) is available as supplementary material.
DISCUSSION
Repeated Cycles of Actin Assembly and Disassembly Occurs on Phagosomes
This is the first study of the spatial and temporal dynamics of actin on phagosomes in live epithelial cells. Previous proteomic and immunofluorescence studies showing the presence of actin on macrophage and epithelial cell phagosomes have focused on fixed or isolated samples (Defacque et al., 2000; Garin et al., 2001; Pizarro-Cerda et al., 2002), overlooking the striking dynamics that we have observed. Based on our observations of cycles of transient actin assembly and disassembly on phagosomes in live epithelial cells, we suggest that the association of actin with phagosomes is highly dynamic.
Previous observations of L. monocytogenes phagosomes in fixed cells have shown that actin is present on the phagosomes of bacteria that are entirely intracellular (Bierne et al., 2001). This observation was proposed to correspond to phagocytic cup closure or residual F-actin from phagocytosis. Our live cell imaging of GFP-actin on phagosomes together with our immunostaining data places these results in a new context. Prior observations in fixed cells of actin on L. monocytogenes phagosomes (Lecuit et al., 2000; Bierne et al., 2001) may in fact be snapshots of flashing. Flashing is unlikely to be associated with phagocytic cup closure because it can occur repeatedly (≥3 times) over tens of minutes on the same phagosome while the phagosome is drifting relative to the cell. If flashing were associated with phagosome closure, it might be hypothesized that the actin would be localized to the point of closure where the phagosome is being pinched off from the membrane. Instead, we observe actin accumulation over the entire phagosome, sometimes in varying spatial patterns on the same phagosome. When actin is localized around the phagosome, the majority of the bacteria are completely intracellular. In addition, some flashing phagosomes form short comet tails and move through the cytoplasm in a manner that suggests that they are unattached to the plasma membrane, similar to actin-based vesicle movement.
Inhibition of PI 3-kinase with LY 294002 had no effect on the flashing of E-cadherin–coated beads, suggesting that flashing is not dependent on PI 3-kinase. Given the robust nature of flashing, we predict that this observation would be true for flashing on the phagosomes of L. monocytogenes and E. coli (invasin). In contrast, efficient InlA- or InlB-mediated entry of L. monocytogenes (Ireton et al., 1996) and invasin-mediated uptake (Mecsas et al., 1998) into nonphagocytic cells requires PI 3-kinase. This suggests that the mechanism of flashing differs from the mechanism of entry into a cell.
Like flashing actin on epithelial cell phagosomes, phosphatidylinositol 3-phosphate (PI3P) cycles on and off macrophage bead phagosomes (Chua and Deretic, 2004) and Salmonella typhimurium epithelial cell phagosomes (Pattni et al., 2001). It has been proposed that the recurring waves of PI3P may play a role in phagosomal maturation (Chua and Deretic, 2004) and that this can be modulated by intracellular pathogens. For example, Mycobacterium tuberculosis alters the timing and characteristics of PI3P waves on its phagosomes as a strategy for blocking phagosome conversion into the phagolysosome (Chua and Deretic, 2004).
Likewise, some intracellular pathogens that survive and replicate within the phagosome modulate the F-actin network around the phagosome. For example, the formation of an F-actin meshwork around the macrophage phagosome is essential for replication of S. typhimurium (Meresse et al., 2001). In contrast to S. typhimurium, inhibition of actin assembly on the phagosome seems to be important for survival of M. tuberculosis and Mycobacterium avium inside the macrophage phagosome (Anes et al., 2003). In these cases where intracellular pathogens modulate actin polymerization on phagosomes for their own advantage, this may be achieved through modulating the endogenous process of actin flashing. Perhaps the small actin comet tails that emerge from flashing bacteria or beads and move away from the flashing object reflect the occurrence of membrane remodeling on the phagosomes associated with phagosome maturation.
