Abstract
Previous studies showed that cultured mouse trophoblast stem cells (mTSCs) have the most rapid proliferation, normal maintenance of stemness/potency, the least spontaneous differentiation, and the lowest level of stress-activated protein kinase (SAPK) when incubated at 2% O2 rather than at the traditional 20% O2 or hypoxic (0.5% and 0% O2) conditions. Switching from 2% O2 induced fast SAPK responses. Here we tested the dose response of AMP-activated protein kinase (AMPK) in its active form (pAMPK Thr172P) at O2 levels from 20–0%, and also tested whether pAMPK levels show similar rapid changes when mTSC cultures were switched from the optimal 2% O2 to other O2 conditions. There was a delayed increase in pAMPK levels ~6–8 h after switching conditions from 20% to 2%, 0.5%, or 0% O2. Altering O2 conditions from 2% to either 20%, 0.5%, or 0% led to rapid increase in pAMPK levels within 1 h, similar to the previously reported SAPK response in mTSC cells removed from 2% O2. Twelve hours of 0.5% O2 exposure led to cell program changes in terms of potency loss and suppressed biosynthesis, as indicated by levels of phosphorylated inactive acetyl CoA carboxylase (pACC). Phosphorylation of ACC was inhibited by the AMPK inhibitor Compound C. However, unlike other stressors, AMPK does not mediate hypoxia-induced potency loss in mTSCs. These results suggest an important aspect of stem cell biology, which demands rapid stress enzyme activation to cope with sudden changes in external environment, e.g., from least stressful (2% O2) to more stressful conditions.
Keywords: AMP-activated protein kinase (AMPK), Hypoxia, Stress, Trophoblast stem cell
5' adenosine monophosphate-activated protein kinase (AMPK) is an essential stress kinase [1]. AMPK activation inhibits cell growth through mammalian target of rapamycin (mTOR), cell cycle checkpoints and other interactions, to promote cell survival in times of stress, as has been previously reviewed [2]. Moreover, AMPK mediates loss of potency in embryonic stem cells (ESC) and trophoblast stem cells (TSC) [3,4,5]. Similar AMPK-mediated stemness/potency loss was found in early mammalian two-cell and blastocyst stage embryos as well, in response to multiple types of stress [4, 5] and drugs such as aspirin and metformin [6]. AMPK-dependent potency loss in two-cell embryos includes both ESC and TSC potency factors [4, 6]. AMPK blocks Warburg metabolism in favor of oxidative metabolism, and this blocks the induction of pluripotency [7, 8]. Although stress-induced high levels of AMPK cause differentiation, increasing AMPK activity in diabetic mice can improve blastocyst development [9]. In addition to potency regulation, AMPK has important roles in the metabolic regulation of early mammalian embryos and their stem cells [10, 11], as well as in somatic cells.
Stemness can be maintained at an O2 niche ≤ 5%, and often at 2–3% [12,13,14]. It was previously established that 2% O2 is the optimal O2 level for mTSC in vitro culture by four criteria: lowest stress (SAPK activation) level, lowest expression of differentiation maker mRNAs, highest growth rate and normal maintenance of potency [15]. Stressors force stem cell differentiation, which has been observed in ESCs and induced pluripotent stem cells [16, 17]. Stress-induced differentiation has also been characterized in mTSCs [18]. In screens for the protein kinases that mediate the stress response of mTSCs, many kinases inhibitors were used; it was found that stress-induced differentiation is mediated through SAPK, which does not affect potency, and that AMPK mediates potency loss [5, 19]. SAPK mediates increased levels of Hand1 mRNA, favoring giant cell differentiation and placental lactogen 1 (PL1) expression, and suppressing later chorionic lineages by decreasing levels of Gcm1 mRNA [11, 20]. PL1 is the hormone that mediates maternal recognition of pregnancy in rodents [21]; this makes it the functional equivalent of chorionic gonadotropin in human, and of interferon-like protein in sheep and cattle [22]. As O2 levels in mTSC culture were switched up or down from 2%, SAPK level showed rapid (1 h) maximal induction when compared to the much slower rates of SAPK activation when O2 levels were switched from 20% to other amounts.
Our hypothesis is that stress induces fast changes in the activity of stress kinases, and that they consequently function to adjust developmental and metabolic programs. Rapid turnover is a feature of many intracellular regulatory and signaling proteins; it enables prompt responses to extracellular or intracellular signals, and rapid cessation of responses upon signal removal. Examples of this include the products of proto-oncogenes, growth factors and inflammatory cytokines [23, 24]. The major regulator of intracellular AMPK activity is the reversible phosphorylation of threonine 172 (Thr172) within the protein′s catalytic α subunit, which activates AMPK [25]. Not surprisingly, AMPK activity also has fast turnover [26]. The level of pAMPK (phosphorylation of AMPKα at Thr172) is often used to indicate AMPK activity [27], and it corresponds with the phosphorylation of its canonical metabolic substrate acetyl CoA carboxylase (ACC Ser79) [28, 29]. ACC catalyzes a rate-limiting reaction in fatty acids synthesis [30]. AMPK phosphorylates ACC at Ser79 and inactivates it, which is an important branch of metabolic regulation by AMPK [31].
Given the central role of AMPK in regulating metabolism, and its emerging role in normal [10] and stressed [4, 5, 32] placental progenitor and stem cell differentiation, we studied the dynamics of AMPK activation in response to O2 changes, using mTSCs as a model. Here, we hypothesize that AMPK also has its lowest activation at 2% O2, similar to SAPK, and that AMPK has faster activation when mTSCs are removed from 2% O2 conditions than when removed from 20% O2 conditions. Because AMPK was found to mediate potency loss and regulate ACC phosphorylation (at Ser79) due to hyperosmolar stress and genotoxic stress [5, 32], we also tested the hypothesis that hypoxic stress induces potency loss and inhibits anabolic metabolism, as exemplified by ACC (Ser79) phosphorylation, and that AMPK is responsible for this outcome.
Materials and Methods
Reagents
Fetal bovine serum, RPMI1640, and fibroblast growth factor 4 (FGF4) were from Gibco (Grand Island, NY, USA). Heparin was purchased from Sigma Chemical (St. Louis, MO, USA). Compound C was purchased from EMD Millipore (Cat# 171260; Billerica, MA, USA). The following antibodies used were from Cell Signaling Technology (Danvers, MA, USA): pAMPK (CS 2535), pACC (Ser79) (CS 3661), β-Actin (CS 4970), anti-rabbit HRP-linked antibody (CS 7074), and anti-mouse HRP-linked antibody (CS 7076). Tubulin (Cat# T 9026, St. Louis, MO, USA) antibody came from Sigma Chemical Co. ErrB and ID2 antibody were purchased from R&D Systems (PP-H6705; Minneapolis, MN, USA) and Santa Cruz Biotechnology (SC-489; Dallas, TX, USA), respectively. Anaerobic bags to create 0% O2 were from Hardy Diagnostics (AN010C; Santa Maria, CA, USA).
