Abstract
The present study measured postnatal ultrasonic vocalization (USV) and gene expression to examine potential changes in communication and/or attachment in the offspring of mothers exposed to morphine during adolescence. Offspring of morphine-exposed (Mor-F1), saline-exposed (Sal-F1), or non-handled control (Con-F1) female Sprague–Dawley rats were tested for separation-induced distress calls and maternal potentiation of distress calls during early postnatal development. We also examined relative expression of dopamine D2 receptor and mu opioid receptor (oprm1) mRNA in the nucleus accumbens and hypothalamus in these offspring, as their activity has been implicated in the regulation of postnatal USV in response to maternal separation. The findings indicate that adolescent experiences of future mothers, including their 10 daily saline or morphine injections, can result in significant region-specific differences in gene expression. In addition, these experiences resulted in fewer numbers of separation-induced distress calls produced by offspring. In contrast, augmented maternal potentiation was only observed in Mor-F1 offspring.
Keywords: rat, attachment, ultrasonic vocalization, opioids, morphine, OPRM1, dopamine D2 receptor, postnatal development
INTRODUCTION
Opioids play an important role in the reproductive axis, including regulating the onset of puberty (Sizonenko, 1987, 1989). Previous experiments have shown that adolescent opiate exposure in female rats delays sexual maturation and induces long-lasting alterations in adult gene expression within the endogenous opioid system (Byrnes, 2008). In addition, significant differences in sensitivity to opiates are observed in the offspring of these adolescent exposed females, suggesting that maternal opiate exposure prior to pregnancy can influence the development of future offspring (Byrnes, 2005; Pickens & Svikis, 1991). The underlying mechanisms involved in the transmission of these offspring effects are unknown but could involve modifications in both the pre and postnatal environment (Nelson & Panksepp, 1998). Indeed, numerous studies have indicated that changes in the postnatal environment can have long-lasting effects on offspring development (Chen, Simar, & Morris, 2009; Clark et al., 2007; Coutellier, Friedrich, Failing, & Würbel, 2008; Crews, Fuller, Mirasol, Pfaff, & Ogawa, 2004; Gorski, Dunn-Meynell, Hartman, & Levin, 2006; Koo et al., 2003).
Previous findings suggest that females exposed to morphine during adolescence display subtle modifications in maternal care, particularly during the dark phase of the light cycle (Johnson, Carini, Schenk, Stewart, & Byrnes, 2011). It is unclear whether these effects on maternal care were due to changes in the dam or were induced by differences in the offspring themselves. It is possible that maternal opiate exposure could influence other aspects of maternal–offspring interactions, including effects in the offspring that may subsequently alter the quality, if not the quantity, of maternal care.
Rat pups use ultrasonic vocalizations (USV) to guide retrieval by the dam during periods of isolation (Brunelli, Shair, & Hofer, 1994) and to elicit maternal behaviors (Hashimoto, Saito, Furudate, & Takahashi, 2001), with dams able to modulate the duration and frequency of these separation-induced distress calls. For example, a pup will initiate USV when separated from the dam, with distress calls subsequently reduced when the two are reunited. If, however, within a short period of time the pup is separated from the mother again, the number of ultrasonic distress calls significantly increases above the level observed during the initial separation. This phenomenon is termed maternal potentiation (Ehret, 2005; Hofer, Brunelli, & Shair, 1994; Kraebel, Brasser, Campbell, Spear, & Spear, 2002; Shair, Brunelli, Masmela, Boone, & Hofer, 2003) and typically develops during the first 2 weeks post-partum (Hofer, Brunelli, Masmela, & Shair, 1996; Hofer, Masmela, Brunelli, & Shair, 1998). It has been suggested that separation-induced distress calls and maternal potentiation may be useful measures of pup-dam communication and attachment, respectively (D’Amato, Scalera, Sarli, & Moles, 2005).