Phagosomes, Endosomes, and Vesicles All Exhibit Actin-based Motility
Flashing objects can nucleate short actin comet tails that propel directional movement, suggesting that there is a connection between flashing and vesicle rocketing. Actin comet tails have been observed in vivo on endosomes and lysosomes stimulated with phorbol 12-myristate 13-acetate (Taunton et al., 2000), endosomes in macrophages treated with lanthanum and zinc ions (Southwick et al., 2003), pinosomes (Merrifield et al., 1999), and Golgi and endocytic vesicles in cells overexpressing phosphatidylinositol phosphate 5-kinase (PI 5-kinase) or treated with pervanadate and platelet-derived growth factor (Rozelle et al., 2000). In addition, actin-based phagosome motility has been observed at a very low frequency (0.6–1.5% of engulfed beads over a 10-min interval) in unstimulated macrophages (Zhang et al., 2002).
Vesicle rocketing, phagosome rocketing, and flashing share several kinetic and molecular features. All are transient events and, under unstimulated conditions, infrequent events. Actin tails on macrophage phagosomes persist for only 1–2 min (Zhang et al., 2002). Actin tails on vesicles induced by PI-5 kinase overexpression persist for an average of 2.2 min (Rozelle et al., 2000). In our study, flashing had rise times of ∼25 s and fall times of ∼30 s; a total duration of ∼1 min. Furthermore, Arp2/3 complex is present throughout the actin tail of endosomes and vesicles (Rozelle et al., 2000; Taunton et al., 2000; Southwick et al., 2003) and colocalizes with flashing actin. Actin-based phagosome motility in macrophages does not depend on the surface coating on the bead (Zhang et al., 2002). Likewise, flashing does not depend on the molecular features of the internalized object. Inhibition of PI-3 kinase does not block comet tail formation (Rozelle et al., 2000) nor in vitro assembly of actin on bead phagosomes (Defacque et al., 2002). Similarly, inhibition of PI 3-kinase had no effect on flashing.
Although there are cases when flashing is asymmetrical and associated with transient periods of directed motion, we did not observe any transition from flashing to persistent directed motion. The preference for nonpolar assembly of actin over the entire phagosome in contrast with the formation of polarized comet tails on vesicles may be due to the different sizes of the objects involved. Actin nucleation on larger objects tends to be nonpolar and uniform. Decreasing the size of the nucleating object increases the probability of symmetry breaking and formation of a polar comet tail (Cameron et al., 1999, 2004). In general, phagosomes are larger than endocytic vesicles, and therefore would have a preference for nonpolar assembly of actin on their surfaces. In addition, variation in the fluidity of the membrane (Giardini et al., 2003) can contribute to differences in the probability of symmetry breaking and the resultant geometry of the actin assembly on the object. Hence, nonpolar and polar forms of actin assembly on membrane-bound organelles may reflect different facets of the same phenomenon.
The ability of phagosomes, endosomes, and vesicles to nucleate actin polymerization in vivo suggests that they share a supramolecular organizational similarity. It has been suggested that sphingolipid-cholesterol rafts may be platforms for PIP2-dependent actin polymerization on the membrane of vesicles (Rozelle et al., 2000). A similar mechanism may exist for actin nucleation on phagosomes, which has been shown to be dependent on PIP2 in vitro (Defacque et al., 2002) and in vivo (Anes et al., 2003) for macrophage phagosomes. Interestingly, proteins associated with lipid rafts, such as flotillin and stomatin, have been identified on phagosomes from macrophages (Garin et al., 2001) and may localize to lipid subdomains (Dermine et al., 2001).
Flashing Reflects Host Cell Actin Dynamics
Actin flashing dynamics are not limited to fully formed phagosomes and also occur on several membrane geometries. Flashing was coincident with the expulsion of unusually long bacteria from cells and reduction in the size of a wound. This suggests that some property of deformed membranes triggers flashing, when the deformations are sufficiently large and prolonged. Furthermore, flashing may be a host cell response involved in the repair of wounds in the cell or maintenance of cellular integrity when foreign objects disrupt the shape of the membrane.
