Abstract
The concentration of circulating anti-Müllerian hormone (AMH) in cattle is a useful endocrine marker for ovarian response to superovulation. Although the AMH concentration undergoes little variation throughout the estrous cycle, its long-term changes remain incompletely understood. Here, we investigated the relationship between superovulation response and plasma AMH concentration in Japanese Black cattle and the long-term changes in plasma AMH concentration of embryo donor cows and heifers. The median, 25th percentile, and 75th percentile of AMH concentrations in 222 mature animals were 0.265, 0.118, and 0.488 ng/ml, respectively. The numbers of ova/embryos, fertilized embryos, and transferable embryos in a total of 295 superovulations were significantly different among the H (AMH ≥ 0.488 ng/ml), M (AMH 0.487–0.119 ng/ml), and L (AMH ≤ 0.118 ng/ml) groups. AMH concentrations during repeated superovulation in ten donor cows were significantly decreased after the third treatment. In heifers, the highest AMH concentration was observed in individuals during 2–13 months of age, with considerable individual variability. AMH concentrations of heifers at 10 or 11 months correlated with the number of ova/embryos during superovulation at 13–18 months (r = 0.641, P < 0.05). These results suggest that the 25th and 75th percentile values of AMH concentration would give a useful rough estimate of ovarian response; however, repeated superovulation may reduce the predictive accuracy of single measurements of AMH concentration. It would be possible to evaluate AMH concentration in heifers after approximately 11 months of age.
Keywords: Anti-Müllerian hormone, Japanese Black cattle, Müllerian-inhibiting substance, Superovulation
Anti-Müllerian hormone (AMH) is a glycoprotein belonging to the transforming growth factor beta family and is produced by granulosa cells of healthy growing follicles [1]. The circulating AMH concentration is strongly associated with the antral follicle count (AFC) and is useful as an endocrine marker for ovarian reserve in cattle [2]. Moreover, it is strongly associated with superovulation response in Holstein cows [3, 4] and Japanese Black cows [5]. Beef heifers and dairy cows with high AFC show higher pregnancy rates than those with low AFC [6, 7]. Recently, Ribeiro et al. [8] reported that the concentration of circulating AMH in dairy cows was positively associated with fertility by artificial insemination and natural service after detection of spontaneous estrus. Thus, the evaluation of circulating AMH concentration would increase the efficiency of genetic improvement and reproductive performance through selection of animals for embryo production and insemination.
Circulating AMH concentration undergoes little variation throughout the estrous cycle [2, 9, 10]. With respect to the long-term trend of circulating AMH concentration, Monniaux et al. [11] reported that AMH concentrations were comparable between the pre-pregnant and post-parturition periods. The stability of AMH concentration permits its determination by a single measurement at random stages of the estrous cycle. Evaluation of the concentration of circulating AMH during an early stage, such as in newborn calves and heifers, is convenient for the selection of heifers for breeding and embryo production. However, the circulating AMH concentration in crossbred beef and Maine-Anjou heifers has been shown to vary greatly with growth [11, 12]. Ribeiro et al. [8] reported that circulating AMH concentrations differed among Holstein, Jersey, and crossbred cows. Thus, the change in circulating AMH concentrations related to growth in Japanese Black cattle warrants further research.
In the present study, we conducted embryo collection tests to establish classification criteria for prediction of the superovulation response in Japanese Black cattle based on the plasma AMH concentration. Furthermore, we examined long-term changes in plasma AMH concentration in heifers and mature animals to improve the predicting performance of the superovulation response.
