Abstract
Several parameters of preoperative complete blood count (CBC) and inflammation-associated blood cell markers derived from them have been reported to correlate with prognosis in patients with epithelial ovarian cancer (EOC), but their prognostic importance and optimal cutoffs are still needed be elucidated. Clinic/pathological parameters, 5-year follow-up data and preoperative CBC parameters were obtained retrospectively in 654 EOC patients underwent primary surgery at Mayo Clinic. Cutoffs for neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and monocyte-to-lymphocyte ratio (MLR) were optimized by receiver operating characteristic (ROC) curve. Prognostic significance for overall survival (OS) and recurrence free survival (RFS) were determined by Cox proportional hazards models and Kaplan-Meier method. Associations of RDW and NLR with clinic/pathological parameters were analyzed using non-parametric tests. RDW with cutoff 14.5 and NLR with cutoff 5.25 had independent prognostic significance for OS, while combined RDW and NLR scores stratified patients into low (RDW-low and NLR-low), intermediate (RDW-high or NLR-high) and high risk (RDW-high and NLR-high) groups, especially in patients with high-grade serous ovarian cancer (HGSOC). Moreover, high NLR was associated with poor RFS as well. Elevated RDW was strongly associated with age, whereas high NLR was strongly associated with stage, preoperative CA125 level and ascites at surgery.
Epithelial ovarian cancer (EOC) is about one-tenth as common as breast cancer, but remains the most lethal gynecologic malignancy which serves as the fifth leading cause of cancer death among women in United States with 21,290 new cases and 14,180 deaths in 20151. Less than 40% of women with EOC are cured1 due to 70% of patients are diagnosed with advanced disease (stage III or IV)2. Primary treatment for patients with advanced stage EOC consists of cytoreductive surgery, followed by platinum and taxane-based chemotherapy3. Unfortunately, the overall survival rate of women with EOC has changed little since platinum based treatment was introduced more than 30 years ago4. Known factors that influence prognosis of patients with EOC include age, FIGO stag, histology, grade and the result of surgical treatment5,6,7. Nevertheless, there are still patients with advanced-stage high-grade cancers survive longer than their contemporaries8,9. Although a serial of molecular signatures was reported to stratify survival in different cohorts of EOC patients10,11,12,13, simple, reproducible and inexpensive biomarkers to generate prognostic model are still unavailable at clinical settings.
Complete blood count (CBC) is one of the most simple, reproducible and inexpensive tests for patients with EOC. In addition to guiding the clinical management of EOC patients who are candidates for surgery, parameters of preoperative CBC, such as platelet count14, hemoglobin15,16, and eosinophil count17, were also reported to correlate with survival of patients. Moreover, with accumulating evidences on the role of inflammation in carcinogenesis and tumor progression18,19, several serum parameters as markers of systemic inflammation, ranging from neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), to monocyte-to-lymphocyte ratio (MLR), have been revealed to possess potential to predict survival in a variety of human cancers20,21,22,23, including EOC24,25,26,27. Quite recently, some other inflammation-associated blood cell markers, such as red cell distribution width (RDW), have also been shown to associate with survival of solid tumors28,29, but have never been studied in EOC. Thus, the aim of the current study was to investigate simultaneously the impact of preoperative CBC parameters and inflammation-associated blood cell markers on survival of a large cohort of EOC patients with 5-year follow-up data.
Results
Patient characteristics
Patient characteristics are outlined in Table 1. A total of 654 patients with EOC were included in the present study. Median age at diagnosis was 63 years (range 28–93). Most patients (533, 81.5%) was of advanced stage (stage III or IV) and underwent cytoreductive surgery, followed by platinum and taxane-based chemotherapy. There were 482 (73.7%) patients with cancer originated from ovary (excluded fallopian tube cancer and primary peritoneal cancer) and among them, 355 (73.7%) were with high-grade serous ovarian cancer (HGSOC), the most common and lethal subtypes of EOC30. Median follow-up time for the current cohort was 49.5 months (range 0.1–175.3).
Table 1. Patient characteristics (N = 654).
| Covariates | No. of Patients (%) |
|---|---|
| Age at diagnosis, years | |
| Mean, Range | 63.0, 28–93 |
| Race | |
| White | 563 (97.2%) |
| Asian | 3 (0.5%) |
| Other | 13 (2.2%) |
| Origin of cancer | |
| Ovary | 482 (73.7%) |
| Fallopian tube | 10 (1.5%) |
| Peritoneum | 162 (24.8%) |
| Stage | |
| I | 87 (13.3%) |
| II | 34 (5.2%) |
| III | 416 (63.6%) |
| IV | 117 (17.9%) |
| Histology | |
| High-grade serous | 525 (80.3%) |
| Low-grade serous | 4 (0.6%) |
| Endometrioid | 71 (10.9%) |
| Clear cell | 37 (5.7%) |
| Mucinous | 17 (2.6%) |
| Grade | |
| 1 | 28 (4.3%) |
| 2 | 54 (8.3%) |
| 3 | 572 (87.5%) |
| Preoperative CA125 level, U/ml | |
| <35 | 50 (9.5%) |
| ≥35 | 475 (90.5%) |
| Unknown | 129 |
| Ascites at surgery | |
| No | 187 (34.2%) |
| Yes | 359 (65.8%) |
| Unknown | 108 |
| Residual disease | |
| None | 266 (41.0%) |
| Macroscopic disease <1 cm | 305 (47.1%) |
| Macroscopic disease >1 cm | 77 (11.9%) |
| Recurrence | |
| No | 276 (42.2%) |
| Yes | 293 (44.8%) |
| Unknown | 85 |
| Vital status | |
| Alive | 197 (30.1%) |
| Dead | 457 (69.9%) |
Numbers may not add to total due to missing values (75 for race, 6 for surgical debulking).
Prognostic significance of preoperative CBC parameters and cut-off determination
To elucidate the prognostic significance of preoperative CBC parameters and inflammation-associated blood cell markers, univariate Cox proportional hazards analyses were performed on continuous data (Supplementary Table 1). Platelet, leukocyte, erythrocyte, neutrophils and lymphocyte counts, along with hemoglobin and hematocrit are significantly associated with OS, while platelet, neutrophils and lymphocyte counts are associated with RFS. Analyses also revealed elevated RDW, PLR, NLR and MLR are associated with both poor OS and RFS. However, no association between monocyte, basophil or eosinophil counts and survival were found.
