Abstract
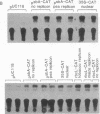
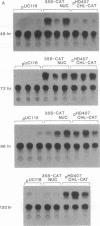
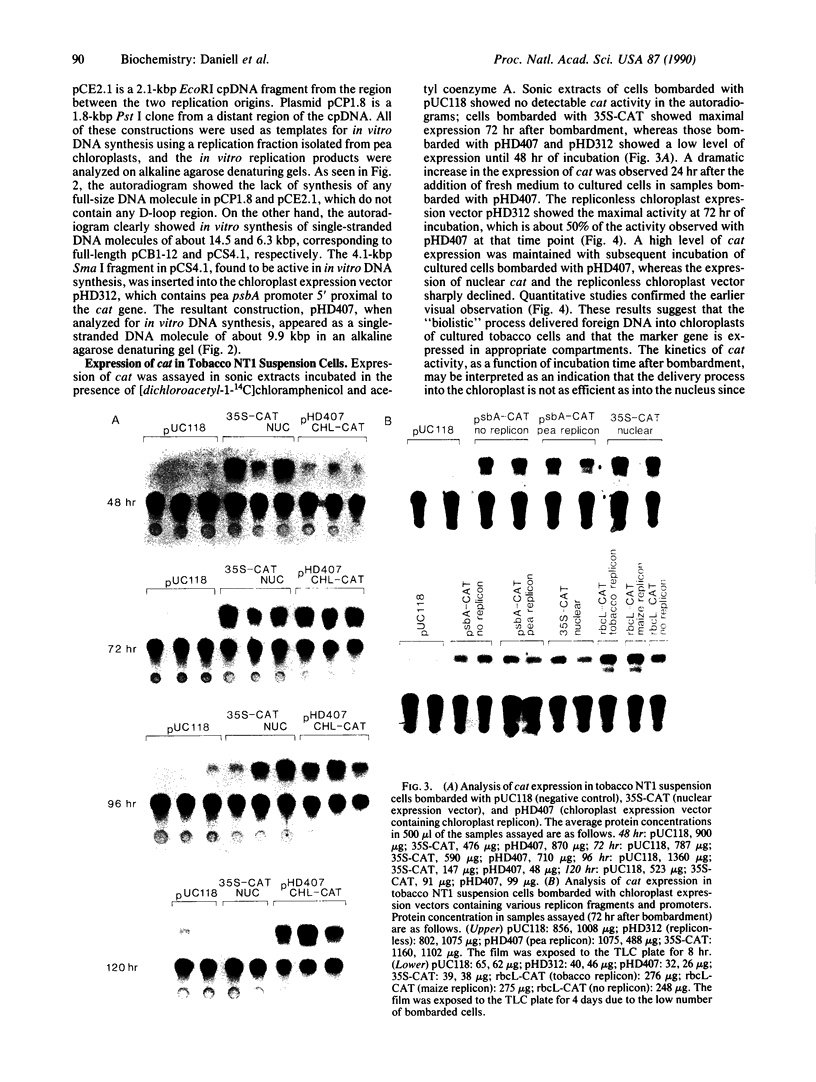
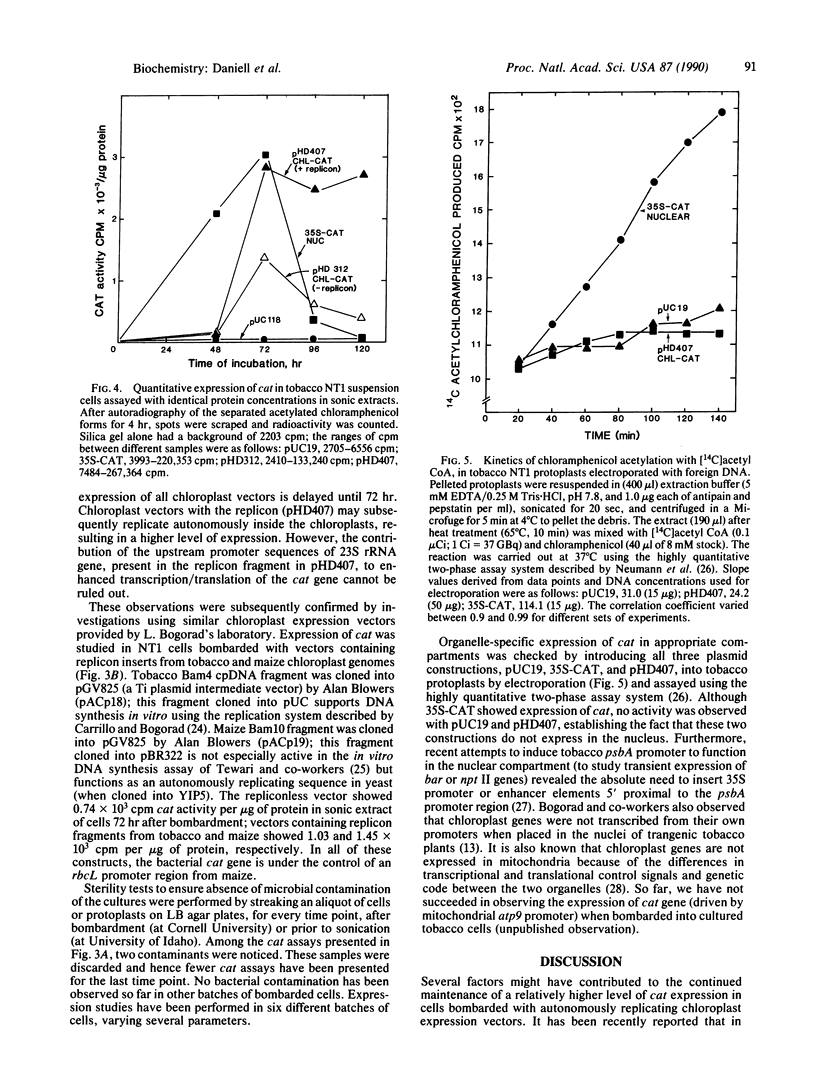
Expression of chloramphenicol acetyltransferase (cat) by suitable vectors in chloroplasts of cultured tobacco cells, delivered by high-velocity microprojectiles, is reported here. Several chloroplast expression vectors containing bacterial cat genes, placed under the control of either psbA promoter region from pea (pHD series) or rbcL promoter region from maize (pAC series) have been used in this study. In addition, chloroplast expression vectors containing replicon fragments from pea, tobacco, or maize chloroplast DNA have also been tested for efficiency and duration of cat expression in chloroplasts of tobacco cells. Cultured NT1 tobacco cells collected on filter papers were bombarded with tungsten particles coated with pUC118 (negative control), 35S-CAT (nuclear expression vector), pHD312 (repliconless chloroplast expression vector), and pHD407, pACp18, and pACp19 (chloroplast expression vectors with replicon). Sonic extracts of cells bombarded with pUC118 showed no detectable cat activity in the autoradiograms. Nuclear expression of cat reached two-thirds of the maximal 48 hr after bombardment and the maximal at 72 hr. Cells bombarded with chloroplast expression vectors showed a low level of expression until 48 hr of incubation. A dramatic increase in the expression of cat was observed 24 hr after the addition of fresh medium to cultured cells in samples bombarded with pHD407; the repliconless vector pHD312 showed about 50% of this maximal activity. The expression of nuclear cat and the repliconless chloroplast vector decreased after 72 hr, but a high level of chloroplast cat expression was maintained in cells bombarded with pHD407. Organelle-specific expression of cat in appropriate compartments was checked by introducing various plasmid constructions into tobacco protoplasts by electroporation. Although the nuclear expression vector 35S-CAT showed expression of cat, no activity was observed with any chloroplast vectors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendich A. J. Why do chloroplasts and mitochondria contain so many copies of their genome? Bioessays. 1987 Jun;6(6):279–282. doi: 10.1002/bies.950060608. [DOI] [PubMed] [Google Scholar]