Materials and Methods
Experiment 1: Plasma AMH concentration and superovulation response in mature animals
Venus blood samples were collected from 222 non-lactating Japanese Black female cattle aged 1.1–16.9 years (average 6.1 years) that were maintained at four farms: 1) the Animal Research Center (Farm A, 136 animals); 2) a commercial farm (Farm B, 67 animals); 3) the Yachiyo public raising farm (Farm C, 10 animals), and; 4) the National Livestock Breeding Center Tokachi Station (Farm D, 9 animals) located in the eastern area of the Hokkaido prefecture. After collection in heparinized tubes, the blood samples were centrifuged at 3,000 × g for 10 min at 4°C to recover the plasma, which was stored at –20°C until the AMH assays were conducted. Among those animals, 122 donors were used for superovulation one to ten times, and a total of 295 superovulation treatments were performed. Superovulation was induced in the animals using follicle-stimulating hormone (FSH) (20 IU/cow, Antorin®R-10, Kyoritsu Seiyaku, Tokyo, Japan) administered twice daily in decreasing doses over 3 days. An injection of prostaglandin F2α (cloprostenol 0.5 mg/cow, Resipron®-C, ASKA Animal Health, Tokyo, Japan or dinoprost 15 mg/cow, Panacelan®·Hi, Meiji Seika Pharma, Tokyo) was administered on the third day of superovulation treatment. A controlled internal drug release (CIDR) device (Eazi-Breed, Pfizer Japan, Tokyo, Japan) was inserted into cows for 7–10 days until the injection of prostaglandin F2α. An injection of estradiol benzoate (1 mg/cow, Kyoritsu Seiyaku) was administered 4 days before the initiation of FSH injections. Donor cows were inseminated 12 or 24 h after the onset of estrus, and embryos were recovered 7–8 days after insemination. The above protocol was applied differently at each farm in that an injection of estradiol benzoate was administered at a dose of 2 mg/cow 5 days before the initiation of FSH injections at Farm B, and a CIDR devise and estradiol benzoate were not used at Farm C. A highly skilled technician specific to each farm performed embryo collection. Embryos were classified according to the International Embryo Transfer Society criteria [13]. Code 1–3 embryos were defined as transferable embryos.
Among the embryo collection tests mentioned above, the data were extracted for ten donor cows aged 2.6–8.8 years (average 5.6 years) that were maintained at the Animal Research Center and used for superovulation six to ten times. These data were used to evaluate the influence of repetition of superovulation on plasma AMH concentration and ovarian responses. Insertion of a CIDR device for subsequent superovulation was performed after detection of one or two spontaneous estrous events following embryo collection. The average interval between superovulation was 1.9 months, and the average total experiment period was 9.7 months. Venous blood samples for AMH measurement in the donor cows were collected within 4 days before initiation of FSH injections.
Experiment 2: Changes in plasma AMH concentration during growth in Japanese Black heifers
To examine changes in plasma AMH concentration associated with growth, the plasma AMH concentrations of 13 Japanese Black heifers maintained at Farm D were analyzed. Blood samples were collected in heparinized tubes every month for heifers aged 1–13 months, except that initiation of blood collection occurred within four and two heifers of the ages of 2 and 3 months, respectively.
To analyze the relationship between plasma AMH concentration and superovulation responses in heifers, embryo collection tests were performed in a total of seven Japanese Black heifers that were maintained at Farms A (four heifers) and D (three heifers). Venus blood samples for AMH measurement were collected in heparinized tubes once only at the age of 10 or 11 months. Three heifers at Farm A was used twice for superovulation and a total of ten superovulation events were performed at the age of 13–18 months (average 15 months) in a similar way to that implemented in experiment 1, except that the dose of FSH was reduced to 16 IU.
Measurement of plasma AMH concentration
AMH concentrations were measured as previously reported with an AMH Gen II ELISA (Beckman Coulter, Brea, CA, USA) [5]. Undiluted plasma (20 µl) was used for the analysis. The assay had a limit of detection of 0.032 ng/ml and a coefficient of variation of 1.9%.
Statistical analysis
All results are presented as means ± standard deviation (SD). The relationships between plasma AMH concentrations and number of ova and embryos (NOE) or the age of cows were evaluated by Pearson′s correlation coefficient. Changes in plasma AMH concentration in donor cows and heifers were evaluated using Steel′s test. A p-value < 0.05 was considered statistically significant. Cut-off points of AMH concentration for NOE and the area under the curve (AUC) were calculated using receiver operating characteristics (ROC) curve analysis. The experimental data were analyzed using the R statistical package.