Flashing is more common in the cell periphery than the cell center, and this may reflect differences in host cell actin dynamics in various parts of the cell. The cell periphery may be a more permissible environment for actin assembly on membrane-bound organelles. For example, endosomal rocketing occurs after formation of the endosome and moves the endosome away from the cell periphery into the cell center, where actin assembly on membranes may be less frequent. In contrast, flashing phagosomes remain in the periphery for longer and drift slowly toward the cell center, giving rise to more opportunities for the assembly of actin on the membrane and repeated cycles of flashing.
Traveling waves of actin have been observed in fibroblasts, epithelial cells, and in Dictyostelium (Vicker, 2002; Krueger et al., 2003; Bretschneider et al., 2004). It has been proposed that they are involved in the creation of protrusive structures and remodeling of the actin cytoskeleton over cellular distances. The rise and fall times of the actin waves in Dictyostelium (Bretschneider et al., 2004) were shorter than those seen for flashing, but the shape of the events was similar, with the rise time being slightly faster than the fall time. One tantalizing possibility is that flashing on phagosomes and disturbed membranes is a result of the actin waves interacting with membrane surfaces. Flashing is sometimes coordinated between phagosomes over large distances, so phagosomes may be sensing both large scale waves that are easily visualized, as well as smaller undetectable waves. These actin waves may be one method cells use to both probe the state of the cell and respond to perturbations.
Supplementary Material
Acknowledgments
We are grateful to D. A. Portnoy, D. M. Monack, T. D. Perez, and M. D. Welch for reagents. We thank members of the Theriot laboratory for stimulating discussions and support throughout the course of the project, in particular K. Keren for assistance with MatLab. We acknowledge the initial observations of flashing made by D. M. Monack and J. R. Robbins. We thank S. R. Pfeffer, I. Mellman, and W. J. Nelson for suggesting the transferrin bead experiment, and J. Swanson for helpful discussions. We greatly appreciate comments on the manuscript from D. A. Aivazian, M. R. Amieva, A. E. Brotcke, R. A. Ihrie, T. D. Perez and S. R. Pfeffer. This work was supported by National Institutes of Health grant AI-36929. P.T.Y. was supported by a Howard Hughes Medical Institute Predoctoral Fellowship, Stanford Graduate Fellowship, and a Skye International Foundation Scholarship.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04–06–0509. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–06–0509.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Alrutz, M.A., Srivastava, A., Wong, K.W., D'Souza-Schorey, C., Tang, M., Ch'Ng, L.E., Snapper, S.B., and Isberg, R.R. (2001). Efficient uptake of Yersinia pseudotuberculosis via integrin receptors involves a Rac1-Arp 2/3 pathway that bypasses N-WASP function. Mol. Microbiol. 42, 689-703. [DOI] [PubMed] [Google Scholar]
- Anes, E., Kuhnel, M.P., Bos, E., Moniz-Pereira, J., Habermann, A., and Griffiths, G. (2003). Selected lipids activate phagosome actin assembly and maturation resulting in killing of pathogenic mycobacteria. Nat. Cell Biol. 5, 793-802. [DOI] [PubMed] [Google Scholar]
- Bierne, H., Gouin, E., Roux, P., Caroni, P., Yin, H.L., and Cossart, P. (2001). A role for cofilin and LIM kinase in Listeria-induced phagocytosis. J. Cell Biol. 155, 101-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, D.K., and Hinrichs, D.J. (1987). Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 139, 2005-2009. [PubMed] [Google Scholar]
- Braun, L., Ghebrehiwet, B., and Cossart, P. (2000). gC1q-R/p32, a C1q-binding protein, is a receptor for the InlB invasion protein of Listeria monocytogenes. EMBO J. 19, 1458-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretschneider, T., Diez, S., Anderson, K., Heuser, J., Clarke, M., Muller-Taubenberger, A., Kohler, J., and Gerisch, G. (2004). Dynamic actin patterns and Arp2/3 assembly at the substrate-attached surface of motile cells. Curr. Biol. 14, 1-10. [DOI] [PubMed] [Google Scholar]