A number of studies have implicated endogenous opioids in the display of separation-induced distress calls and these effects appear to be opiate-receptor specific (Barr, Wang, & Carden, 1994; Carden, Davachi, & Hofer, 1994; Moles, Kieffer, & D’Amato, 2004). Currently, it remains unclear to what extent specific opioid receptor subtypes are involved in the regulation of maternal potentiation (Shair, Brunelli, & Hofer, 2005). Several studies, however, indicate that activity at the dopamine D2 receptor subtype can modulate maternal potentiation (Muller, Brunelli, Moore, Myers, & Shair, 2005; Muller, Moore, Myers, & Shair, 2009) and these effects appear to be localized within the ventral striatum (Muller, Moore, Myers, & Shair, 2008). Offspring of dams exposed to morphine during puberty demonstrate significant modifications in the functional activation and transcription of both the mu opioid receptor (OPRM1) and the dopamine D2 receptor (Byrnes, 2005; Byrnes, Babb, Scanlan, & Byrnes, 2011; Byrnes, Johnson, Carini, & Byrnes, 2013; Vassoler, Johnson-Collins, Carini, & Byrnes, 2014). When these effects develop or to what extent they are modified by the postnatal environment remains to be determined. It is possible that separation-induced distress calls and/or maternal potentiation could be modified in these offspring, which could impact the quality of pup–dam interactions. As changes in the maternal environment can have long-lasting effects on subsequent neurodevelopment, differences in USV could play a role in behavioral alterations observed in adulthood.
The purpose of this study was to measure separation-induced USV (i.e., distress calls) and maternal potentiation of USV during early development to determine whether the offspring of females exposed to morphine during adolescence display altered call rates in response to maternal separation. Additionally, quantitative PCR was conducted to examine potential modifications in both OPRM1 and dopamine (DA) D2 receptor gene expression in both the nucleus accumbens and mediobasal hypothalamus at postnatal Day (PND) 12. A non-handled control group was also included to parse potential differences associated with morphine exposure and those that may be associated with daily handling/injections during adolescent development.
METHODS
Subjects
Thirty-two female Sprague–Dawley rats were purchased from Charles River Laboratories (Kingston, NY). Beginning at PND30, 12 subjects began treatment with morphine sulfate (Butler Company, Columbus, OH) using an increasing dose regimen for a total of 10 days. On Day 1 of morphine treatment, rats (Mor) received 5 mg/kg subcutaneously (sc) once per day. Every other day the dose of morphine was increased by 5 mg/kg such that by the final day of treatment (PND39) subjects received a 25 mg/kg injection. Other females (Sal, n = 12) served as age-matched controls and received saline injections (sc) once a day with volumes adjusted to match those of the drug-treated females or were left undisturbed to serve as age-matched, non-handled controls (Con, n = 8). Injected females were weighed daily. All subjects then remained undisturbed in their home cage until reaching 60 days of age.
On or after 60 days of age, Mor, Sal, and Con females were housed with males from our colony. Pregnancy was determined by presence of sperm in the vaginal lavage (gestation Day 1). All pregnant females were then individually housed. On PND1 (parturition = PND0), dams were weighed and all litters were culled to a minimum of four males and four females and a maximum of five males and five females. Only one male and one female per litter were used on any dependent measure to minimize potential litter effects.
Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, NRC Publication, 2011 edition. The Cummings School of Veterinary Medicine at Tufts University is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
Ultrasonic Vocalizations
Thirty-two female rats were exposed to either morphine (n = 12), saline (n = 12), or served as nonhandled controls (n = 8) during adolescent development and were mated as detailed above. USV were measured on postnatal days 3, 6, 9, and 12. On the testing day, individual pups were removed from the home cage and placed in a novel rectangular polypropylene container (11 × 13 × 2 cm3). The container was placed inside a sound proof box maintained at room temperature. A heterodyne bat detector (Mini-3; Ultra Sound Advice, London, United Kingdom) was located 10 cm above the pup. Rat pups emit vocalizations in the 30–50 kHz range. The detector was connected to a PC with data recorded on Soundwave software (Softonic International, Barcelona, Spain). USV were measured for 5 min and the number of calls was analyzed. Recording began 20 s after the pup was placed in the container to avoid the effects of handling. Following the initial test, pups were marked using a low-odor marker on their dorsal aspect and reunited with the dam for 5 min. After this brief reunion, pups were once again removed and tested as described above. Maternal behaviors were not quantified during the reunion period; however, all pups were confirmed to have been immediately retrieved by the dam upon return to the home-cage. All tested pups were earmarked after the completion of testing to prevent retesting of the same pup at older ages. Bodyweights for tested pups were recorded on each test day. Difference scores between the number of calls after the second separation minus those after the first separation were used as a measure of maternal potentiation. Dam body weights on PND1 were analyzed using a one-way ANOVA. Behavioral data and pup body weights were analyzed using a three-way ANOVA with sex, postnatal day, and maternal condition as factors. Post hoc analyses were performed using the Tukey’s test or a one-sample t-test (for potentiation data) with significance defined as p < .05.