Results
Experiment 1: Plasma AMH concentration and superovulatory response in mature animals
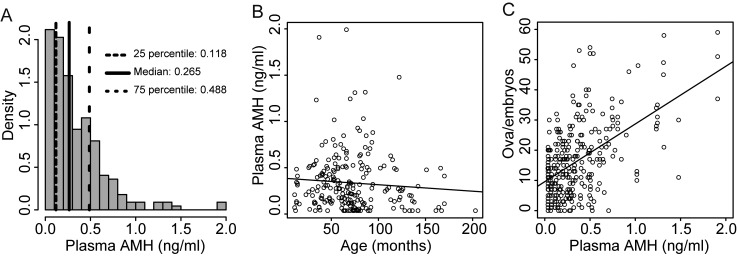
Plasma AMH concentrations in Japanese Black cows ranged from 0.032 to 1.992 ng/ml (Fig. 1A). The mean ± SD and median concentrations were 0.334 ± 0.318 ng/ml and 0.265 ng/ml, respectively. Samples comprising the lowest 25%, intermediate, and the highest 25% of concentrations were classified as the AMH concentration L, M, and H groups, respectively. There was no correlation of plasma AMH concentration with age (r = –0.081, P > 0.05) (Fig. 1B). A positive correlation was observed between plasma AMH concentration and NOE (r = 0.537, P < 0.001) (Fig. 1C).
Fig. 1.
Plasma anti-Müllerian hormone (AMH) concentration and ovarian response to superovulation in Japanese Black cows. (A) Histogram of the frequency distribution of plasma AMH concentration (n = 222). (B) Correlation between age in months at blood collection and plasma AMH concentration (n = 222, r = –0.082, P > 0.05). (C) Correlation between plasma AMH concentration and the number of ova and embryos after treatments (n = 295, r = 0.537, P < 0.001).
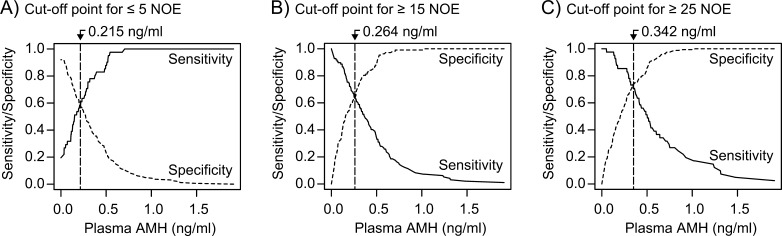
Cut-off points of plasma AMH concentration for cows producing ≤ 5, ≥ 15, and ≥ 25 NOE were 0.215, 0.264, and 0.342 ng/ml, respectively (Fig. 2). The sensitivity and specificity at the cut-off points were 0.584 for ≤ 5 NOE, 0.638 for ≥ 15 NOE, and 0.731 for ≥ 25 NOE. The AUC values at those cut-off points for ≤ 5, ≥ 15, and ≥ 25 NOE were 0.636, 0.717, and 0.799, respectively. These results indicated that the predictive ability of NOE increases with increasing prediction criteria of NOE.
Fig. 2.
Estimated cut-off points of plasma anti-Müllerian hormone (AMH) concentration for donor cows that produce ≤ 5, ≥ 15, and ≥ 25 ova and embryos in response to superovulation, based on sensitivity and specificity by the receiver operating characteristics.
NOE, the number of fertilized embryos (NFE), and the number of transferable embryos (NTE) in the four farms were significantly higher in the H group than in M and L groups (Table 1). These were also significantly higher in the M group than in the L group. By excluding Farms C and D, which had small sample sizes, Farm A showed significant differences in NOE, NFE, and NTE among the L, M, and H groups. The H group from Farm B showed significantly higher NOE and NFE than did the M group, whereas no significant difference was detected between the H or M groups and the L group. In the L group, NOE, NFE, and NTE were significantly higher for Farm B than Farm A.