- Blowers A. D., Bogorad L., Shark K. B., Sanford J. C. Studies on Chlamydomonas chloroplast transformation: foreign DNA can be stably maintained in the chromosome. Plant Cell. 1989 Jan;1(1):123–132. doi: 10.1105/tpc.1.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutry M., Nagy F., Poulsen C., Aoyagi K., Chua N. H. Targeting of bacterial chloramphenicol acetyltransferase to mitochondria in transgenic plants. Nature. 1987 Jul 23;328(6128):340–342. doi: 10.1038/328340a0. [DOI] [PubMed] [Google Scholar]
- Boynton J. E., Gillham N. W., Harris E. H., Hosler J. P., Johnson A. M., Jones A. R., Randolph-Anderson B. L., Robertson D., Klein T. M., Shark K. B. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science. 1988 Jun 10;240(4858):1534–1538. doi: 10.1126/science.2897716. [DOI] [PubMed] [Google Scholar]
- Carrillo N., Bogorad L. Chloroplast DNA replication in vitro: site-specific initiation from preferred templates. Nucleic Acids Res. 1988 Jun 24;16(12):5603–5620. doi: 10.1093/nar/16.12.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. Y., Bogorad L., Van Montagu M., Schell J. Relocating a gene for herbicide tolerance: A chloroplast gene is converted into a nuclear gene. Proc Natl Acad Sci U S A. 1988 Jan;85(2):391–395. doi: 10.1073/pnas.85.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K., Werner-Washburne M., Lubben T. H., Keegstra K. Precursors to two nuclear-encoded chloroplast proteins bind to the outer envelope membrane before being imported into chloroplasts. J Biol Chem. 1985 Mar 25;260(6):3691–3696. [PubMed] [Google Scholar]
- Coen D. M., Bedbrook J. R., Bogorad L., Rich A. Maize chloroplast DNA fragment encoding the large subunit of ribulosebisphosphate carboxylase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5487–5491. doi: 10.1073/pnas.74.12.