Cell lines and culture conditions
The mouse trophoblast stem cell isolate was a gift from Dr. Rossant (Samuel Lunenfeld Research Institute, Ontario, Canada). mTSCs were cultured as described previously [20, 33]. Routine culture conditions were 20% O2, with 25 ng/ml FGF4 and 70% embryonic fibroblast conditioned medium. The cells were passaged approximately 24 h before the start of each experiment to allow recovery from passage stress. In the group of experiments where cells were switched from 20% O2 to other O2 levels, post-passage culturing was conducted at 20% O2. Alternately, if cells were planned to be switched from 2% O2 to other O2 levels, they were placed in 2% O2 for 24 h. The starting cell confluence was around 20–30% prior to the switch. After switching, cells were cultured for various length of time and were then lysed for immunoblot analysis. Culture at 20% O2 was conducted in a conventional CO2 incubator; 2% and 0.5% O2 conditions were achieved using commercial gas mixtures containing [2% O2 /5% CO2 ] or [0.5% O2 /5% CO2 ], balanced with N2. All culture media were pre-equilibrated for 24 h, at the specified O2 levels before use.
Western blot
Cells were washed twice with ice-cold PBS and were then lysed with RIPA buffer (Thermo Scientific). Fifty micrograms of whole-cell extracts were separated by electrophoresis on a 4–20% SDS-PAGE gel using Precast TGX mini gels (Bio-Rad, Hercules, CA, USA), and were then transferred to PVDF membranes using a Bio-Rad Semi-dry Transfer cell. We found that semi-dry transfer was not very efficient in transferring ACC (280 kDa), and so wet transfer was conducted for this protein using a Bio-Rad Trans-blot cell. The membranes were blocked with 5% fat-free milk at room temperature for 1 h, and were probed with primary antibody at 4°C overnight. The dilution of each antibody was pAMPK (1:250), ErrB (1:1000), ID2 (1:300), pACC (1:1000), β-Actin (1:1500), and Tubulin (1: 20000). Horseradish peroxidase (HRP)-conjugated secondary antibody (1:15000) was incubated at room temperature for 1.5 h. Primary antibodies were diluted with 3% BSA/TBST; secondary antibodies were diluted with 2% fat-free milk/TBST. The protein bands were visualized using enhanced chemiluminescence (ECL) (Amersham/GE Healthcare Life Sciences, Marlborough, MA, USA).
Statistical analysis
Data collected over three independent experiments were subjected to the SPSS Statistics software package (version 22.0) for distribution examination and statistical analysis. Treatments were compared to controls using one-way ANOVA. If statistical significance was found, ANOVA was followed by the least square significance (LSD) post hoc test. Data on changing O2 levels from 20% O2 to 2% O2 (at nine time intervals) were logarithmically transformed to meet the normality assumption of ANOVA prior to analysis. Values are presented as mean +/– standard error (SE). Differences between treatments were considered significant if P < 0.05.
Results
During the study of SAPK activation, we found that 2% O2 enabled a growth rate that was 2.5-fold higher than that of 20% O2 for mTSC culture in vitro, but the media was very acidic by 24 h [15]. Therefore, in this experiment, the final duration of time after removing cells from 20% O2 was set to 12 h. The switch from 20% to lower O2 levels was designed to emulate the changes that might occur during re-implantation of in vitro-cultured embryos into normal or hypoxic implantation sites. The kinetics of AMPK activation were investigated after changing from 20% O2 to 2% O2 (Fig. 1A), to 0.5% O2 (Fig. 1B), or to 0% O2 (Fig. 1C). There were consistent increases in pAMPK levels, starting by 6 h, in all O2 groups. For the 20% to 2% O2 switch, pAMPK levels fluctuated ~4-fold throughout the 12 h period, after reaching this level by 6 h (Fig. 1A). After switching to 0.5% or to 0% O2, pAMPK levels continued to increase, with the peak observed at 12 h (Fig. 1B, Fig. 1C). Total AMPK protein levels did not change after the three sets of switch from 20% O2 during the period studied (Supplementary Fig. 1: online only). Thus, removing cells from 20% O2 produced consistent increases in pAMPK levels, starting at 6 h independent of what O2 level the cells were moved into.
Fig. 1.