- Cameron, L.A., Footer, M.J., van Oudenaarden, A., and Theriot, J.A. (1999). Motility of ActA protein-coated microspheres driven by actin polymerization. Proc. Natl. Acad. Sci. USA 96, 4908-4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron, L.A., Robbins, J.R., Footer, M.J., and Theriot, J.A. (2004). Biophysical parameters influence actin-based movement, trajectory, and initiation in a cell-free system. Mol. Biol. Cell 15, 2312-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X., and Wang, Z. (2001). Regulation of intracellular trafficking of the EGF receptor by Rab5 in the absence of phosphatidylinositol 3-kinase activity. EMBO Rep. 2, 68-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.T., and Nelson, W.J. (1996). Continuous production of soluble extracellular domain of a type-I transmembrane protein in mammalian cells using an Epstein-Barr virus Ori-P-based expression vector. Anal. Biochem. 242, 276-278. [DOI] [PubMed] [Google Scholar]
- Chua, J., and Deretic, V. (2004). Mycobacterium tuberculosis reprograms waves of phosphatidylinositol 3-phosphate on phagosomal organelles. J. Biol. Chem. 279, 36982-36992. [DOI] [PubMed] [Google Scholar]
- Cossart, P., and Lecuit, M. (1998). Interactions of Listeria monocytogenes with mammalian cells during entry and actin-based movement: bacterial factors, cellular ligands and signaling. EMBO J. 17, 3797-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart, P., and Sansonetti, P.J. (2004). Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304, 242-248. [DOI] [PubMed] [Google Scholar]
- Defacque, H., Bos, E., Garvalov, B., Barret, C., Roy, C., Mangeat, P., Shin, H.W., Rybin, V., and Griffiths, G. (2002). Phosphoinositides regulate membrane-dependent actin assembly by latex bead phagosomes. Mol. Biol. Cell 13, 1190-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defacque, H., et al. (2000). Involvement of ezrin/moesin in de novo actin assembly on phagosomal membranes. EMBO J. 19, 199-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermine, J.F., Duclos, S., Garin, J., St-Louis, F., Rea, S., Parton, R.G., and Desjardins, M. (2001). Flotillin-1-enriched lipid raft domains accumulate on maturing phagosomes. J. Biol. Chem. 276, 18507-18512. [DOI] [PubMed] [Google Scholar]
- Finlay, B.B., Ruschkowski, S., and Dedhar, S. (1991). Cytoskeletal rearrangements accompanying Salmonella entry into epithelial cells. J. Cell Sci. 99, 283-296. [DOI] [PubMed] [Google Scholar]
- Freitag, N.E., Rong, L., and Portnoy, D.A. (1993). Regulation of the prfA transcriptional activator of Listeria monocytogenes: multiple promoter elements contribute to intracellular growth and cell-to-cell spread. Infect. Immun. 61, 2537-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto, L.M., Roth, R., Heuser, J.E., and Schmid, S.L. (2000). Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis in mammalian cells. Traffic 1, 161-171. [DOI] [PubMed] [Google Scholar]
- Gaillard, J.L., Berche, P., Mounier, J., Richard, S., and Sansonetti, P. (1987). In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect. Immun. 55, 2822-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garin, J., Diez, R., Kieffer, S., Dermine, J.F., Duclos, S., Gagnon, E., Sadoul, R., Rondeau, C., and Desjardins, M. (2001). The phagosome proteome: insight into phagosome functions. J. Cell Biol. 152, 165-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedde, M.M., Higgins, D.E., Tilney, L.G., and Portnoy, D.A. (2000). Role of listeriolysin O in cell-to-cell spread of Listeria monocytogenes. Infect. Immun. 68, 999-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardini, P.A., Fletcher, D.A., and Theriot, J.A. (2003). Compression forces generated by actin comet tails on lipid vesicles. Proc. Natl. Acad. Sci. USA 100, 6493-6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton, K., Payrastre, B., Chap, H., Ogawa, W., Sakaue, H., Kasuga, M., and Cossart, P. (1996). A role for phosphoinositide 3-kinase in bacterial invasion. Science 274, 780-782. [DOI] [PubMed] [Google Scholar]