Table 1. Effects of anti-Müllerian hormone (AMH) concentrations on superovulatory responses in Japanese Black cattle.
| AMH levels * | Farms | No. of animals | No. of superovulation | No. of ova/embryos | No. of fertilized embryos | No. of transferable embryos |
| L | Total | 25 | 71 | 9.3 ± 6.7 A | 7.6 ± 6.5 A | 5.9 ± 5.6 A |
| A | 19 | 59 | 8.1 ± 5.7 A,a | 6.4 ± 5.4 A,a | 5.1 ± 5.1 A,a | |
| B | 4 | 10 | 16.3 ± 8.4 AB,b | 14.6 ± 8.1 AB,b | 10.8 ± 6.1 b | |
| D | 2 | 2 | 7.0 | 7.0 | 4.0 | |
| M | Total | 66 | 148 | 15.6 ± 10.6 B | 12.5 ± 9.0 B | 9.0 ± 7.0 B |
| A | 25 | 69 | 13.0 ± 8.3 B | 10.5 ± 7.1 B | 8.5 ± 6.4 B | |
| B | 32 | 70 | 19.0 ± 11.9 A | 15.2 ± 10.0 A | 10.1 ± 7.4 | |
| C | 3 | 3 | 7.0 ± 4.1 A | 2.7 ± 1.7 A | 2.0 ± 1.4 A | |
| D | 6 | 6 | 10.8 ± 6.1 | 8.3 ± 6.7 | 6.0 ± 4.8 | |
| H | Total | 31 | 76 | 24.3 ± 14.2 C | 19.4 ± 13.7 C | 13.2 ± 9.9 C |
| A | 10 | 30 | 21.3 ± 14.9 C | 17.3 ± 14.7 B | 12.7 ± 11.6 B | |
| B | 17 | 42 | 26.7 ± 13.5 B | 21.6 ± 13.0 B | 13.7 ± 8.8 | |
| C | 3 | 3 | 26.3 ± 4.5 B | 15.0 ± 2.4 B | 13.3 ± 2.5 B | |
| D | 1 | 1 | 6.0 | 5.0 | 4.0 | |
*AMH levels were classified as L, M and H based on the 25th and 75th percentile values of plasma AMH concentration in Fig. 1A. Different uppercase letters represent statistical differences among anti-Müllerian hormone (AMH) levels in each farm. Different lowercase letters represent statistical differences among farms at each AMH level.
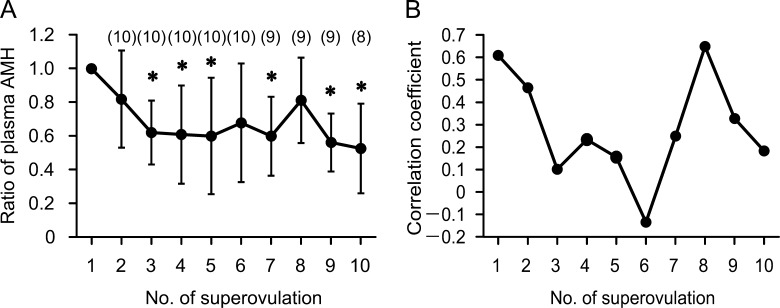
As shown in Figure 3A, plasma AMH concentrations during repeated sessions of superovulation were significantly decreased after the third treatment. The correlation coefficient between plasma AMH concentration and NOE in overall data of repeated sessions was 0.532 (P < 0.001). The correlation coefficients between plasma AMH concentration before the first treatment and NOE in repeated treatments decreased gradually, except for NOE in the eighth treatment (Fig. 3B).
Fig. 3.
Changes in plasma anti-Müllerian hormone (AMH) concentration in donor cows following repeated superovulation sessions. (A) Plasma AMH concentrations before treatments are presented as the proportions of that before the first treatment. Asterisks indicate significant difference (P < 0.05) between the first treatment and following treatments. (B) Value of the correlation coefficient of plasma AMH concentration before the first treatment with number of ova and embryos in following treatments.