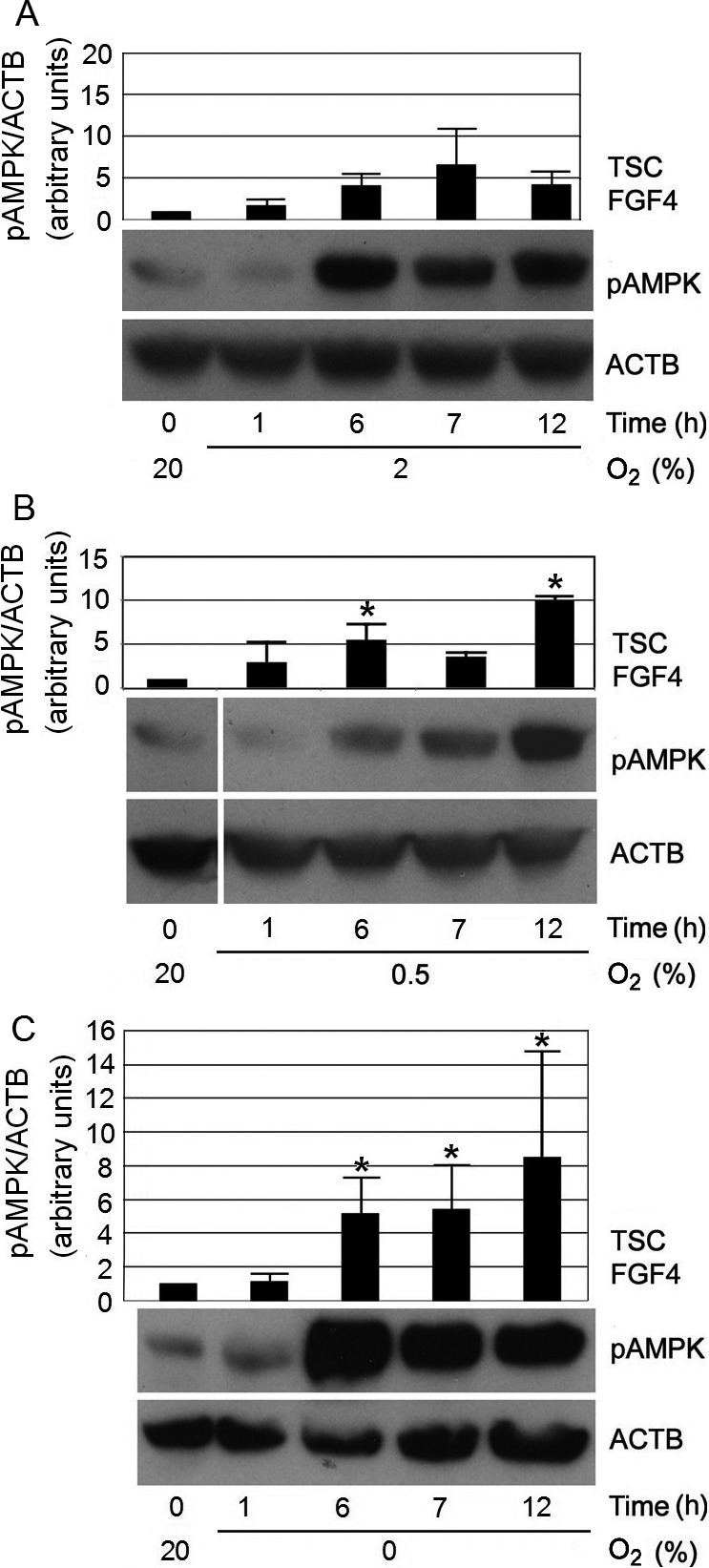
Switching O2 conditions from 20% to 2%, 0.5%, or 0% led to increases in pAMPK levels at 6 h. mTSCs were cultured as indicated, lysed, fractionated by SDS-PAGE, and probed with antibodies against pAMPK Thr172. ACTB was used as a loading control. Histograms show the average pAMPK level of three independent biological experiments, with error bars indicating standard error. A) Change from 20% to 2% O2 led to a delayed increase in pAMPK levels, to ~4-fold over baseline at 6 h; pAMPK remained at these higher levels until 12 h. B) Change from 20% to 0.5% O2 led to an increase in pAMPK levels, to ~5-fold above baseline at 6 h, and reached a peak of ~10-fold at 12 h. “*” indicates statistical significance compared with time zero at 20% O2. C) After changing from 20% to 0% O2, pAMPK levels increased to ~5-fold at 6 h, and reached a peak of ~9-fold over baseline, at 12 h. “*” indicates statistical significance compared with time zero at 20% O2.
Because there was rapid proliferation and metabolic waste accumulation when cells were cultured at 2% O2 [15], we took advantage of AMPK as a reporter of metabolic stress to evaluate how often the culture medium should be changed when cells are cultured at 2% O2. The result showed that changing to 2% O2 did not activate AMPK by 1–3 h, but pAMPK levels became high between 6–8 h, and maintained high levels throughout the 12 h period (Fig. 2). These results suggest that the intake of nutrients and the accumulation of metabolic waste may have already become evident, and been sensed by the cells, after 6–8 h of 2% O2 culture. Without microfluidic equipment to provide constant nutrient support and waste removal, it would be difficult to routinely culture mTSCs at 2% O2. Currently in vitro culturing of mTSCs is commonly carried out at 20% O2 [33], and this does not pose any problems for the isolation, maintenance, or in vivo differentiation capabilities of mTSCs [34].
Fig. 2.
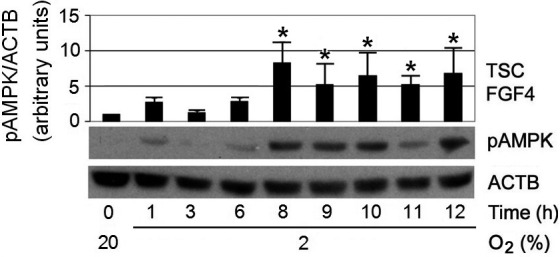
pAMPK reached peak levels at 8 h after changing from 20% to 2% O2, then stabilized at ~5-fold over baseline during the 12 h period. pAMPK levels were normalized to ACTB. “*” indicates statistical significance compared with time zero at 20% O2.
It should be noted that even though changing cells from 20% O2 to 2%, 0.5%, and 0% O2 conditions all produced higher pAMPK levels after 6–8 h, the biological processes underlying these increases are unlikely to be the same. It is likely that the high pAMPK levels observed after 6–8 h of 2% O2 reflect the metabolic needs initiated by rapid cell proliferation. There was minimal net cell growth in the 0.5% and 0% O2 groups, as reported previously for mTSCs [15]. Increased pAMPK levels supposedly report other signals raised by hypoxic stress, other than the need of biosynthesis for cell division in these conditions. Further studies are needed to investigate the mechanisms underlying pAMPK level increases in each condition.
To test the hypothesis that moving away from the least stressful 2% O2 condition would also produce faster AMPK activation (as was observed for SAPK), the kinetics of AMPK activation were studied within a time frame similar to that used in studies of SAPK [15]. Time points of 0.5, 1, 2, 3, and 24 h were chosen. Four early time points (0.5, 1, 2, 3 h) were selected to detect when AMPK activation first occurs. The period of 24 h after removing cells from 2% O2 was also studied to enable comparisons between our data and the published SAPK data. The results showed that pAMPK levels were near maximum after removing cells from 2% O2 for 1 h, regardless of the O2 level that the cells were moved into. The results of switches from 2% O2 to either 20%, 0.5%, or 0% O2 are shown in Figs. 3A, 3B, and 3C, respectively. Total AMPK protein levels did not change after the three sets of switch from 2% O2 during the period studied (Supplementary Fig. 2: online only). Interestingly, the 2% to 20% O2 switch increased pAMPK levels significantly at 1–3 h, but by 24 h the pAMPK levels returned to baseline (Fig. 3A). This indicates that changing cells from the least stressful condition of 2% O2 to the more stressful 20% O2 required a rapid AMPK response. However, unlike in the stressful O2 levels below 2%, cells eventually adapted to 20% O2, and their pAMPK levels decreased.
Fig. 3.