Cutoff values for PLR applied to predict survival in EOC patients range from 20026,27 to 30031, while those for NLR vary from 2.624 to 3.7732, or even a trend rather than specific values25 that cripples their prognostic values for clinical use. Moreover, there is no report concerning the prognostic significance, yet the cutoff values for RDW and MLR in EOC until now. Thus, we decide to optimize cutoff for RDW, PLR, NLR and MLR on this study cohort with ROC curve analysis (Materials and Methods). Cutoff values as 14.15 for RDW (P = 5.6e-4, HR = 141), 5.25 for NLR (P = 1e-4, HR = 1.48), 273.5 for PLR (P = 5e-8, HR = 1.68) and 0.45 for MLR (P = 8.5e-8, HR = 1.66) were then optimized respectively.
RDW and NLR have independent prognostic significance
Univariate Cox proportional hazards analyses also revealed age at diagnosis (stratified into four groups according to interquartile range), origin of cancer (EOC, fallopian tube cancer (FTC), and primary peritoneal cancer (PPC)), stage, histology, grade, preoperative CA125 level (≥35 vs <35 U/ml, P < 0.001), ascites at surgery (yes vs no, P < 0.001) and residual disease were significantly associated with OS (Table 2), while all except age were significantly associated with RFS (Supplemental Table 2). We then included those clinical/pathological parameters except preoperative CA125 and ascites at surgery because of a large amount of missing values, into subsequent multivariate Cox proportional hazards models.
Table 2. Overall survival of ovarian cancer patients stratified according to RDW, NLR, PLR and MLR cut-offs, together with other prognostic parameters (N = 654).
| Parameter | Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| RDW | ||||
| Low (<14.15) | 1 (reference) | 1 (reference) | ||
| High (≥14.15) | 1.412 (1.160–1.720) | 0.001 | 1.235 (1.008–1.513) | 0.042 |
| NLR | ||||
| Low (<5.25) | 1 (reference) | 1 (reference) | ||
| High (≥5.25) | 1.478 (1.212–1.801) | <0.001 | 1.391 (1.133–1.708) | 0.002 |
| PLR | ||||
| Low (<273.5) | 1 (reference) | 1 (reference) | ||
| High (≥273.5) | 1.680 (1.392–2.028) | <0.001 | 1.102 (0.900–1.348) | 0.347 |
| MLR | ||||
| Low (<0.45) | 1 (reference) | 1 (reference) | ||
| High (≥0.45) | 1.565 (1.412–20.69) | <0.001 | 1.129 (0.923–1.381) | 0.236 |
| Combined RDW+NLR | ||||
| RDW-low + NLR-low | 1 (reference) | 1 (reference) | ||
| RDW-high or NLR-high | 1.595 (1.307–1.946) | <0.001 | 1.332 (1.087–1.633) | 0.006 |
| RDW-high + NLR-high | 1.734 (1.261–2.385) | 0.001 | 1.670 (1.207–2.311) | 0.002 |
| Age at diagnosis, years | ||||
| <55 | 1 (reference) | 1 (reference) | ||
| 55–63 | 1.351 (1.019–1.790) | 0.036 | 1.032 (0.767–1.389) | 0.834 |
| 63–72 | 1.762 (1.356–2.290) | <0.001 | 1.216 (0.925–1.597) | 0.161 |
| ≥72 | 2.539 (1.955–3.296) | <0.001 | 1.948 (1.476–2.571) | <0.001 |
| Origin of cancer | ||||
| Ovary | 1 (reference) | 1 (reference) | ||
| Fallopian tube | 0.790 (0.352–1.774) | 0.568 | 0.962 (0.424–2.182) | 0.925 |
| Peritoneum | 1.872 (1.531–2.289) | <0.001 | 1.218 (0.985–1.507) | 0.069 |
| Stage | ||||
| I | 1 (reference) | 1 (reference) | ||
| II | 1.752 (0.871–3.522) | 0.116 | 1.260 (0.598–2.654) | 0.543 |
| III | 5.980 (3.799–9.415) | <0.001 | 2.647 (1.486–4.714) | 0.001 |
| IV | 9.971 (6.161–16.136) | <0.001 | 4.201 (2.281–7.736) | <0.001 |
| Histology | ||||
| High-grade serous | 1 (reference) | 1 (reference) | ||
| Low-grade serous | 0.175 (0.025–1.247) | 0.082 | 0.344 (0.036–3.262) | 0.353 |
| Endometrioid | 0.338 (0.232–0.494) | <0.001 | 1.039 (0.653–1.655) | 0.870 |
| Clear cell | 0.497 (0.306–0.808) | 0.005 | 1.260 (0.706–2.248) | 0.435 |
| Mucinous | 0.082 (0.020–0.330) | <0.001 | 0.369 (0.085–1.846) | 0.238 |
| Grade | ||||
| 1 | 1 (reference) | 1 (reference) | ||
| 2 | 1.667 (0.662–4.200) | 0.278 | 0.607 (0.201–1.839) | 0.378 |
| 3 | 6.592 (2.940–14.781) | <0.001 | 1.249 (0.422–3.703) | 0.688 |
| Residual disease | ||||
| None | 1 (reference) | 1 (reference) | ||
| Macroscopic disease <1 cm | 2.981 (2.399–3.705) | <0.001 | 1.823 (1.432–2.320) | <0.001 |
| Macroscopic disease >1 cm | 5.427 (4.037–7.296) | <0.001 | 2.939 (2.123–4.069) | <0.001 |
Univariate and multivariate analysis performed using Cox proportional hazards models. RDW, NLR, PLR, MLR and combined RDW+NLR were adjusted separately in models that included age at diagnosis, origin of cancer, stage, histology, grade and residual disease. Preoperative CA125 level and ascites at surgery were excluded because of missing values (19.7% and 16.5%, respectively). Results from multivariate model which included combined RDW+NLR score are indicated in bold. Abbreviations: RDW = red blood cell distribution width; NLR = neutrophil-to-lymphocyte ratio; PLR = platelet-to-lymphocyte ratio; MLR = monocyte-to-lymphocyte ratio; HR = hazard ratio; CI = confidence interval.