Experiment 2: Changes in plasma AMH concentration during growth in Japanese Black heifers
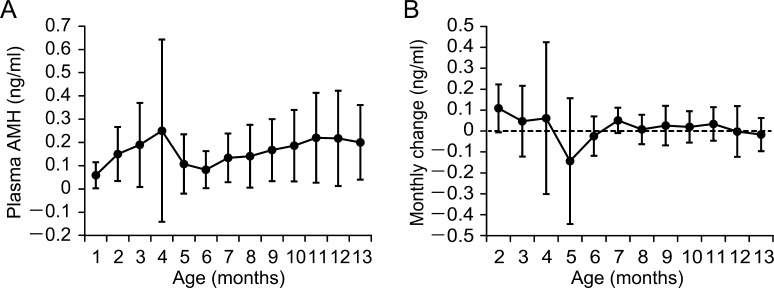
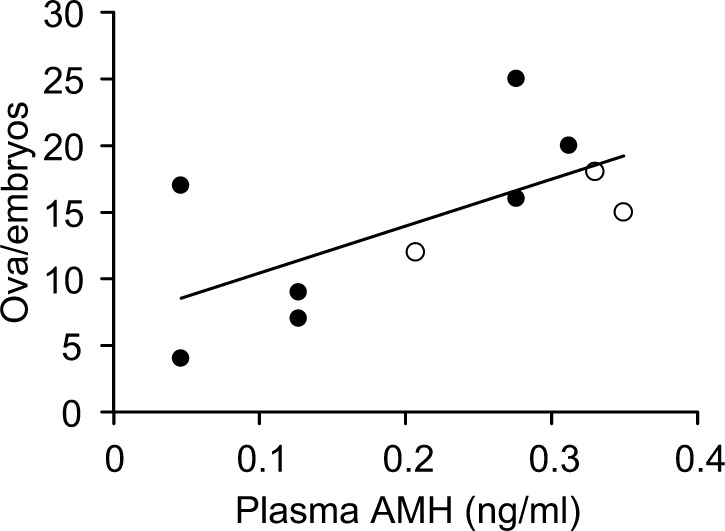
Although average plasma AMH concentrations during 2–13 months of age were higher than the average concentration at 1 month of age, no significant change in plasma AMH concentration was observed (Fig. 4A). The highest concentration of plasma AMH in individuals was observed during 2–13 months of age (average 6.3 months) with considerable individual variation, except for one heifer with plasma AMH concentration below the limit of detection throughout the period. Although positive and negative monthly changes (differences from previous months) in plasma AMH concentration with considerable individual variation were observed during 2–7 months of age, monthly changes were stable, and individual differences decreased after 8 months of age (Fig. 4B). Plasma AMH concentrations at 10 or 11 months of age were positively correlated with NOE in the superovulation treatment at 13–18 months of age (r = 0.641, P < 0.05) (Fig. 5).
Fig. 4.
Changes in plasma anti-Müllerian hormone (AMH) concentration during growth in Japanese Black heifers. (A) Plasma samples were collected every month for the ages of 1–13 months, except for four and two heifers at the ages of 1 and 2 months, respectively. (B) Monthly change (differences from past month) in plasma AMH concentration.
Fig. 5.
Relationship between plasma anti-Müllerian hormone (AMH) concentration and number of ova and embryos after superovulation in Japanese Black heifers. The correlation coefficient was r = 0.641 (P < 0.05). Blood samples were collected at the age of 10 or 11 months and superovulation was performed at 13–18 months. Filled circles and open circles indicate heifers maintained at Farms A and D, respectively.