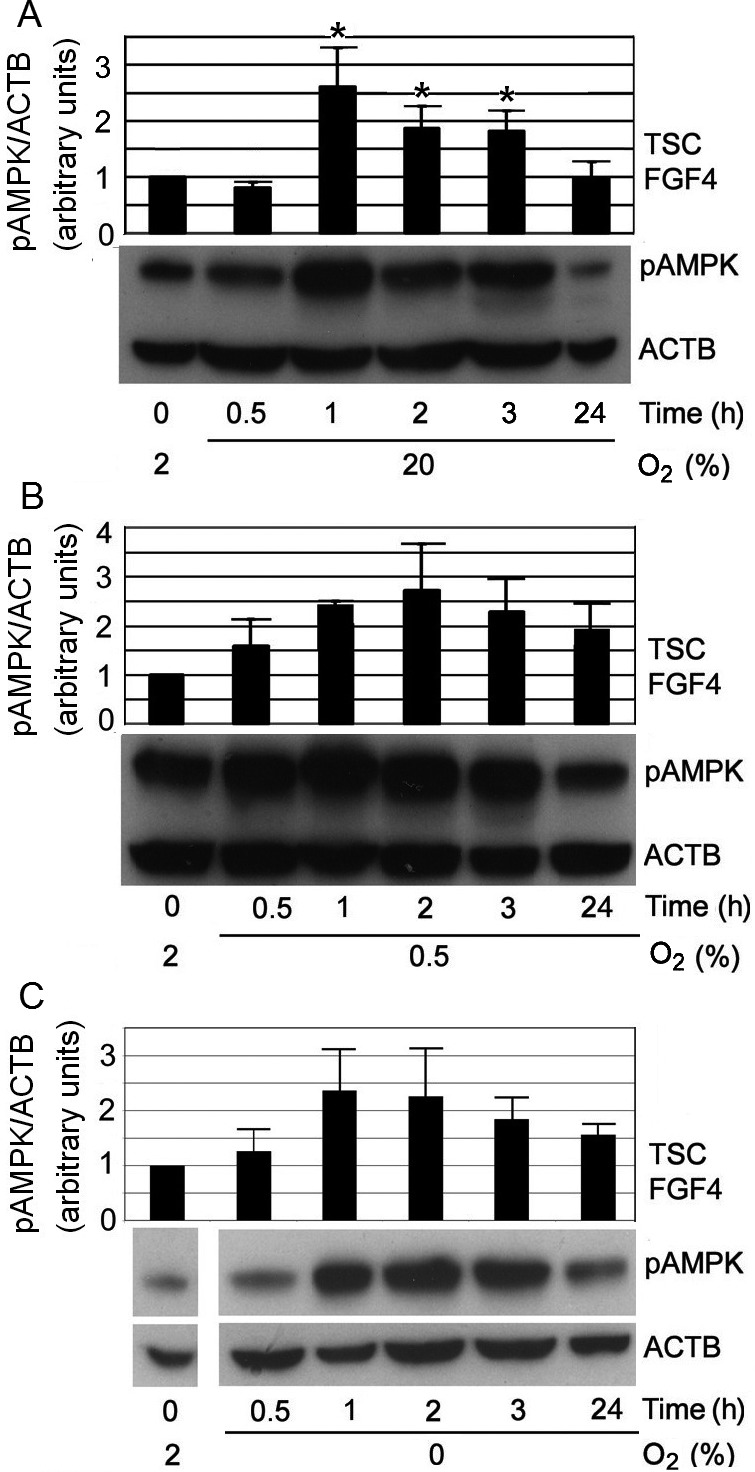
Changing O2 from 2% up to 20%, or down to 0.5% and 0% led to rapid increases in pAMPK levels at 1 h. A) Changing from 2% to 20% O2 induced rapid increases in pAMPK levels at 1 h, which subsequently returned to baseline level at 24 h. “*” indicates statistical significance compared with time zero at 2% O2. B) Changing from optimal 2% to 0.5% O2 induced rapid increases in pAMPK levels, to ~2.5-fold over baseline (at 2% O2) at 1 h; this was maintained throughout the 24 h period, but no statistical significance was found. C) Changing from optimal 2% to 0% O2 induced rapid increases in pAMPK levels to ~2.5-fold over baseline (at 2% O2) at 1 h; and this was maintained throughout the 24 h period, but no statistical significance was found.
The maximal stimulation index in the switches from 2% to other O2 levels was only ~2–3 fold, whereas it was ~8–10 fold following the switches away from 20% O2, as shown in Fig. 1. This was the case even when the terminal O2 level following the switch was the same. This is most likely due to the difference in pAMPK levels observed between 2% and 20% O2 at the 0 h baseline, prior to the switch. Because 2% O2 facilitates faster cell proliferation than 20% O2, it is possible that the high metabolic needs of mTSCs cultured at 2% O2 prior to O2 change have already led to a higher pAMPK baseline. As a result, the relative fold changes in pAMPK levels from baseline were smaller after cells were moved away from 2% O2 (Fig. 3).
In Fig. 4A, we superimposed the dynamics and magnitude of pAMPK levels, based on data represented in Fig. 1 and Fig. 3. This shows average pAMPK levels after 12 h of different O2 treatment compared to the 0 h baseline. pAMPK levels were lowest at 20% O2, and highest at 0.5–0% O2, with an approximate 10-fold change. pAMPK levels at 2% O2 were between those of the 20% and 0.5–0% O2 conditions, which produced an S-shaped curve. The speed of pAMPK (Fig. 4A) activation after moving cells away from 20% or 2% O2 to other O2 levels mimicked the pSAPK response observed in a similar experimental condition (Fig. 4B), which was cited with permission from a previous publication [11]. Sudden changes in O2 environments, from the least stressful 2% to either higher or lower O2 levels, induced rapid AMPK and SAPK activation at 1 h. When cells were moved from 20% O2 to 0% O2, the levels of pAMPK and pSAPK increased at 6–8 h.
Fig. 4.