Brain Dissection and Tissue Preparation
PND12 brains were harvested from each litter. None of the animals used for the gene expression studies were tested in the USV paradigm. Animals were decapitated, and brains were rapidly removed, frozen in methylbutane, and stored at −80°C. To collect samples for analysis of gene expression, frozen brains were mounted on a cryostat and bilateral micropunches were taken from the nucleus accumbens (NAc) (1 mm; starting at: +2.5 mm A/P, ±1.5 mm M/L, −7 mm D/V, a region encompassing both core and shell) and hypothalamus (1 mm; starting at: −1.4 mm A/P, ±0.5 mm M/L, −8 mm D/V) (Paxinos & Watson, 1997). Tissue punches were then homogenized in lysis buffer and total RNA extracted using the RNeasy kit from Qiagen (Valencia, CA) according to the manufacturer’s protocol. Complementary DNA (cDNA) was synthesized using the RETROscript kit from Applied Biosystems (Carlsbad, CA).
Real Time Quantitative Polymerase Chain Reaction (qPCR)
All qPCR was performed using an AB 7500 (Applied Biosystems) under standard amplification conditions: 2 min at 50°C, 10 min at 95°C, 40 cycles of 15 s at 95°C, and 60 s at 60°C. All qPCR primers were TaqMan® Gene Expression Assays purchased from Applied Biosystems. The amplification efficiency of each of these assays has been validated by Applied Biosystems and averages 100% (±10). Assay ID and accession numbers were as follows: Gapdh: Rn01775763_g1, oprm1: Rn00565144_m1, D2: Rn01418275_m1.
Quantification of Gene Transcription
Final quantification of mRNA was obtained using the comparative cycle threshold (CT) method (User Bulletin #2, Applied Biosystems). Data are reported as relative transcription or the N-fold difference relative to a calibrator cDNA. In brief, the housekeeping gene for the rat brain tissue, GapDH, was used as an internal control against which each target signal was normalized; this is referred to as the ΔCT. Validation studies confirmed that the raw CT values of GapDH did not vary by treatment group, confirming GAPDH as an appropriate housekeeping gene. The ΔCT was then normalized against a calibrator (i.e., the mean of the male Con-F1 group for the target gene in each brain region) and data are presented as ΔΔCT. Data were analyzed using a two-way ANOVA with sex and maternal treatment as factors. Significance was set at p < .05.
RESULTS
Dam and Pup Body Weights
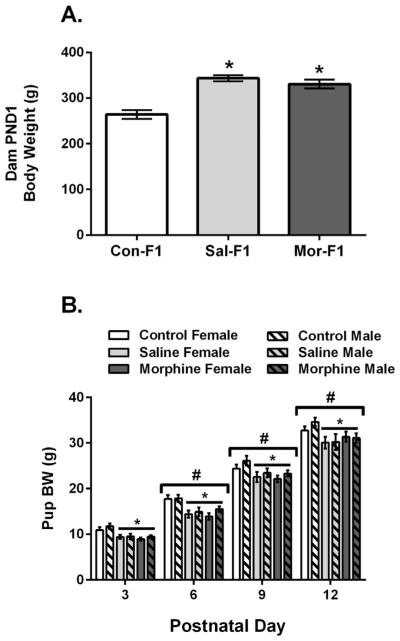
Dam body weights were measured on PND1. A one-way ANOVA revealed a significant main effect of maternal condition (F[2,31] = 19.73, p < .001). These data are shown in Figure 1A. Post hoc analyses indicate that both Sal-F1 and Mor-F1 dams were significantly heavier than Con-F1 dams (both p’s < .001). Pup body weight data are shown in Figure 1B. A three-way ANOVA revealed a significant main effect of pup sex (F[1,251] = 4.41, p < .05) with males heavier than females. There was also a significant main effect of day (F[3,251] = 650.5, p < .001) with body weight significantly increased at each postnatal day (all p’s < .001). Finally, there was a significant main effect of maternal condition (F[2,251] = 19.85, p < .001) with both Sal-F1 and Mor-F1 offspring weighing less than Con-F1 offspring (both p’s < .001) but not different from each other (p = .94).