Discussion
The mean, median, and distribution of plasma AMH concentrations in Japanese Black cows were comparable with those previously reported for lactating dairy cows [8]. No effect of the age on plasma AMH concentrations was found in the present study. In agreement with previous reports on Holstein cows [3, 4], in the present study, plasma AMH concentrations were useful markers for the prediction of superovulation responses in Japanese Black cows. In addition, the present study used a larger sample size and wider age distribution and the findings corroborate those of our previous study [5] on Japanese Black cows.
ROC curve analysis for ≤ 5, ≥ 15, and ≥ 25 NOE indicated that the sensitivity and specificity of cut-off points of plasma AMH concentration were higher in the selection of donor cows with high superovulatory responses than in the exclusion of cows with low superovulatory responses. AUC values of ROC curve analysis were in the range of 0.636–0.799, and the accuracies of prediction with those cut-off points were moderate. Therefore, we used the 25th (0.118 ng/ml) and 75th (0.488 ng/ml) percentile values of plasma AMH concentration for classification of cows with low and high superovulatory responses. These values were more severe compared with the cut-off points 0.215 and 0.342 ng/ml according to the ROC curve analysis for ≤ 5 and ≥ 25 NOE. These quartile points would be useful for rough estimation of ovarian response to superovulation, as in the report of high-producing dairy cows [3]. Although the numbers of donor cows used in the present study differed among farms, we feel that the data based on several farms would provide useful information for practitioners related to embryo transfer.
In the L group, NOE, NFE, and NTE on Farm B were significantly higher than those on Farm A. It is unclear if these differences could be due to the difference in environmental and genetic factors such as superovulation protocol, the feeding condition, or pedigree. However, the possibility that a slight difference of superovulation protocol between farm A and B was the cause of the difference of ovarian response cannot be discounted. Although the embryo recovery rate relative to the number of corpus luteum was not available, we believe that differences of technician and the methods used to recover embryos had little effect on the results, as highly skilled technicians performed embryo collection. In cattle, follicle growth above 3–4 mm in diameter is considered to be gonadotropin dependent [14, 15], and the number of small follicles has been found to significantly correlate with NOE during superovulation [16, 17]. On the other hand, circulating AMH is mainly supplied by granulosa cells of preantral and small antral follicles [18]. Therefore, we infer that the proportion of follicular recruitment into follicles ≥3 mm in diameter from secondary follicles could be higher in farm B than in farm A.
Rico et al. [4] found no significant effect of repetition number of ovum pickup (OPU) following gonadotropin treatment on the numbers of large follicles or plasma AMH concentrations over a 1-y period. In contrast, repeated superovulation in Japanese Black cows resulted in a decrease in the average plasma AMH concentration with a decrease of NOE. Although plasma AMH concentration by measurement for each superovulation treatment and NOE showed a significant positive correlation, correlation coefficients between plasma AMH concentration at the first treatment and NOE in repeated treatments decreased gradually. These results suggest that the prediction accuracy of ovarian response by single measurement of AMH concentration decreases gradually during repeated superovulation. In the study by Rico et al. [4], OPU was performed using Holstein dairy cows, and the age of cows (4–9 years) was comparable with that of the cows used in the present study. We found no effect of the parity of plasma AMH concentrations in the present study (data not shown). The plasma AMH concentration before gonadotropin treatment was distinctly different between the previous report (mean ± standard error of the mean, 0.177 ± 0.012 ng/ml) [4] and the present study (0.437 ± 0.107 ng/ml). We infer that levels of ovarian reserves before gonadotropin treatment affect the decrease in serum AMH concentration with repeated gonadotropin treatment, and that donor cows bearing abundant ovarian reserves are highly sensitive to repetition of gonadotropin treatment.