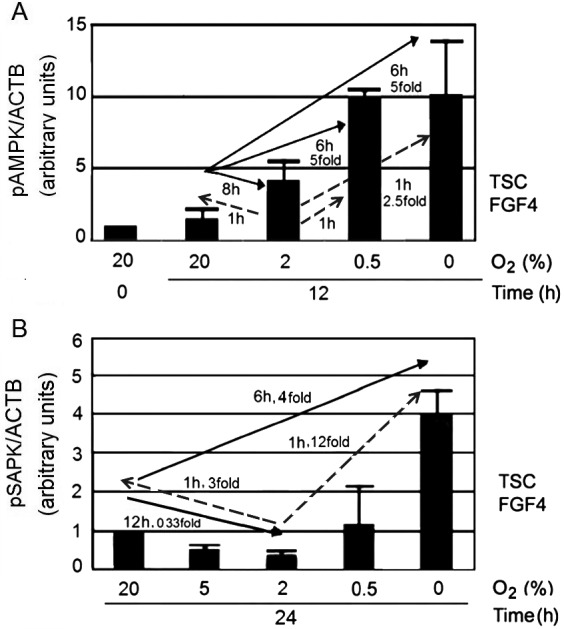
O2 stresses induced an S-shaped curve for pAMPK levels, and a U-shaped curve for pSAPK levels, but activation of both enzymes was most rapid when O2 was changed from the least stressful condition at 2% O2. A) Summary of the pAMPK dose and kinetic responses to changes in O2 levels, based on the data presented in Figs. 1–3. The tail of the arrow is the O2 level at time zero before the change in O2 conditions, and the head of the arrow is the level of O2 into which the cells were moved. mTSCs responded to culture at 20%, 2%, 0.5%, and 0% O2 with an S-shaped pAMPK dose-response curve, with a maximal increase of ~10-fold over baseline after 12 h of culture. B) Summary of the SAPK dose and kinetic responses to changes in O2 levels, based on a published figure we cite with permission [11]. The tail of the arrow is the O2 level at time zero before the change in O2 conditions, and the head of the arrow is the level of O2 into which the cells were moved. pSAPK levels were lowest at 2% O2 and increased rapidly within 1 h, when the cells were removed from 2% O2. The change from 20% to 0% O2 produced a slower activation of SAPK.
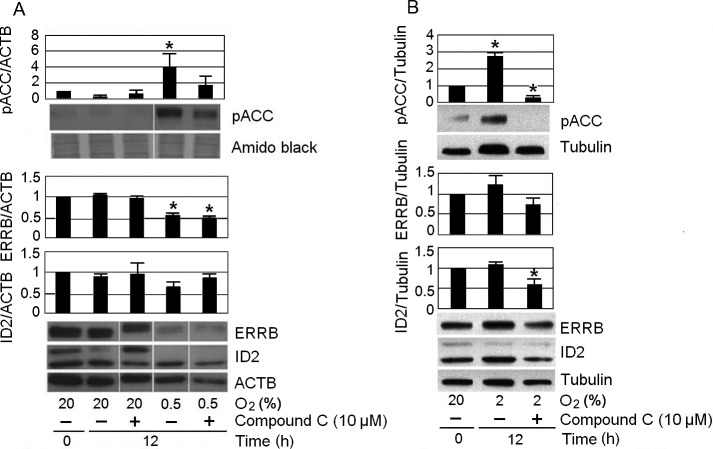
The stem cell state of mTSCs is characterized by the expression of potency factors. Loss of potency predisposes mTSCs to differentiation. We investigated the biological consequences of hypoxic exposure at 0.5% O2 on the levels of two mTSC potency factors: inhibition of differentiation 2 (ID2), and estrogen-related receptor beta (ERRB) (Fig. 5A). The time point of 12 h was chosen because, in 0.5% O2 conditions, pAMPK reached peak levels at 12 h. In addition, to study whether inhibition of AMPK could reverse potency loss, we needed to choose a time point when potency loss had already occurred. In the 0.5% O2 culture, there was no appreciable potency loss in the two time points (4 h and 8 h) prior to 12 h (Supplementary Fig. 3: online only). mTSC incubated at 0.5% O2 for 12 h led to a significant (~50%) loss of ERRB and a not significant (~35%) loss of ID2. AMPK inhibitor compound C did not reverse the loss of ERRB caused by hypoxic stress. Thus, the hypothesis that stress induces potency loss was supported. However, unlike potency loss caused by other stressors, inhibiting AMPK cannot reverse potency loss induced by hypoxic stress. Consistent with a previous report [15], mTSCs maintained their potency at 2% O2 (Fig. 5B). After 12 h of 2% O2 culture, levels of ERRB and ID2 were comparable to those observed in the 20% control group at 0 h. Unexpectedly, adding 10 µM compound C to 2% O2 culture significantly decreased the level of ID2 by approximately 45%. There were significant increases (~4-fold and 2.7-fold) in the levels of ACC (Ser79) phosphorylation after 12 h of 0.5% and 2% O2 culture, respectively. These increases in pACC are consistent with the increases in pAMPK shown in Fig. 2, which are more likely to reflect the needs of cells engaged in active metabolism rather than pathologic hypoxia. This was because, unlike that observed in cells cultured at 0.5% O2, after 12 h of 2% O2 treatment, the levels of potency factors were comparable to those of the 20% O2 control group. The increase in pACC levels observed at 0.5% O2 was reversed by ~50% via introduction of the AMPK inhibitor compound C, while the increase in pACC level observed at 2% O2 was reversed completely by compound C.
Fig. 5.
Hypoxia at 0.5% O2 significantly increased the pACC levels, and decreased the levels of potency factor ERRB. mTSCs were cultured for 12 h at 20% O2, 0.5% O2, or 2% O2, with or without 10 μM compound C. Increased pACC levels due to 0.5% O2 or 2% O2 exposure were mitigated by compound C; however, the loss of ERRB protein at 0.5% O2 was not reversed. “*” indicates statistical significance compared with time zero at 20% O2.
Discussion
AMPK mediates profound changes in mTSC metabolic regulation and stemness/differentiation balance [11]. It is not surprising that AMPK was activated rapidly when cultured mTSCs were from optimal 2% O2 conditions to hyperoxic 20% O2 or hypoxic 0.5% O2. The highest stimulation index of both pAMPK and pSAPK occurred at 0–0.5% O2 (Fig. 4), suggesting that hypoxia at O2 conditions below 2% is more stressful for mTSCs than that at the 20% O2 condition. Because the stimulus response of an enzyme is a product of speed (i.e., the “direness” index) and magnitude (i.e., the “stimulation” index), the most powerful AMPK response occurred when mTSCs were moved from 2% to below 2% O2. It is interesting that switching mTSCs from the less ideal 20% O2 environment induced slower activation of AMPK at 6–8 h, while switching them from the least stressful 2% O2 environment induced fast AMPK activation. We call the similar kinetic patterns of the relatively faster AMPK and SAPK responses a “direness response” when cells were moved out of the optimal 2% O2 environment. The speed of change in kinase activation is based on AMPK sensing the dissimilar cellular state at starting 2% or 20% O2, not the final AMPK activity state at 0% or 0.5% O2 (which is reflected by the similar stimulation index). The speed with which stress enzymes were activated after cells were moved out of the least stressful 2% O2 condition may reflect the profound stress initiated by deviating from this ideal cellular condition. In times of noxious environmental stimuli, cells must quickly change programs to adapt, or else they die. AMPK and SAPK play important roles in sensing environmental cues and determining cell fate [35, 36].