- Isberg, R.R., and Falkow, S. (1985). A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature 317, 262-264. [DOI] [PubMed] [Google Scholar]
- Isberg, R.R., and Leong, J.M. (1990). Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell 60, 861-871. [DOI] [PubMed] [Google Scholar]
- Kocks, C., Gouin, E., Tabouret, M., Berche, P., Ohayon, H., and Cossart, P. (1992). L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell 68, 521-531. [DOI] [PubMed] [Google Scholar]
- Krueger, E.W., Orth, J.D., Cao, H., and McNiven, M.A. (2003). A dynamincortactin-Arp2/3 complex mediates actin reorganization in growth factor-stimulated cells. Mol. Biol. Cell 14, 1085-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit, M., Hurme, R., Pizarro-Cerda, J., Ohayon, H., Geiger, B., and Cossart, P. (2000). A role for alpha-and beta-catenins in bacterial uptake. Proc. Natl. Acad. Sci. USA 97, 10008-10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit, M., Vandormael-Pournin, S., Lefort, J., Huerre, M., Gounon, P., Dupuy, C., Babinet, C., and Cossart, P. (2001). A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science 292, 1722-1725. [DOI] [PubMed] [Google Scholar]
- Markelonis, G.J., Bradshaw, R.A., Oh, T.H., Johnson, J.L., and Bates, O.J. (1982). Sciatin is a transferrin-like polypeptide. J. Neurochem. 39, 315-320. [DOI] [PubMed] [Google Scholar]
- Marquis, H., Doshi, V., and Portnoy, D.A. (1995). The broad-range phospholipase C and a metalloprotease mediate listeriolysin O-independent escape of Listeria monocytogenes from a primary vacuole in human epithelial cells. Infect. Immun. 63, 4531-4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecsas, J., Raupach, B., and Falkow, S. (1998). The Yersinia Yops inhibit invasion of Listeria, Shigella and Edwardsiella but not Salmonella into epithelial cells. Mol. Microbiol. 28, 1269-1281. [DOI] [PubMed] [Google Scholar]
- Mengaud, J., Ohayon, H., Gounon, P., Mege, R.M., and Cossart, P. (1996). E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84, 923-932. [DOI] [PubMed] [Google Scholar]
- Meresse, S., Unsworth, K.E., Habermann, A., Griffiths, G., Fang, F., Martinez-Lorenzo, M.J., Waterman, S.R., Gorvel, J.P., and Holden, D.W. (2001). Remodelling of the actin cytoskeleton is essential for replication of intravacuolar Salmonella. Cell Microbiol. 3, 567-577. [DOI] [PubMed] [Google Scholar]
- Merrifield, C.J., Moss, S.E., Ballestrem, C., Imhof, B.A., Giese, G., Wunderlich, I., and Almers, W. (1999). Endocytic vesicles move at the tips of actin tails in cultured mast cells. Nat. Cell Biol. 1, 72-74. [DOI] [PubMed] [Google Scholar]
- Monack, D.M., and Theriot, J.A. (2001). Actin-based motility is sufficient for bacterial membrane protrusion formation and host cell uptake. Cell Microbiol. 3, 633-647. [DOI] [PubMed] [Google Scholar]
- Mounier, J., Ryter, A., Coquis-Rondon, M., and Sansonetti, P.J. (1990). Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect. Immun. 58, 1048-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins, R.D., Heuser, J.A., and Pollard, T.D. (1998). The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl. Acad. Sci. USA 95, 6181-6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Riordan, M., Yi, C.H., Gonzales, R., Lee, K.D., and Portnoy, D.A. (2002). Innate recognition of bacteria by a macrophage cytosolic surveillance pathway. Proc. Natl. Acad. Sci. USA 99, 13861-13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattni, K., Jepson, M., Stenmark, H., and Banting, G. (2001). A PtdIns(3)P-specific probe cycles on and off host cell membranes during Salmonella invasion of mammalian cells. Curr. Biol. 11, 1636-1642. [DOI] [PubMed] [Google Scholar]