In the previous study [11] using Maine-Anjou beef heifers, plasma AMH concentrations increased from the 1st to the 3rd month, and the concentration was maintained until the 6th month, following which AMH concentrations started to decline until the 12th month. Another study using ewes at prepubertal age showed a similar profile for plasma AMH concentration [19]. In the present study, although Japanese Black heifers showed a progressive increase in average plasma AMH concentration after 2 months of age, no statistically significant change in AMH concentration was observed. Changes in plasma AMH concentration showed high individual variation, and the age when plasma AMH concentration was the highest ranged from 2 to 13 months of age. Although the physiological mechanism behind these changes in AMH concentration remains unclear, the changes may reflect the activation of recruitment into preantral and small antral follicles that secrete large amounts of AMH [18]. In addition, our results indicate that plasma AMH concentration in Japanese Black heifers becomes stable at approximately 11 months. In this breed, puberty occurs at approximately 13 months. Evaluation of AMH concentration at an early stage corresponded to the ovarian response to superovulation after puberty. We conclude that evaluation of AMH concentration during the early stages is valuable for the selection of candidate embryo donors in Japanese Black cattle.
In summary, we suggest that the 25th and 75th percentile values of plasma AMH concentration would be useful for a rough estimation of ovarian response to superovulation. However, it appears that repeated superovulation sessions reduce the prediction accuracy of ovarian response by a single measurement of AMH concentration, given that ovarian response and plasma AMH concentration change progressively. AMH concentrations in heifers may be evaluated after the age of approximately 11 months, during which plasma AMH concentration becomes stable. The findings of the present study may provide practical information for using AMH concentrations for the selection of candidate embryo donors in Japanese Black cattle.
Acknowledgments
The authors thank the staff of the Yachiyo public rearing farm and the commercial farm for technical assistance and providing blood samples.
References
- 1.La Marca A, Volpe A. Anti-Müllerian hormone (AMH) in female reproduction: is measurement of circulating AMH a useful tool? Clin Endocrinol (Oxf) 2006; 64: 603–610. [DOI] [PubMed] [Google Scholar]
- 2.Ireland JLH, Scheetz D, Jimenez-Krassel F, Themmen APN, Ward F, Lonergan P, Smith GW, Perez GI, Evans ACO, Ireland JJ. Antral follicle count reliably predicts number of morphologically healthy oocytes and follicles in ovaries of young adult cattle. Biol Reprod 2008; 79: 1219–1225. [DOI] [PubMed] [Google Scholar]
- 3.Souza AH, Carvalho PD, Rozner AE, Vieira LM, Hackbart KS, Bender RW, Dresch AR, Verstegen JP, Shaver RD, Wiltbank MC. Relationship between circulating anti-Müllerian hormone (AMH) and superovulatory response of high-producing dairy cows. J Dairy Sci 2015; 98: 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rico C, Drouilhet L, Salvetti P, Dalbiès-Tran R, Jarrier P, Touzé JL, Pillet E, Ponsart C, Fabre S, Monniaux D. Determination of anti-Müllerian hormone concentrations in blood as a tool to select Holstein donor cows for embryo production: from the laboratory to the farm. Reprod Fertil Dev 2012; 24: 932–944. [DOI] [PubMed] [Google Scholar]
- 5.Hirayama H, Kageyama S, Naito A, Fukuda S, Fujii T, Minamihashi A. Prediction of superovulatory response in Japanese Black cattle using ultrasound, plasma anti-Müllerian hormone concentrations and polymorphism in the ionotropic glutamate receptor AMPA1/GRIA1. J Reprod Dev 2012; 58: 380–383. [DOI] [PubMed] [Google Scholar]