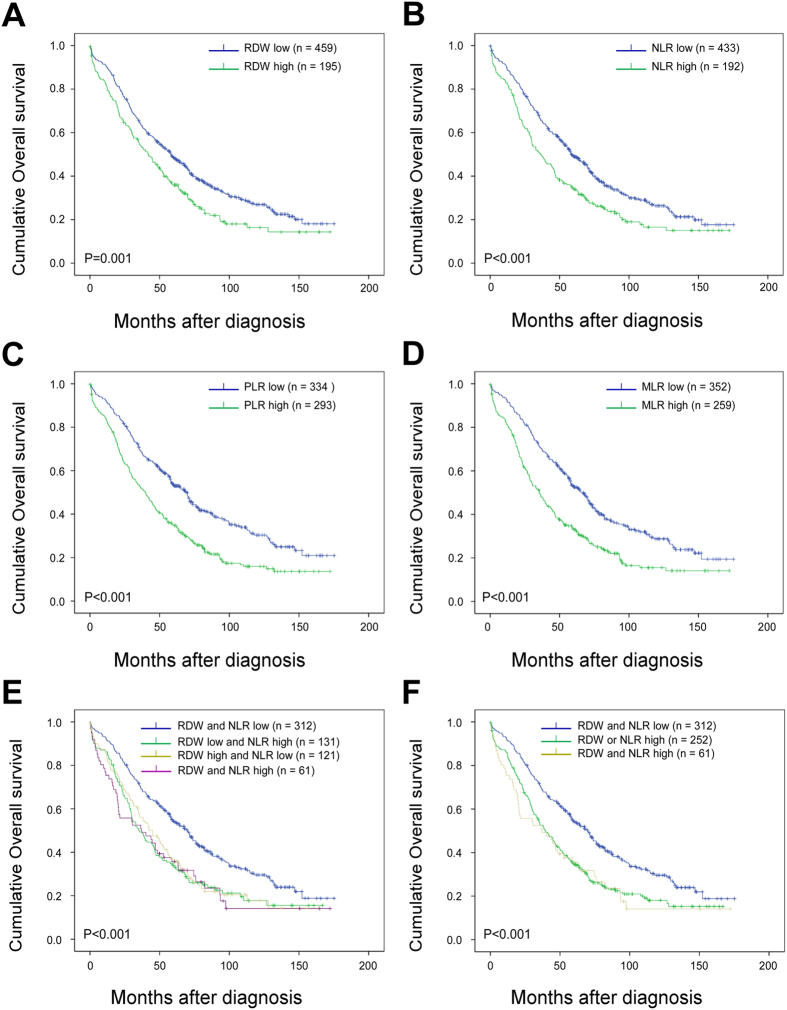
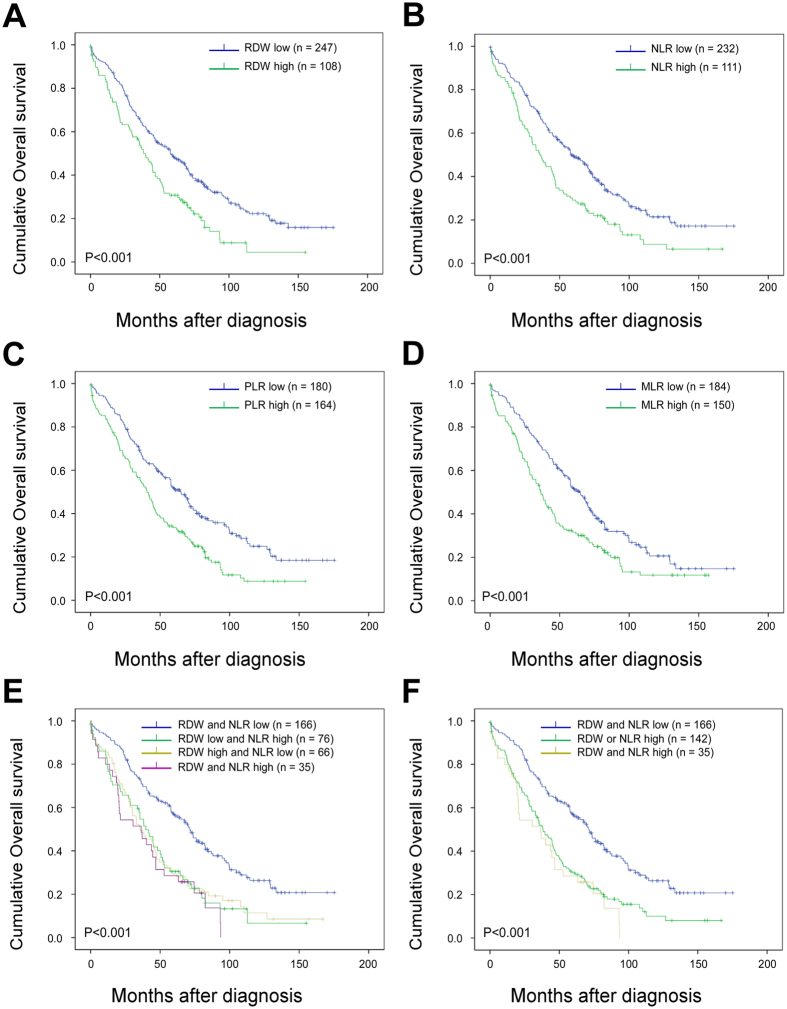
Kaplan-Meier analysis and log-rank test demonstrated that high preoperative RDW, NLR, PLR and MLR significantly predicted poorer OS both in all EOC patients (including FTC and PPC, Fig. 1A to D) and in HGSOC patients (Fig. 2A to D). Patients with different combination of RDW and NLR according to their dichotomized values had extra prognostic values both in all EOC patients (including FTC and PPC, Fig. 1E) and in HGSOC patients (Fig. 2E). We then combined RDW+NLR by stratifying patients into three, rather than four groups for more distinctive patients’ stratification and easier clinical usage33,34 as: (1) RDW-low and NLR-low; (2) RDW-high or NLR-high; and (3) RDW-high and NLR-high. The simplest and most effective way succeeded in identifying low, intermediate and high risk groups, especially in HGSOC patients, with estimated cumulative 5-year OS rates of 58.4%, 31.4% and 24.4%, respectively (Fig. 2F), as well as 53.8%, 34.5% and 35.7%, respectively, in all EOC patients (Fig. 1F). So, we used three groups strategy rather than four groups to summarize combined RDW+NLR in the following analyses.
Figure 1. Overall survival of ovarian cancer patients stratified according to RDW, NLR and MLR cut-offs (N = 654).