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen M., Vandewiele M. Nuclear transcriptional activity of the tobacco plastid psbA promoter. Nucleic Acids Res. 1989 Jan 11;17(1):19–29. doi: 10.1093/nar/17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi G., Broglie R., Edwards C., Chua N. H. Tissue-specific and light-regulated expression of a pea nuclear gene encoding the small subunit of ribulose-1,5-bisphosphate carboxylase. EMBO J. 1984 Aug;3(8):1671–1679. doi: 10.1002/j.1460-2075.1984.tb02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H., McFadden B. A. Uptake and expression of bacterial and cyanobacterial genes by isolated cucumber etioplasts. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6349–6353. doi: 10.1073/pnas.84.18.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm M., Taylor L. P., Walbot V. Expression of genes transferred into monocot and dicot plant cells by electroporation. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5824–5828. doi: 10.1073/pnas.82.17.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor J. J., Still C. C. Subcellular localization of rice leaf aryl acylamidase activity. Plant Physiol. 1983 May;72(1):80–85. doi: 10.1104/pp.72.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold B., Carrillo N., Tewari K. K., Bogorad L. Nucleotide sequence of a preferred maize chloroplast genome template for in vitro DNA synthesis. Proc Natl Acad Sci U S A. 1987 Jan;84(1):194–198. doi: 10.1073/pnas.84.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
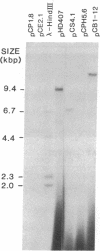
- Meeker R., Nielsen B., Tewari K. K. Localization of replication origins in pea chloroplast DNA. Mol Cell Biol. 1988 Mar;8(3):1216–1223. doi: 10.1128/mcb.8.3.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C., Soll J. Protein transport in intact, purified pea etioplasts. Arch Biochem Biophys. 1986 May 15;247(1):211–220. doi: 10.1016/0003-9861(86)90550-3. [DOI] [PubMed] [Google Scholar]
- Shah D. M., Horsch R. B., Klee H. J., Kishore G. M., Winter J. A., Tumer N. E., Hironaka C. M., Sanders P. R., Gasser C. S., Aykent S., Siegel N. R., Rogers S. G., Fraley R. T. Engineering herbicide tolerance in transgenic plants. Science. 1986 Jul 25;233(4762):478–481. doi: 10.1126/science.233.4762.478. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]