- 6.Mossa F, Walsh SW, Butler ST, Berry DP, Carter F, Lonergan P, Smith GW, Ireland JJ, Evans ACO. Low numbers of ovarian follicles ≥3 mm in diameter are associated with low fertility in dairy cows. J Dairy Sci 2012; 95: 2355–2361. [DOI] [PubMed] [Google Scholar]
- 7.Cushman RA, Allan MF, Kuehn LA, Snelling WM, Cupp AS, Freetly HC. Evaluation of antral follicle count and ovarian morphology in crossbred beef cows: investigation of influence of stage of the estrous cycle, age, and birth weight. J Anim Sci 2009; 87: 1971–1980. [DOI] [PubMed] [Google Scholar]
- 8.Ribeiro ES, Bisinotto RS, Lima FS, Greco LF, Morrison A, Kumar A, Thatcher WW, Santos JEP. Plasma anti-Müllerian hormone in adult dairy cows and associations with fertility. J Dairy Sci 2014; 97: 6888–6900. [DOI] [PubMed] [Google Scholar]
- 9.Ireland JJ, Smith GW, Scheetz D, Jimenez-Krassel F, Folger JK, Ireland JLH, Mossa F, Lonergan P, Evans ACO. Does size matter in females? An overview of the impact of the high variation in the ovarian reserve on ovarian function and fertility, utility of anti-Müllerian hormone as a diagnostic marker for fertility and causes of variation in the ovarian reserve in cattle. Reprod Fertil Dev 2011; 23: 1–14. [DOI] [PubMed] [Google Scholar]
- 10.Rico C, Fabre S, Médigue C, di Clemente N, Clément F, Bontoux M, Touzé J-L, Dupont M, Briant E, Rémy B, Beckers J-F, Monniaux D. Anti-mullerian hormone is an endocrine marker of ovarian gonadotropin-responsive follicles and can help to predict superovulatory responses in the cow. Biol Reprod 2009; 80: 50–59. [DOI] [PubMed] [Google Scholar]
- 11.Monniaux D, Drouilhet L, Rico C, Estienne A, Jarrier P, Touzé J-L, Sapa J, Phocas F, Dupont J, Dalbiès-Tran R, Fabre S. Regulation of anti-Müllerian hormone production in domestic animals. Reprod Fertil Dev 2012; 25: 1–16. [DOI] [PubMed] [Google Scholar]
- 12.Mossa F, Carter F, Walsh SW, Kenny DA, Smith GW, Ireland JLH, Hildebrandt TB, Lonergan P, Ireland JJ, Evans ACO. Maternal undernutrition in cows impairs ovarian and cardiovascular systems in their offspring. Biol Reprod 2013; 88: 92. [DOI] [PubMed] [Google Scholar]
- 13.Wright JM. Manual of the International Embryo Transfer Society. 4th ed. Stringfellow D, Daniel Givens M (eds.), Champaign (IL): International Embryo Transfer Society; 2010.
- 14.Ginther OJ, Beg MA, Bergfelt DR, Donadeu FX, Kot K. Follicle selection in monovular species. Biol Reprod 2001; 65: 638–647. [DOI] [PubMed] [Google Scholar]
- 15.Burns DS, Jimenez-Krassel F, Ireland JLH, Knight PG, Ireland JJ. Numbers of antral follicles during follicular waves in cattle: evidence for high variation among animals, very high repeatability in individuals, and an inverse association with serum follicle-stimulating hormone concentrations. Biol Reprod 2005; 73: 54–62. [DOI] [PubMed] [Google Scholar]
- 16.Kawamata M. Relationships between the number of small follicles prior to superovulatory treatment and superovulatory response in Holstein cows. J Vet Med Sci 1994; 56: 965–967. [DOI] [PubMed] [Google Scholar]
- 17.Singh J, Domínguez M, Jaiswal R, Adams GP. A simple ultrasound test to predict the superstimulatory response in cattle. Theriogenology 2004; 62: 227–243. [DOI] [PubMed] [Google Scholar]
- 18.Rico C, Médigue C, Fabre S, Jarrier P, Bontoux M, Clément F, Monniaux D. Regulation of anti-Müllerian hormone production in the cow: a multiscale study at endocrine, ovarian, follicular, and granulosa cell levels. Biol Reprod 2011; 84: 560–571. [DOI] [PubMed] [Google Scholar]
- 19.Lahoz B, Alabart JL, Cocero MJ, Monniaux D, Echegoyen E, Sánchez P, Folch J. Anti-Müllerian hormone concentration in sheep and its dependence of age and independence of BMP15 genotype: an endocrine predictor to select the best donors for embryo biotechnologies. Theriogenology 2014; 81: 347–357. [DOI] [PubMed] [Google Scholar]