Kaplan–Meier overall survival (OS) curves with log-rank P-values for patients stratified using red blood cell distribution width (RDW) cutoff of 14.15 (A), neutrophil-to-lymphocyte ratio (NLR) cutoff of 5.25 (B), platelet-to-lymphocyte ratio (PLR) cutoff of 242.9 (C), monocyte-to-lymphocyte ratio (MLR) cutoff of 0.45 (D) and combined RDW + NLR (four groups, E; three groups, F) with cutoffs defined above.
Figure 2. Overall survival of high-grade serous ovarian cancer patients stratified according to RDW, NLR and MLR cut-offs (N = 355).
Kaplan–Meier overall survival (OS) curves with log-rank P-values for patients stratified using red blood cell distribution width (RDW) cutoff of 14.15 (A), neutrophil-to-lymphocyte ratio (NLR) cutoff of 5.25 (B), platelet-to-lymphocyte ratio (PLR) cutoff of 242.9 (C), monocyte-to-lymphocyte ratio (MLR) cutoff of 0.45 (D) and combined RDW+NLR (four groups, E; three groups, F) with cutoffs defined above.
Given that NLR, PLR and MLR were strongly correlated with each other (Spearman’s rho coefficients of 0.425 (NLR vs PLR), 0.511 (NLR vs MLR) and 0.514 (PLR vs MLR; all P < 0.001), and all of them were derived from CBC parameters such as platelet, neutrophils, monocyte and lymphocyte counts, all CBC parameters and inflammation-associated blood cell markers that had significantly impact on survival in univariate Cox proportional hazards analyses (P < 0.05) were adjusted separately in multivariable Cox proportional hazards models included (1) age at diagnosis, origin of cancer, stage, histology, grade and residual disease for OS in all EOC patients (Table 2); (2) age at diagnosis, stage and residual disease for OS in HGSOC patients (Table 3); and (3) origin of cancer, stage, histology, grade and residual disease for RFS in all EOC patients (Supplemental Table 2). RDW, NLR and combined RDW+NLR were then revealed as independent prognostic factors for OS both in all EOC patients and in HGSOC patients, while more highly significant in HGSOC patients (except for RDW in HGSOC patients, P = 0.064). However, no association between other CBC parameters and inflammation-associated blood cell markers with OS was identified by multivariate Cox proportional hazards analyses. On the contrary, only NLR had independent prognostic value for RFS both in all EOC patients (Supplemental Fig. 1A) and in HGSOC patients (Supplemental Fig. 1B).
Table 3. Overall survival of high-grade serous ovarian cancer patients stratified according to RDW, NLR, PLR and MLR cut-offs, together with other prognostic parameters (N = 355).
| Parameter | Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| RDW | ||||
| Low (<14.15) | 1 (reference) | 1 (reference) | ||
| High (≥14.15) | 1.682 (1.299–2.180) | <0.001 | 1.292 (0.985–1.694) | 0.064 |
| NLR | ||||
| Low (<5.25) | 1 (reference) | 1 (reference) | ||
| High (≥5.25) | 1.663 (1.287–2.149) | <0.001 | 1.414 (1.087–1.838) | 0.010 |
| PLR | ||||
| Low (<273.5) | 1 (reference) | 1 (reference) | ||
| High (≥273.5) | 1.683 (1.313–2.158) | <0.001 | 1.228 (0.935–1.613) | 0.139 |
| MLR | ||||
| Low (<0.45) | 1 (reference) | 1 (reference) | ||
| High (≥0.45) | 1.600 (1.245–2.057) | <0.001 | 1.156 (0.883–1.514) | 0.291 |
| Combined RDW+NLR | ||||
| RDW-low+NLR-low | 1 (reference) | 1 (reference) | ||
| RDW-high or NLR-high | 1.900 (1.458–2.477) | <0.001 | 1.392 (1.058–1.830) | 0.018 |
| RDW-high+NLR-high | 2.342 (1.553–3.530) | <0.001 | 1.844 (1.213–2.804) | 0.004 |
| Age at diagnosis, years | ||||
| <55 | 1 (reference) | 1 (reference) | ||
| 55–63 | 1.351 (1.019–1.790) | 0.036 | 1.099 (0.749–1.610) | 0.630 |
| 63–72 | 1.762 (1.356–2.290) | <0.001 | 1.232 (0.866–1.753) | 0.246 |
| ≥72 | 2.539 (1.955–3.296) | <0.001 | 2.233 (1.566–3.184) | <0.001 |
| Stage | ||||
| I | 1 (reference) | 1 (reference) | ||
| II | 1.752 (0.871–3.522) | 0.116 | 1.597 (0.574–4.444) | 0.370 |
| III | 5.980 (3.799–9.415) | <0.001 | 2.452 (1.050–5.725) | 0.038 |
| IV | 9.971 (6.161–16.136) | <0.001 | 4.649 (1.908–11.327) | 0.001 |
| Residual disease | ||||
| None | 1 (reference) | 1 (reference) | ||
| Macroscopic disease <1 cm | 2.981 (2.399–3.705) | <0.001 | 1.491 (1.094–2.032) | 0.011 |
| Macroscopic disease >1 cm | 5.427 (4.037–7.296) | <0.001 | 2.908 (1.929–4.384) | <0.001 |
Univariate and multivariate analysis performed using Cox proportional hazards models. RDW, NLR, PLR, MLR, and combined RDW+NLR were adjusted separately in models that included age at diagnosis, stage and residual disease. Preoperative CA125 level and ascites at surgery were excluded because of missing values (16.3% and 18.3%, respectively). Results from multivariate model which included combined RDW+NLR score are indicated in bold. Abbreviations: RDW = red blood cell distribution width; NLR = neutrophil-to-lymphocyte ratio; PLR = platelet-to-lymphocyte ratio; MLR = monocyte-to-lymphocyte ratio; HR = hazard ratio; CI = confidence interval.
Associations of RDW and NLR with other clinic/pathological parameters
Finally, associations of RDW and NLR with other clinic/pathological parameters were investigated (Table 4). While RDW was significantly associated with age (<0.001) and significantly elevated in patients aged ≥72 years compared with those aged <55 years, NLR was significantly associated with features of high tumor burden, such as stage (P = 0.006), preoperative CA125 level (P = 0.001) and ascites at surgery (P < 0.001).