FIGURE 1.
Mean (±SEM) Dam (A) and pup (B) body weights in grams. (A) There was a significant main effect of dam body weight as measured on PND1 with both Sal dams (n = 12) and Mor dams (n = 12) significantly heavier than Con dams (n = 8) (* p < .001). (B) Con-F1 (n = 14–16/day), Sal-F1 (n = 14–18/day), and Mor-F1 (n = 14–18/day) pups demonstrated the expected increase in body weight over the postnatal days examined (#p < .01 as compared to all other days). There was also a significant main effect of maternal condition with both Sal-F1 and Mor-F1 offspring weighing less than Con-F1 offspring at all postnatal days (*p < .001).
Ultrasonic Vocalizations
The number of distress-calls elicited following the initial separation from the dam/litter are shown in Figure 2. A three-way ANOVA revealed no main effect of sex (F1,251 = .97, p = .33) and no sex by day, sex by maternal condition or sex by day by maternal condition interaction (all p’s > .9). Therefore these data are presented collapsed across sex. A significant main effect of day was observed (F3,251 = 15.4, p < .001) with the number of calls increasing across the postnatal period. There was also a main effect of maternal condition (F2,251 = 16.5, p < .01) which was due to decreased call numbers in both Sal-F1 and Mor-F1 when compared to Con-F1 (both p’s < .01). No significant group by day interaction was observed (F6,251 = 1.4, p = .24).
FIGURE 2.
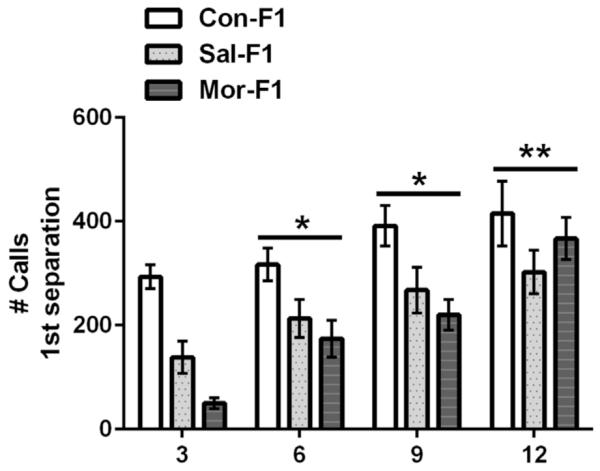
Mean (±SEM) number of distress calls following an initial separation from the dam/litter in Con-F1 (n = 14–16/day), Sal-F1 (n = 14–18/day), and Mor-F1 (n = 14–18/day) animals at PND3, 6, 9, and 12. One male and one female per litter were examined on each postnatal day and no animals were tested twice (data are collapsed across sex). The number of calls increased across postnatal days; *p < .05 as compared to PND3 and PND12, **p < .05 when compared to all other days. There was a main effect of maternal condition with both Sal-F1 and Mor-F1 producing fewer calls (p < .001).