Although both AMPK and SAPK are stress kinases, they do not always respond to stress in the same way [37]. SAPK is ubiquitously expressed, and is activated by multiple types of stress, including UV radiation, hyperosmolarity, ischemia/reperfusion injury, and stimulation by TNF-α [11]. SAPK participates in intracellular signaling pathways that control cell proliferation, differentiation, apoptosis, cytoskeletal integrity, and other functions, as has been reviewed in [35]. By contrast, AMPK is first and foremost a kinase — with the primary role of maintaining ATP balance by regulating anabolic and catabolic metabolism [1]. The relatively higher level of pAMPK observed at 2% O2 may be due to the depletion of energy substrates and/or the accumulation of acidic, metabolic wastes; alternatively, it may have occurred in anticipation of the energy requirements of rapid cell proliferation [38]. At 2% O2, the increase in pACC level was not accompanied by the loss of potency, supporting the new state that the cells must respond to; this is in contrast with the outcome for cells in 0.5% O2. Further studies are needed to elucidate the mechanism of AMPK activation that occurs when cells are moved into the least stressful 2% O2 environment.
Many kinases demonstrate early and late activation, and mediate distinct downstream events at different activation times [39−42]. During a microarray study of mTSC responses to hyperosmolar stress, we found that the early stress response (within 30 min of sorbitol treatment) is to downregulate highly changing mRNAs (all 31 genes with significant change were downregulated). However, by 24 h, 158 genes were upregulated and 130 were downregulated, including genes involved in the cell cycle, apoptosis, macromolecular synthesis, and differentiation [43]. The direct effects of AMPK were not investigated in the microarray study. Because AMPK is activated under hyperosmolar stress [4], it is likely to have a role in the hyperosmolar stress-induced changes that cells undergo. If rapid and early responses to stress have been successful, cells may regain their balance after the stress subsides; or if the stress persists, larger-scale changes in transcription, cell cycle, and differentiation may follow, leading to irreversible changes in cell program. In mTSCs, mESCs, and early mouse embryos under hyperosmotic and genotoxic stress, as well as to certain drugs or diet supplements, AMPK downregulates cellular potency factors [4, 5] and predisposes the cells to differentiation. The fact that moving cells from 2% O2 to 20% O2 induced early increases in pAMPK levels that eventually fell back to baseline is informative. This suggests that cells are capable of coping with sudden environmental changes and that if the new environment is not too stressful, they regain their balance.
To further understand the biological consequences of hypoxic stress and AMPK activation, we studied the effects of an AMPK inhibitor, and the levels of two potency factors, ID2 [44] and ERRB [34, 45], in cells cultured at traditional 20% O2, 0.5% O2, or 2% O2 conditions. ID2 is an mTSC stemness marker, and is a key potency-maintenance gene. Forced expression of proteins in the ID family inhibits the differentiation of human cytotrophoblasts [44]. ERRB is also a mTSC stemness marker, and it is involved in chorionic lineage specification and potency maintenance following implantation [45]. With FGF4 removal, normally differentiated mTSCs lose expression of ID2 and ERRB, which is consistent with their role as potency factors [20, 34]. Hypoxic stress at 0.5% O2 also drove ID2 and ERRB downregulation, despite the presence of the potency-maintaining growth factor FGF4; however, AMPK inhibition did not reverse potency loss at 0.5% O2. Unlike the minimal effects that compound C had on the levels of ERRB and ID2 in cells cultured at 20% O2 and 0.5% O2, both potency factors were reduced by compound C in cells at 2% O2 (although the decrease in ERRB did not reach statistical significance). We know that AMPK activity was inhibited by compound C at 2% O2, because pACC (Ser79) level was significantly reduced. ACC carries the classical AMPK substrate motif [46], and the level of ACC phosphorylation at Ser79 indicates the activity of AMPK. However, in addition to being an AMPK inhibitor, compound C is known to have AMPK-independent effects on multiple cellular processes, such as cell cycle progression [47], mitochondrial respiration [48], and autophagy [49]. Further studies are required to elucidate the unique metabolic features that mTSCs possess at 2% O2 that make them more susceptible to the effects of compound C.
The general hypothesis that stress drives mTSC differentiation applies to hypoxic stress, as well as to hyperosmolar and genotoxic stress [5, 32]. Hypoxic stress inhibits anabolic metabolism, as represented by the inhibition of lipid synthesis, by inducing ACC phosphorylation and inactivation. ACC is a rate-limiting enzyme in the very early step of lipid synthesis [30]. Compound C partially blocked the phosphorylation of ACC at Ser79, suggesting that AMPK regulates metabolism during hypoxia via mechanisms similar to those used during other forms of stress [11]. The unique feature of hypoxic stress is that it affects the essential process of cellular energy production. The energy level of a cell is a fundamental signal, regulating every aspect of cell metabolism; therefore, it must be tightly regulated. AMPK sits at the center of cellular energy regulation by affecting multiple anabolic and catabolic pathways [1, 50]. Even for normal in vitro cell culture, occasional stressors exist, and AMPK functions are needed. Knockdown of AMPK catalytic α subunits leads to reduced cell growth in SM10 mouse placental progenitor cells presumably due to lack of culture adaptaion [10]. Inhibiting AMPK function may compromise the ability of mTSCs to adapt to hypoxic stress, and may produce severely maladaptive hypoxic cells that suffer irreversible loss of their potency factor proteins.
Limitations of this study, and future directions
Here, we studied the dynamics of AMPK activation and found that moving cells out of 20% O2 conditions activated AMPK at a slower rate than when they were moved out of the least stressful conditions at 2% O2. We did not study the upstream events that mediate the early (1 h) and late (6–8 h) AMPK response, nor did we study how the 2% O2 environment differs from that of 20% O2 in causing these changes. Because 2% O2 is associated with faster mTSC proliferation (~7 h doubling time), anabolic processes such as transcription, translation, and DNA replication should also be more active at 2% O2. Bacterial studies showed that rapidly growing cells operate close to their optimal energy efficiency [51], which is necessary to support the high rates of biosynthesis during cell proliferation [52, 53]. We speculate that the demands of high energy turnover that are associated with rapid cell growth may make mTSCs more susceptible to perturbations in the environment. An alternate hypothesis is that healthier cells, cultured in the least stressful environment, are inherently more capable of sensing stress and mounting a rapid adaptive response. Different cell types and stimuli should be used to gain better understanding of whether this is a generalized phenomenon, and future studies are needed to test these hypotheses.