Table 4. Associations of RDW and NLR with other clinic/pathological parameters (N = 654).
| Parameter | n%# | RDW, Median (IQR) | P | NLR, Median (IQR) | P |
|---|---|---|---|---|---|
| Age at diagnosis, years | |||||
| <55 | 180 (27.5) | 13.70 (12.60–14.10)4 | <0.001 | 5.13 (2.83–6.06) | 0.210 |
| 55–63 | 151 (23.1) | 13.71 (12.70–14.40) | 5.04 (3.09–5.71) | ||
| 63–72 | 171 (26.1) | 13.83 (12.80–14.30) | 4.71 (2.88–5.64) | ||
| ≥72 | 152 (23.2) | 14.16 (13.10–14.80)1 | 5.13 (2.96–5.21) | ||
| Origin of cancer | |||||
| Ovary | 482 (73.7) | 13.88 (12.80–14.50) | 0.576 | 4.94 (2.94–5.67) | 0.359 |
| Fallopian tube | 10 (1.5) | 14.17 (12.60–15.83) | 3.34 (2.11–4.74) | ||
| Peritoneum | 162 (24.8) | 13.72 (12.80–14.30) | 4.87 (3.19–5.67) | ||
| Stage | |||||
| I | 87 (13.3) | 14.00 (12.90–14.10) | 0.441 | 4.07 (2.55–5.20)4 | 0.006 |
| II | 34 (5.2) | 13.41 (12.65–14.33) | 3.64 (2.49–4.20)3,4 | ||
| III | 416 (63.6) | 13.82 (12.70–14.50) | 4.86 (2.94–5.70)2,4 | ||
| IV | 117 (17.9) | 13.94 (12.90–14.60) | 5.96 (3.70–6.22)1,2,3 | ||
| Histology | |||||
| High-grade serous | 525 (80.3) | 13.80 (12.75–14.40) | 0.499 | 4.99 (2.97–5.66) | 0.378 |
| Low-grade serous | 4 (0.6) | 14.80 (13.60–14.70) | 2.95 (2.55-NA) | ||
| Endometrioid | 71 (10.9) | 14.06 (12.90–14.50) | 4.33 (2.62–5.74) | ||
| Clear cell | 37 (5.7) | 14.06 (12.90–14.80) | 5.14 (3.13–6.43) | ||
| Mucinous | 17 (2.6) | 13.53 (12.75–14.25) | 3.86 (2.99–4.94) | ||
| Grade | |||||
| 1 | 28 (4.3) | 14.41 (12.93–14.75) | 0.437 | 3.82 (2.55–4.94) | 0.149 |
| 2 | 54 (8.3) | 13.51 (12.80–14.10) | 3.79 (2.32–4.85) | ||
| 3 | 572 (87.5) | 13.85 (12.80–14.50) | 5.04 (3.04–5.70) | ||
| Preoperative CA125 level, U/ml | |||||
| <35 | 50 (7.6) | 13.98 (12.95–14.25) | 0.788* | 3.23 (1.92–4.41) | 0.001* |
| ≥35 | 475 (72.6) | 13.77 (12.70–14.30) | 5.09 (3.11–5.72) | ||
| Missing | 129 (19.7) | — | — | ||
| Ascites at surgery | |||||
| No | 187 (28.6) | 13.71 (12.80–14.00) | 0.004* | 4.10 (2.56–5.10) | <0.001* |
| Yes | 359 (54.9) | 13.91 (12.80–14.60) | 5.44 (3.31–6.27) | ||
| Missing | 108 (16.5) | — | — | ||
| Residual disease | |||||
| None | 266 (40.7) | 13.75 (12.80–14.10) | 0.234 | 4.26 (2.61–5.45) | 0.348 |
| Macroscopic disease <1 cm | 305 (46.6) | 13.85 (12.80–14.40) | 5.09 (3.06–5.74) | ||
| Macroscopic disease >1 cm | 77 (11.8) | 14.01 (12.70–14.95) | 6.45 (3.80–6.97) | ||
| Missing | 6 (0.9) | — | — | ||
#Values for RDW. *P-values from Mann–Whitney U-test (all other P-values are from Kruskall–Wallis tests). 1,2,3,4Categories significant differences between one another following post-hoc Mann–Whitney U-tests with Bonferroni corrections for multiple comparisons. Abbreviations: RDW = red blood cell distribution width; NLR = neutrophil-to-lymphocyte ratio; NA = no data available due to small sample size in that category.
Discussion
Here is the first study to investigate the prognostic value of preoperative RDW in EOC, and the largest study to investigate the prognostic role of preoperative CBC parameters and inflammation-associated blood cell markers including NLR, PLR and MLR in patients with EOC, especially in patients with HGSOC. We revealed that elevated preoperative RDW and NLR predict poor OS in patients with EOC, and combined high RDW+NLR provides additional patient stratification, especially in patients with HGSOC. On the contrary, only high NLR predicts poor RFS in EOC and HGSOC.
Inflammation has been recognized as one of the hallmarks of nearly all human cancers35. Tumor-related inflammatory microenvironment could facilitate tumor growth and metastasis by sustaining proliferation, inhibiting apoptosis, inducing epithelial-to-mesenchymal transition (EMT), initiating angiogenesis, and suppressing host-anti-tumor immunity18,19. For EOC, epidemiological studies revealed pelvic inflammatory diseases might increase risk36, while inflammation caused by incessant ovulation remains one of the well-accepted hypotheses of EOC carcinogenesis37. However, the biologic mechanisms underlying the correlation between NLR, PLR, MLR, systemic inflammation and tumorigenesis in EOC remains poorly understood. In fact, intratumoral neutrophils had been shown to be associated with unfavorable survival in many human cancers ranging from hepatocellular carcinoma, gastric carcinoma, colorectal cancer, to non-small-cell lung cancer and renal cell carcinoma38. Cancer cells facilitate recruitment of tumor-associated neutrophils (TANs) by expressing various of chemokines and cytokines, including CXCL5, CXCL6, and CXCL839, along with ligands that recognize receptors such as CXCR2 expressed by TANs40. TANs recruited by tumor then release pro-growth and pro-invasion factors including hepatocyte growth factor (HGF), reactive oxygen species (ROS), reactive nitrogen species (RNS), neutrophil elastase (NE), neutrophil collagenase (MMP8), and gelatinase B (MMP9). In addition, TANs also release cytokines like Oncostatin M, which induce VEGF and then stimulate angiogenesis to support tumor metastasis41. On the contrary, lymphocytes, especially CD8+T cells, which represents host anti- tumor immune response, had been recognized as a predictor of favorable survival in a variety of human cancers42, including EOC43. However, neutrophils recruited by tumor could interact with CD8+T cells to counteract their protective effect that result in procancer immunosuppressive microenvironment41. That may explain, why high preoperative NLR, in terms of more neutrophils and less lymphocytes, was significantly associated with features of high tumor burden, including stage (P = 0.006), preoperative CA125 level (P = 0.001) and ascites at surgery (P < 0.001), and predicted both poor RFS and OS in EOC patients in the current study. Quite recently, two independent studies conducted in colorectal cancer added evidence to the hypothesis mentioned above. Chen, Z. Y. et al. indicated NLR > 5 was associated with poor prognosis in metastatic colorectal cancer and high NLR was correlated with increased expression of inflammatory cytokines such as interleukin 6 (IL-6), IL-8, IL-2Ra, HGF, macrophage-colony stimulating factor (M-CSF), and vascular epidermal growth factor (VEGF)44. Pine, J. K. and colleagues found NLR ≥ 5 predicted lower overall survival and greater disease recurrence while lower NLR was associated with pronounced lymphocytic reaction at the invasive margin (IM) in colorectal cancer tissues45. Those results inspired us to further study the cytokines profile and tumor associated local lymphocytic response in this EOC cohort to better understand the possible mechanism behind preoperative high NLR as a risk factor predicting poor prognosis in EOC patients.