Maternal potentiation data are presented in Figure 3. A three-way ANOVA again revealed no significant main effect of sex (F1,251 = 1.44, p = .23) and no sex by day, sex by maternal condition or sex by day by maternal condition interaction (all p’s > .07). Therefore, these data are also presented collapsed across sex in Figure 3. There was a significant main effect of day (F3,251 = 3.3, p < .05) which was due to an increase in maternal potentiation on PND9 when compared PND3 (p’s < .01). There was also a significant main effect of maternal condition (F2,251 = 6.14, p < .01) which was due to significantly increased maternal potentiation in Mor-F1 offspring when compared to Sal-F1 offspring (p < .01) but not Con-F1 (p = .6). No significant difference between Sal-F1 and Con-F1 were observed (p = .08). There was no significant day by maternal condition interaction (F6,251 = .72, p = .64). While there was no significant interaction, to determine when during postnatal development significant levels of maternal potentiation could be discerned in these three groups, we conducted one sample t-tests for each group at all of the postnatal days examined. All p-values were then corrected using Bonferroni correction for multiple comparisons (.05/12—p set to < .004). The Mor-F1 group displayed significant maternal potentiation with significant effects observed on PND9 (t[25] = 5.45, p < .001) and PND12 (t[22] = 5.43, p < .001). Con-F1 animals had significant maternal potentiation compared to Sal-F1 animals at PND12 (t[15] = 2.34, p = .03).
FIGURE 3.
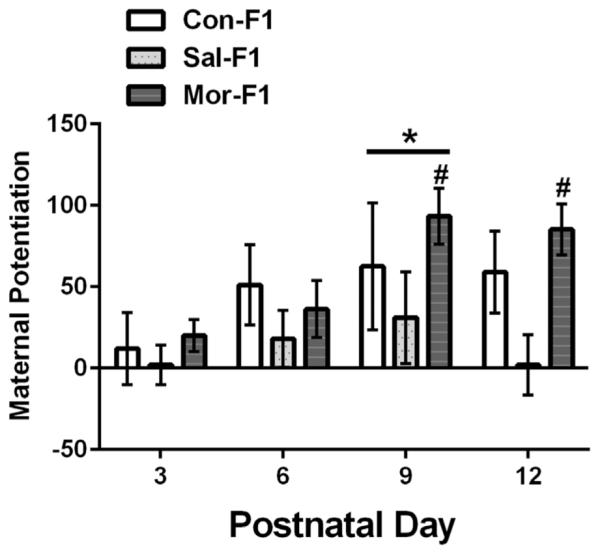
Mean (±SEM) maternal potentiation score (i.e., the number of USVs during second separation—number of USVs during first separation) for Con-F1 (n = 14–16), Sal-F1 (n = 14–18), and Mor-F1 (n = 14–18) animals at PND3, 6, 9, and 12. One male and one female per litter were examined on each postnatal day and no animals were tested twice (data are collapsed across sex). When compared across days, significantly higher levels of maternal potentiation were observed on PND9 (* p < .01 as compared to PND3 and PND6). There was a main effect of maternal condition with Mor-F1 demonstrating higher levels of maternal potentiation when compared to Sal-F1 across all postnatal (p < .01). Significant levels of maternal potentiation (i.e., a significant increase in the number of calls following a second separation) were only displayed by Mor-F1 animals on PND9 and PND12 (#p < .001).
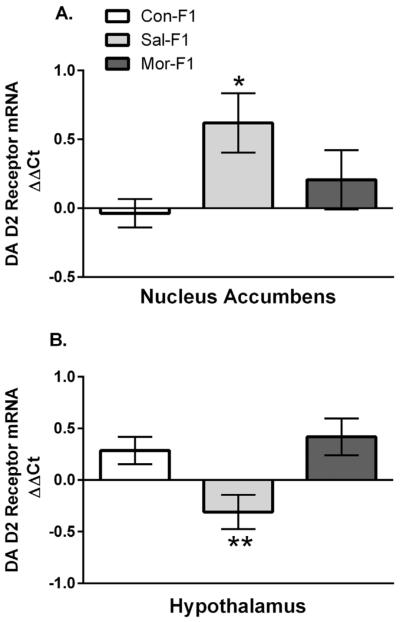
D2 Receptor mRNA Expression
Dopamine receptor D2 mRNA expression was examined using qPCR in naïve (i.e., untested) littermates sacrificed on PND12 (see Fig. 4). Two-way ANOVAs of expression data from the nucleus accumbens and hypothalamus revealed no significant main effect of sex (F1,47 = 1.55; p = .22 and F1,49 = .95, p = .14, respectively) and no sex by maternal condition interaction (F1,47 = 1.4; p = .27 and F1,49 = 1.0, p = .38, respectively); thus data are presented collapsed across sex. There was a significant main effect of maternal condition in both brain regions (F2,47 = 3.88; p = .03 and F2,49 = 6.3; p < .01, respectively). As shown in Figure 4A, in the nucleus accumbens, Sal-F1 offspring have significantly increased expression when compared to controls (p < .05) but not when compared to Mor-F1 offspring (p = .026). No differences were observed between Con-F1 and Mor-F1 (p = .59). In contrast, in the hypothalamus (Fig. 4B) Sal-F1 offspring having decreased D2 receptor expression as compared to both Con-F1 and Mor-F1 offspring (p < .05 and p < .01, respectively). Again, no differences between Con-F1 and Mor-F1 were observed (p = .83).