Supplementary
Acknowledgments
Thanks to Drs Husam Abu-Soud and Awoniyi Awonuga for analysis and comments on the manuscript. This research was supported by grants to DAR from NIH (1R03HD061431 02) and from the Office of the Vice President for Research at Wayne State University.
References
- 1.Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology 2003; 144: 5179–5183. [DOI] [PubMed] [Google Scholar]
- 2.Sanli T, Steinberg GR, Singh G, Tsakiridis T. AMP-activated protein kinase (AMPK) beyond metabolism: a novel genomic stress sensor participating in the DNA damage response pathway. Cancer Biol Ther 2014; 15: 156–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chae HD, Lee MR, Broxmeyer HE. 5-Aminoimidazole-4-carboxyamide ribonucleoside induces G(1)/S arrest and Nanog downregulation via p53 and enhances erythroid differentiation. Stem Cells 2012; 30: 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie Y, Awonuga A, Liu J, Rings E, Puscheck EE, Rappolee DA. Stress induces AMPK-dependent loss of potency factors Id2 and Cdx2 in early embryos and stem cells [corrected]. Stem Cells Dev 2013; 22: 1564–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong W, Xie Y, Abdallah M, Awonuga AO, Slater JA, Sipahi L, Puscheck EE, Rappolee DA. Cellular stress causes reversible, PRKAA1/2-, and proteasome-dependent ID2 protein loss in trophoblast stem cells. Reproduction 2010; 140: 921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolnick A, Abdulhasan M, Kilburn B, Xie Y, Howard M, Andresen P, Shamir AM, Dai J, Puscheck EE, Rappolee DA. Commonly used fertility drugs, a diet supplement, and stress force AMPK-dependent block of stemness and development in cultured mammalian embryos. J Assist Reprod Genet 2016; 33: 1027–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vazquez-Martin A, Corominas-Faja B, Cufi S, Vellon L, Oliveras-Ferraros C, Menendez OJ, Joven J, Lupu R, Menendez JA. The mitochondrial H(+)-ATP synthase and the lipogenic switch: new core components of metabolic reprogramming in induced pluripotent stem (iPS) cells. Cell Cycle 2013; 12: 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vazquez-Martin A, Vellon L, Quirós PM, Cufí S, Ruiz de Galarreta E, Oliveras-Ferraros C, Martin AG, Martin-Castillo B, López-Otín C, Menendez JA. Activation of AMP-activated protein kinase (AMPK) provides a metabolic barrier to reprogramming somatic cells into stem cells. Cell Cycle 2012; 11: 974–989. [DOI] [PubMed] [Google Scholar]
- 9.Louden ED, Luzzo KM, Jimenez PT, Chi T, Chi M, Moley KH. TallyHO obese female mice experience poor reproductive outcomes and abnormal blastocyst metabolism that is reversed by metformin. Reprod Fertil Dev 2014; 27: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carey EA, Albers RE, Doliboa SR, Hughes M, Wyatt CN, Natale DR, Brown TL. AMPK knockdown in placental trophoblast cells results in altered morphology and function. Stem Cells Dev 2014; 23: 2921–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puscheck EE, Awonuga AO, Yang Y, Jiang Z, Rappolee DA. Molecular biology of the stress response in the early embryo and its stem cells. Adv Exp Med Biol 2015; 843: 77–128. [DOI] [PubMed] [Google Scholar]
- 12.Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 2010; 7: 150–161. [DOI] [PubMed] [Google Scholar]
- 13.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell 2007; 129: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazumdar J, OBrien WT, Johnson RS, LaManna JC, Chavez JC, Klein PS, Simon MC. O2 regulates stem cells through Wnt/β-catenin signalling. Nat Cell Biol 2010; 12: 1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou S, Xie Y, Puscheck EE, Rappolee DA. Oxygen levels that optimize TSC culture are identified by maximizing growth rates and minimizing stress. Placenta 2011; 32: 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung HW, Chen A, Choo AB, Reuveny S, Oh SK. Agitation can induce differentiation of human pluripotent stem cells in microcarrier cultures. Tissue Eng Part C Methods 2011; 17: 165–172. [DOI] [PubMed] [Google Scholar]
- 17.Toh YC, Voldman J. Fluid shear stress primes mouse embryonic stem cells for differentiation in a self-renewing environment via heparan sulfate proteoglycans transduction. FASEB J 2011; 25: 1208–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Arenas-Hernandez M, Gomez-Lopez N, Dai J, Parker GC, Puscheck EE, Rappolee DA. Hypoxic stress forces irreversible differentiation of a majority of mouse trophoblast stem cells to giant cell fate despite FGF4. Biol Reprod 2016; biolreprod.116.138412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Awonuga AO, Zhong W, Abdallah ME, Slater JA, Zhou SC, Xie YF, Puscheck EE, Rappolee DA. Eomesodermin, HAND1, and CSH1 proteins are induced by cellular stress in a stress-activated protein kinase-dependent manner. Mol Reprod Dev 2011; 78: 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie Y, Zhou S, Jiang Z, Dai J, Puscheck EE, Lee I, Parker G, Hüttemann M, Rappolee DA. Hypoxic stress induces, but cannot sustain trophoblast stem cell differentiation to labyrinthine placenta due to mitochondrial insufficiency. Stem Cell Res (Amst) 2014; 13(3 Pt A): 478–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soares MJ, Müller H, Orwig KE, Peters TJ, Dai G. The uteroplacental prolactin family and pregnancy. Biol Reprod 1998; 58: 273–284. [DOI] [PubMed] [Google Scholar]
- 22.Farin CE, Imakawa K, Hansen TR, McDonnell JJ, Murphy CN, Farin PW, Roberts RM. Expression of trophoblastic interferon genes in sheep and cattle. Biol Reprod 1990; 43: 210–218. [DOI] [PubMed] [Google Scholar]
- 23.Alberts B. Molecular biology of the cell. New York: Garland Science; 2002: xxxiv, 1548 p. [Google Scholar]
- 24.Shaw G, Kamen R. A conserved AU sequence from the 3 untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell 1986; 46: 659–667. [DOI] [PubMed] [Google Scholar]
- 25.Carling D, Sanders MJ, Woods A. The regulation of AMP-activated protein kinase by upstream kinases. Int J Obes 2008; 32(Suppl 4): S55–S59. [DOI] [PubMed] [Google Scholar]