Previous studies in EOC26,27,31,46 and many other human cancers22 established NLR’s role in predicting survival, but the wide range of NLR cutoff from 1.9 to 5.020 limited its usage in clinical field. This study employed ROC curve analysis to optimize cutoff for NLR as 5.25, which succeeded in stratifying 654 EOC patients independently into two distinctive survival groups both for RFS (P = 0.026, HR = 1.331, 95% CI = 1.035–1.712, multivariate) and OS (P = 0.002, HR = 1.391, 95% CI = 1.133–1.708). While studies in independent cohort to determine the optimized cutoffs for NLR in EOC are still warranted.
Also known as erythrocytes, red blood cells (RBCs) are the most common type of blood cells47. Red cell distribution width (RDW) indicates the size variation of RBCs, and is calculated by dividing the mean corpuscular volume (MCV) by the standard deviation (SD) of the RBC and then multiplied for 100, to express data as a percentage48. Traditionally, RDW is used in laboratory hematology for differential diagnosis of anemias49, while quite recently, growing evidence indicated that high RDW is associated with systematic inflammation49 and elevated RDW harbored the potential to predict poor survival in a variety of human cancers, consisting of breast cancer50,51,52, lung cancer53,54,55, prostate cancer56, endometrial cancer57 and upper tract urothelial carcinoma58. In the present study, we demonstrated, for the first time, that preoperative RDW, with cutoff 14.15 determined by ROC curve, acts as a risk factor for shorten OS in patients with EOC. Moreover, combined high RDW+NLR provides additional patient stratification, especially in patients with HGSOC (estimated cumulative 5-year OS rates of 58.4%, 31.4% and 24.4%, respectively), even though RDW itself lost impact on OS in multivariate model in HGSOC patients (P = 0.064). Given that 81.5% patients underwent adjuvant platinum and taxane-based chemotherapy after surgery, these data suggest that patients with high preoperative RDW and NLR might be potential candidates for clinical trials employing more intensive treatments, including maintain chemotherapy, target therapy and immune therapy to delay recurrence and obtain desirable prognosis. However, RDW’s significant association with age (<0.001) in this EOC cohort indicated that RDW may serve as a surrogate for conditions like poor performance/nutrition status and the presence of comorbidities, which needs to be elucidated in studies involving cancer-specific death analyses.
In conclusion, the current study highlights the role of RDW and NLR as additional prognostic factors in EOC patients. These simple, reproducible and inexpensive markers, though need further investigations, may harbor the potential to identify high-risk EOC patients as candidate for more intensive therapies after standard treatment.
Material and Methods
Patients and follow-up
Patients who underwent primary surgery for invasive EOC, fallopian tube cancer (FTC), or primary peritoneal cancer (PPC) from 2000 to 2010 at departments of gynecologic surgery at Mayo Clinic in Rochester, MN were recruited. FTC and PPC are less common neoplasms that are managed in a similar manner to epithelial ovarian cancer3. The research was approved by the institutional review board (IRB) of Mayo Clinic. All methods were performed in accordance with the relevant guidelines and regulations. Patients provided written informed consent and permission for active follow-up concerning of recurrence and vital status changes. Patients were excluded if they (1) underwent neoadjuvant chemotherapy prior to surgery; (2) underwent prior surgery for their cancer elsewhere; (3) were treated as recurrent disease; (4) had non-epithelial or non-ovarian malignancies; (5) had no preoperative CBC parameters tested by Mayo Clinic in Rochester within 30 days prior to primary surgery or (6) did not consent to the use of their medical records for research purposes. Perioperative CBC parameters were collected retrospectively. Patient cohort identification and data query were supported by our data management and analysis platform named Integrating Biology and the Bedside (i2b2)59, an NIH-funded software framework allowing collaborative exchange of data including electronic health records, lab results, genetic and research data. Details of cohort identification and data query will be described in our other publications. Recurrence and vital status were updated every six months using medical records and active follow-up. The end of follow-up was the time of last follow-up (April 2015) or death.
Statistical analysis
Overall survival (OS) was defined as time from diagnosis to death (all causes). Recurrence free survival (RFS) was defined as time from surgery to the first recurrence, and patients who had persistent disease after primary treatment (surgery alone or surgery and adjuvant chemotherapy) were treated as censored. Cutoff optimization of RDW, NLR, PLR and MLR were performed using the software package Cutoff Finder33 based on R version 2.15.0 (R Core Team, 2012), and the standard receiver operating characteristic (ROC) curve based on binary outcome (vital status), using Manhattan distance to calculate optimal cut-offs was employed. Univariate and multivariate survival analyses were performed using Cox proportional hazards models. Kaplan-Meier method and corresponding log rank test were used for survival analyses on categorical variables. Correlations between RDW, NLR, PLR and MLR were performed using Spearman’s rho test. Associations of RDW and NLR with other categorized clinic/pathological parameters were determined using either Mann-Whitney U-tests or Kruskal-Wallis tests followed by post-hoc pairwise Mann-Whitney U-tests.
All statistical tests were two-sided, and P-values < 0.05 were considered significant. Statistical analysis was performed using IBM SPSS package (Statistical Package for the Social Sciences; Version 22, Armonk, NY).