- Pizarro-Cerda, J., Jonquieres, R., Gouin, E., Vandekerckhove, J., Garin, J., and Cossart, P. (2002). Distinct protein patterns associated with Listeria monocytogenes InlA- or InlB-phagosomes. Cell Microbiol. 4, 101-115. [DOI] [PubMed] [Google Scholar]
- Robbins, J.R., Barth, A.I., Marquis, H., de Hostos, E.L., Nelson, W.J., and Theriot, J.A. (1999). Listeria monocytogenes exploits normal host cell processes to spread from cell to cell. J. Cell Biol. 146, 1333-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozelle, A.L., Machesky, L.M., Yamamoto, M., Driessens, M.H., Insall, R.H., Roth, M.G., Luby-Phelps, K., Marriott, G., Hall, A., and Yin, H.L. (2000). Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP-Arp2/3. Curr. Biol. 10, 311-320. [DOI] [PubMed] [Google Scholar]
- Shen, Y., Naujokas, M., Park, M., and Ireton, K. (2000). InIB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell 103, 501-510. [DOI] [PubMed] [Google Scholar]
- Skoble, J., Auerbuch, V., Goley, E.D., Welch, M.D., and Portnoy, D.A. (2001). Pivotal role of VASP in Arp2/3 complex-mediated actin nucleation, actin branch-formation, and Listeria monocytogenes motility. J. Cell Biol. 155, 89-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoble, J., Portnoy, D.A., and Welch, M.D. (2000). Three regions within ActA promote Arp2/3 complex-mediated actin nucleation and Listeria monocytogenes motility. J. Cell Biol. 150, 527-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, G.A., Portnoy, D.A., and Theriot, J.A. (1995). Asymmetric distribution of the Listeria monocytogenes ActA protein is required and sufficient to direct actin-based motility. Mol. Microbiol. 17, 945-951. [DOI] [PubMed] [Google Scholar]
- Smith, G.A., Theriot, J.A., and Portnoy, D.A. (1996). The tandem repeat domain in the Listeria monocytogenes ActA protein controls the rate of actin-based motility, the percentage of moving bacteria, and the localization of vasodilator-stimulated phosphoprotein and profilin. J. Cell Biol. 135, 647-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick, F.S., Li, W., Zhang, F., Zeile, W.L., and Purich, D.L. (2003). Actin-based endosome and phagosome rocketing in macrophages: activation by the secretagogue antagonists lanthanum and zinc. Cell Motil. Cytoskeleton 54, 41-55. [DOI] [PubMed] [Google Scholar]
- Sun, A.N., Camilli, A., and Portnoy, D.A. (1990). Isolation of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect. Immun. 58, 3770-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons, M.H., and Mitchison, T.J. (1991). Control of actin polymerization in live and permeabilized fibroblasts. J. Cell Biol. 114, 503-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taunton, J., Rowning, B.A., Coughlin, M.L., Wu, M., Moon, R.T., Mitchison, T.J., and Larabell, C.A. (2000). Actin-dependent propulsion of endosomes and lysosomes by recruitment of N-WASP. J. Cell Biol. 148, 519-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney, L.G., and Portnoy, D.A. (1989). Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J. Cell Biol. 109, 1597-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicker, M.G. (2002). Eukaryotic cell locomotion depends on the propagation of self-organized reaction-diffusion waves and oscillations of actin filament assembly. Exp. Cell Res. 275, 54-66. [DOI] [PubMed] [Google Scholar]
- Welch, M.D., DePace, A.H., Verma, S., Iwamatsu, A., and Mitchison, T.J. (1997a). The human Arp2/3 complex is composed of evolutionarily conserved subunits and is localized to cellular regions of dynamic actin filament assembly. J. Cell Biol. 138, 375-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch, M.D., Iwamatsu, A., and Mitchison, T.J. (1997b). Actin polymerization is induced by Arp2/3 protein complex at the surface of Listeria monocytogenes. Nature 385, 265-269. [DOI] [PubMed] [Google Scholar]
- Zhang, F., Southwick, F.S., and Purich, D.L. (2002). Actin-based phagosome motility. Cell Motil. Cytoskeleton 53, 81-88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.