- 26.Rasmussen BB, Hancock CR, Winder WW. Postexercise recovery of skeletal muscle malonyl-CoA, acetyl-CoA carboxylase, and AMP-activated protein kinase. J Appl Physiol (1985) 1998; 85: 1629–1634. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, Qi Q, Chan CB, Zhou W, Chen J, Luo HR, Appin C, Brat DJ, Ye K. Fyn-phosphorylated PIKE-A binds and inhibits AMPK signaling, blocking its tumor suppressive activity. Cell Death Differ 2016; 23: 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barb D, Neuwirth A, Mantzoros CS, Balk SP. Adiponectin signals in prostate cancer cells through Akt to activate the mammalian target of rapamycin pathway. Endocr Relat Cancer 2007; 14: 995–1005. [DOI] [PubMed] [Google Scholar]
- 29.LeBrasseur NK, Kelly M, Tsao TS, Farmer SR, Saha AK, Ruderman NB, Tomas E. Thiazolidinediones can rapidly activate AMP-activated protein kinase in mammalian tissues. Am J Physiol Endocrinol Metab 2006; 291: E175–E181. [DOI] [PubMed] [Google Scholar]
- 30.Wakil SJ, Stoops JK, Joshi VC. Fatty acid synthesis and its regulation. Annu Rev Biochem 1983; 52: 537–579. [DOI] [PubMed] [Google Scholar]
- 31.Ha J, Daniel S, Broyles SS, Kim KH. Critical phosphorylation sites for acetyl-CoA carboxylase activity. J Biol Chem 1994; 269: 22162–22168. [PubMed] [Google Scholar]
- 32.Xie Y, Abdallah ME, Awonuga AO, Slater JA, Puscheck EE, Rappolee DA. Benzo(a)pyrene causes PRKAA1/2-dependent ID2 loss in trophoblast stem cells. Mol Reprod Dev 2010; 77: 533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quinn J, Kunath T, Rossant J. Mouse trophoblast stem cells. Methods Mol Med 2006; 121: 125–148. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science 1998; 282: 2072–2075. [DOI] [PubMed] [Google Scholar]
- 35.Nishina H, Wada T, Katada T. Physiological roles of SAPK/JNK signaling pathway. J Biochem 2004; 136: 123–126. [DOI] [PubMed] [Google Scholar]
- 36.Alexander A, Walker CL. The role of LKB1 and AMPK in cellular responses to stress and damage. FEBS Lett 2011; 585: 952–957. [DOI] [PubMed] [Google Scholar]
- 37.López JM. Digital kinases: A cell model for sensing, integrating and making choices. Commun Integr Biol 2010; 3: 146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamás P, Hawley SA, Clarke RG, Mustard KJ, Green K, Hardie DG, Cantrell DA. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med 2006; 203: 1665–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Susa M, Olivier AR, Fabbro D, Thomas G. EGF induces biphasic S6 kinase activation: late phase is protein kinase C-dependent and contributes to mitogenicity. Cell 1989; 57: 817–824. [DOI] [PubMed] [Google Scholar]
- 40.Fritz G, Kaina B. Late activation of stress kinases (SAPK/JNK) by genotoxins requires the DNA repair proteins DNA-PKcs and CSB. Mol Biol Cell 2006; 17: 851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones SM, Klinghoffer R, Prestwich GD, Toker A, Kazlauskas A. PDGF induces an early and a late wave of PI 3-kinase activity, and only the late wave is required for progression through G1. Curr Biol 1999; 9: 512–521. [DOI] [PubMed] [Google Scholar]
- 42.Gustin MC, Albertyn J, Alexander M, Davenport K. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol Mol Biol Rev 1998; 62: 1264–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J, Xu W, Sun T, Wang F, Puscheck E, Brigstock D, Wang QT, Davis R, Rappolee DA. Hyperosmolar stress induces global mRNA responses in placental trophoblast stem cells that emulate early post-implantation differentiation. Placenta 2009; 30: 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janatpour MJ, McMaster MT, Genbacev O, Zhou Y, Dong J, Cross JC, Israel MA, Fisher SJ. Id-2 regulates critical aspects of human cytotrophoblast differentiation, invasion and migration. Development 2000; 127: 549–558. [DOI] [PubMed] [Google Scholar]
- 45.Luo J, Sladek R, Bader JA, Matthyssen A, Rossant J, Giguère V. Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-beta. Nature 1997; 388: 778–782. [DOI] [PubMed] [Google Scholar]
- 46.Ducommun S, Deak M, Sumpton D, Ford RJ, Núñez Galindo A, Kussmann M, Viollet B, Steinberg GR, Foretz M, Dayon L, Morrice NA, Sakamoto K. Motif affinity and mass spectrometry proteomic approach for the discovery of cellular AMPK targets: identification of mitochondrial fission factor as a new AMPK substrate. Cell Signal 2015; 27: 978–988. [DOI] [PubMed] [Google Scholar]
- 47.Liu X, Chhipa RR, Nakano I, Dasgupta B. The AMPK inhibitor compound C is a potent AMPK-independent antiglioma agent. Mol Cancer Ther 2014; 13: 596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Emerling BM, Viollet B, Tormos KV, Chandel NS. Compound C inhibits hypoxic activation of HIF-1 independent of AMPK. FEBS Lett 2007; 581: 5727–5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vucicevic L, Misirkic M, Janjetovic K, Vilimanovich U, Sudar E, Isenovic E, Prica M, Harhaji-Trajkovic L, Kravic-Stevovic T, Bumbasirevic V, Trajkovic V. Compound C induces protective autophagy in cancer cells through AMPK inhibition-independent blockade of Akt/mTOR pathway. Autophagy 2011; 7: 40–50. [DOI] [PubMed] [Google Scholar]
- 50.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 2012; 13: 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maitra A, Dill KA. Bacterial growth laws reflect the evolutionary importance of energy efficiency. Proc Natl Acad Sci USA 2015; 112: 406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Warner JR. The economics of ribosome biosynthesis in yeast. Trends Biochem Sci 1999; 24: 437–440. [DOI] [PubMed] [Google Scholar]
- 53.García-Martínez J, Delgado-Ramos L, Ayala G, Pelechano V, Medina DA, Carrasco F, González R, Andrés-León E, Steinmetz L, Warringer J, Chávez S, Pérez-Ortín JE. The cellular growth rate controls overall mRNA turnover, and modulates either transcription or degradation rates of particular gene regulons. Nucleic Acids Res 2016; 44: 3643–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.