Additional Information
How to cite this article: Li, Z. et al. Preoperative red cell distribution width and neutrophil-to-lymphocyte ratio predict survival in patients with epithelial ovarian cancer. Sci. Rep. 7, 43001; doi: 10.1038/srep43001 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81402146, Z.L.), the Research Fund for the Doctoral Program of Higher Education of China (20125317120002, Z.L.), China Scholarship Council (201408535054), Science and Technology Planning Project of Yunnan Province (2014FB195, Z.L.), the Scientific Research Foundation for Doctors of Yunnan Tumor Hospital (BSJJ201402, Z.L.) and grand from National Institutes of Health (1U01CA180940-01A1, G.J.).
Footnotes
The authors declare no competing financial interests.
Author Contributions Z.L. and N.H. wrote the main manuscript text, N.H. and M.B. accomplished data query, Z.L., N.H. and C.W. analyzed the results, Z.L., C.W. and G.J. prepared Tables 1–4 and Figures 1 and 2. All authors reviewed the manuscript.
References
- Torre L. A. et al. Global cancer statistics, 2012. CA Cancer J Clin 65, 87–108, doi: 10.3322/caac.21262 (2015). [DOI] [PubMed] [Google Scholar]
- Howlader N. et al. SEER Cancer Statistics Review, 1975–2012, based on November 2014 SEER data submission, posted to the SEER web site, http://seer.cancer.gov/csr/1975_2012/ (April 2015).
- Richard R., Barakat A. B., Maurie Markman & Marcus E. Randall. Principles and Practice of Gynecologic Oncology. 6th edition edn, 1118 (Lippincott Williams & Wilkins 2013). [Google Scholar]
- Vaughan S. et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer 11, 719–725, doi: 10.1038/nrc3144 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter W. E. 3rd et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 25, 3621–3627, doi: 10.1200/JCO.2006.10.2517 (2007). [DOI] [PubMed] [Google Scholar]
- Bristow R. E., Tomacruz R. S., Armstrong D. K., Trimble E. L. & Montz F. J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 20, 1248–1259 (2002). [DOI] [PubMed] [Google Scholar]
- du Bois A. et al. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 115, 1234–1244, doi: 10.1002/cncr.24149 (2009). [DOI] [PubMed] [Google Scholar]
- Cress R. D., Chen Y. S., Morris C. R., Petersen M. & Leiserowitz G. S. Characteristics of Long-Term Survivors of Epithelial Ovarian Cancer. Obstet Gynecol 126, 491–497, doi: 10.1097/AOG.0000000000000981 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkelspiel H. E. et al. Long-Term Mortality Among Women with Epithelial Ovarian Cancer. Gynecologic oncology, doi: 10.1016/j.ygyno.2015.06.005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothill R. W. et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res 14, 5198–5208, doi: 10.1158/1078-0432.CCR-08-0196 (2008). [DOI] [PubMed] [Google Scholar]
- Integrated genomic analyses of ovarian carcinoma. Nature 474, 609–615, doi: 10.1038/nature10166 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaak R. G. et al. Prognostically relevant gene signatures of high-grade serous ovarian carcinoma. J Clin Invest 123, 517–525, doi: 10.1172/JCI65833 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron L. et al. Comparative meta-analysis of prognostic gene signatures for late-stage ovarian cancer. J Natl Cancer Inst 106, doi: 10.1093/jnci/dju049 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zhang L., Liu W. X. & Liu X. Y. Prognostic significance of preoperative anemia, leukocytosis and thrombocytosis in chinese women with epithelial ovarian cancer. Asian Pac J Cancer Prev 16, 933–939 (2015). [DOI] [PubMed] [Google Scholar]
- Obermair A. et al. Significance of pretreatment serum hemoglobin and survival in epithelial ovarian cancer. Oncol Rep 7, 639–644 (2000). [DOI] [PubMed] [Google Scholar]
- Munstedt K., Kovacic M., Zygmunt M. & Von Georgi R. Impact of hemoglobin levels before and during chemotherapy on survival of patients with ovarian cancer. Int J Oncol 23, 837–843 (2003). [PubMed] [Google Scholar]
- Bishara S. et al. Pre-treatment white blood cell subtypes as prognostic indicators in ovarian cancer. Eur J Obstet Gynecol Reprod Biol 138, 71–75, doi: 10.1016/j.ejogrb.2007.05.012 (2008). [DOI] [PubMed] [Google Scholar]
- Coussens L. M. & Werb Z. Inflammation and cancer. Nature 420, 860–867, doi: 10.1038/nature01322 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Allavena P., Sica A. & Balkwill F. Cancer-related inflammation. Nature 454, 436–444, doi: 10.1038/nature07205 (2008). [DOI] [PubMed] [Google Scholar]
- Templeton A. J. et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 106, dju124, doi: 10.1093/jnci/dju124 (2014). [DOI] [PubMed] [Google Scholar]
- Templeton A. J. et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 23, 1204–1212, doi: 10.1158/1055-9965.EPI-14-0146 (2014). [DOI] [PubMed] [Google Scholar]
- Zhou X. et al. Prognostic value of PLR in various cancers: a meta-analysis. PLoS One 9, e101119, doi: 10.1371/journal.pone.0101119 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. et al. A large cohort study reveals the association of elevated peripheral blood lymphocyte-to-monocyte ratio with favorable prognosis in nasopharyngeal carcinoma. PLoS One 8, e83069, doi: 10.1371/journal.pone.0083069 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H. et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother 58, 15–23, doi: 10.1007/s00262-008-0516-3 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. A. et al. Prognostic significance and predictors of the neutrophil-to-lymphocyte ratio in ovarian cancer. Gynecol Oncol 132, 542–550, doi: 10.1016/j.ygyno.2014.01.026 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raungkaewmanee S., Tangjitgamol S., Manusirivithaya S., Srijaipracharoen S. & Thavaramara T. Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer. J Gynecol Oncol 23, 265–273, doi: 10.3802/jgo.2012.23.4.265 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. W., Liu K. J., Hu G. L. & Liang W. J. Preoperative platelet/lymphocyte ratio is a superior prognostic factor compared to other systemic inflammatory response markers in ovarian cancer patients. Tumour Biol, doi: 10.1007/s13277-015-3533-9 (2015). [DOI] [PubMed] [Google Scholar]
- Riedl J. et al. Red cell distribution width and other red blood cell parameters in patients with cancer: association with risk of venous thromboembolism and mortality. PLoS One 9, e111440, doi: 10.1371/journal.pone.0111440 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D. et al. A monocyte/granulocyte to lymphocyte ratio predicts survival in patients with hepatocellular carcinoma. Sci Rep 5, 15263, doi: 10.1038/srep15263 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowtell D. D. et al. Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer 15, 668–679, doi: 10.1038/nrc4019 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher V., Lee J., Innamaa A. & Bali A. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin Transl Oncol 13, 499–503, doi: 10.1007/s12094-011-0687-9 (2011). [DOI] [PubMed] [Google Scholar]
- Wang Y. et al. Preoperative neutrophil-to-lymphocyte ratio predicts response to first-line platinum-based chemotherapy and prognosis in serous ovarian cancer. Cancer Chemother Pharmacol 75, 255–262, doi: 10.1007/s00280-014-2622-6 (2015). [DOI] [PubMed] [Google Scholar]
- Budczies J. et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One 7, e51862, doi: 10.1371/journal.pone.0051862 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings M. et al. Preoperative neutrophil:lymphocyte and platelet:lymphocyte ratios predict endometrial cancer survival. Br J Cancer 113, 311–320, doi: 10.1038/bjc.2015.200 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. & Weinberg R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674, doi: 10.1016/j.cell.2011.02.013 (2011). [DOI] [PubMed] [Google Scholar]
- Lin H. W. et al. Risk of ovarian cancer in women with pelvic inflammatory disease: a population-based study. Lancet Oncol 12, 900–904, doi: 10.1016/s1470-2045(11)70165-6 (2011). [DOI] [PubMed] [Google Scholar]
- Landen C. N. Jr., Birrer M. J. & Sood A. K. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol 26, 995–1005, doi: 10.1200/JCO.2006.07.9970 (2008). [DOI] [PubMed] [Google Scholar]
- Shen M. et al. Tumor-associated neutrophils as a new prognostic factor in cancer: a systematic review and meta-analysis. PLoS One 9, e98259, doi: 10.1371/journal.pone.0098259 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola A., Sarukhan A., Bronte V. & Molon B. The pros and cons of chemokines in tumor immunology. Trends Immunol 33, 496–504, doi: 10.1016/j.it.2012.05.007 (2012). [DOI] [PubMed] [Google Scholar]
- Raccosta L. et al. The oxysterol-CXCR2 axis plays a key role in the recruitment of tumor-promoting neutrophils. J Exp Med 210, 1711–1728, doi: 10.1084/jem.20130440 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D. R. & Huttenlocher A. Neutrophils in the Tumor Microenvironment. Trends Immunol, doi: 10.1016/j.it.2015.11.008 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley D. A. & Kristensen V. Predicting prognosis and therapeutic response from interactions between lymphocytes and tumor cells. Mol Oncol 9, 2054–2062, doi: 10.1016/j.molonc.2015.10.003 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang W. T., Adams S. F., Tahirovic E., Hagemann I. S. & Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol 124, 192–198, doi: 10.1016/j.ygyno.2011.09.039 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. Y. et al. Cytokine profile and prognostic significance of high neutrophil-lymphocyte ratio in colorectal cancer. Br J Cancer 112, 1088–1097, doi: 10.1038/bjc.2015.61 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine J. K. et al. Systemic neutrophil-to-lymphocyte ratio in colorectal cancer: the relationship to patient survival, tumour biology and local lymphocytic response to tumour. Br J Cancer 113, 204–211, doi: 10.1038/bjc.2015.87 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokcu A. et al. May the platelet to lymphocyte ratio be a prognostic factor for epithelial ovarian cancer? Asian Pac J Cancer Prev 15, 9781–9784 (2014). [DOI] [PubMed] [Google Scholar]
- Franco R. S. The measurement and importance of red cell survival. Am J Hematol 84, 109–114, doi: 10.1002/ajh.21298 (2009). [DOI] [PubMed] [Google Scholar]
- Evans T. C. & Jehle D. The red blood cell distribution width. J Emerg Med 9 Suppl 1, 71–74 (1991). [DOI] [PubMed] [Google Scholar]
- Montagnana M., Cervellin G., Meschi T. & Lippi G. The role of red blood cell distribution width in cardiovascular and thrombotic disorders. Clin Chem Lab Med 50, 635–641, doi: 10.1515/cclm.2011.831 (2012). [DOI] [PubMed] [Google Scholar]
- Seretis C., Seretis F., Lagoudianakis E., Gemenetzis G. & Salemis N. S. Is red cell distribution width a novel biomarker of breast cancer activity? Data from a pilot study. J Clin Med Res 5, 121–126, doi: 10.4021/jocmr1214w (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. et al. Prognostic value of preoperative inflammatory markers in Chinese patients with breast cancer. Onco Targets Ther 7, 1743–1752, doi: 10.2147/OTT.S69657 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. et al. Racial disparity in breast cancer survival: the impact of pre-treatment hematologic variables. Cancer Causes Control 26, 45–56, doi: 10.1007/s10552-014-0481-4 (2015). [DOI] [PubMed] [Google Scholar]
- Koma Y. et al. Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer. PLoS One 8, e80240, doi: 10.1371/journal.pone.0080240 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwick R. et al. Preoperative red cell distribution width in patients undergoing pulmonary resections for non-small-cell lung cancer. Eur J Cardiothorac Surg 45, 108–113, doi: 10.1093/ejcts/ezt275 (2014). [DOI] [PubMed] [Google Scholar]
- Xie D. et al. Nomograms Predict Overall Survival for Patients with Small-Cell Lung Cancer Incorporating Pretreatment Peripheral Blood Markers. J Thorac Oncol 10, 1213–1220, doi: 10.1097/JTO.0000000000000585 (2015). [DOI] [PubMed] [Google Scholar]
- Albayrak S. et al. Red cell distribution width as a predictor of prostate cancer progression. Asian Pac J Cancer Prev 15, 7781–7784 (2014). [DOI] [PubMed] [Google Scholar]
- Kemal Y. et al. The value of red blood cell distribution width in endometrial cancer. Clin Chem Lab Med 53, 823–827, doi: 10.1515/cclm-2014-0699 (2015). [DOI] [PubMed] [Google Scholar]
- Cheng Y. C. et al. The Prognostic Significance of Inflammation-Associated Blood Cell Markers in Patients with Upper Tract Urothelial Carcinoma. Ann Surg Oncol, doi: 10.1245/s10434-015-4781-z (2015). [DOI] [PubMed] [Google Scholar]
- Murphy S. et al. Instrumenting the health care enterprise for discovery research in the genomic era. Genome Res 19, 1675–1681, doi: 10.1101/gr.094615.109 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.