FIGURE 4.
Mean (±SEM) relative expression (ΔΔCT) of the Dopamine (DA) D2 receptor gene as measured in tissue punches from the nucleus accumbens (A) or hypothalamus (B) of PND12 Con-F1 (n = 16), Sal-F1 (n = 17), and Mor-F1 (16) animals. (A) In the nucleus accumbens, there was a significant main effect of maternal condition with Sal-F1 having significantly increased expression when compared to Con-F1 animals (* p < .05). (B) In the hypothalamus, Sal-F1 animals have decreased DA D2 receptor expression when compared to either Con-F1 or Mor-F1 groups (**p < .05).
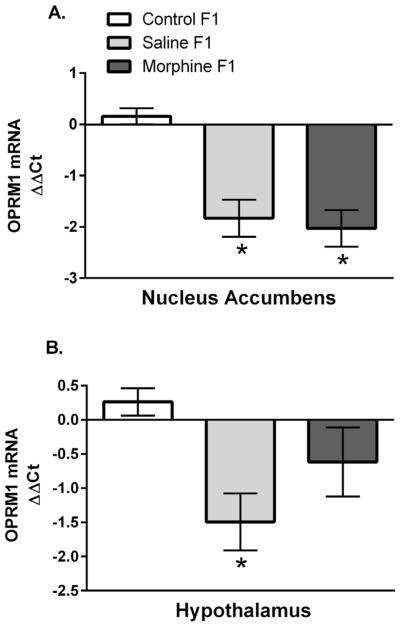
OPRM1 mRNA Expression
OPRM1 expression on PND12 in the nucleus accumbens and hypothalamus are shown in Figure 5. Two-way ANOVAs revealed no significant effect of sex in either region (F1,47 = .09; p = .76 and F1,49 = .11, p = .74, respectively) and no sex by maternal condition interaction (F1,47 = .29; p = .75 and F1,49 = .59, p = .56, respectively); thus data are presented collapsed across sex. In the nucleus accumbens (Fig. 5A), there was a significant main effect of maternal condition (F2,47 = 16.2; p < .001) which was due to significantly decreased OPRM1 expression in both Sal-F1 and Mor-F1 animals when compared to Con-F1 animals (both p’s < .001) with similar expression levels observed between these two groups (p = .9). In the hypothalamus (Fig. 5B), there was also a main effect of maternal condition (F1,49 = 4.65, p < .05). Post hoc analyses indicate that this effect was largely driven by a significant decrease in OPRM1 expression in Sal-F1 animals as compared to Con-F1 animals (p = .01) with no differences observed between Con-F1 and Mor-F1 animals (p = .29) or between Sal-F1 and Mor-F1 animals (p = .28).
FIGURE 5.
Mean (±SEM) relative expression (ΔΔCT) of OPRM1) as measured in tissue punches from the nucleus accumbens (A) or hypothalamus (B) of PND12 Con-F1 (n = 16), Sal-F1 (n = 17), and Mor-F1 (n = 16) animals. (A) There was a main effect of maternal condition due to significantly decreased OPRM1 expression in both Sal-F1 and Mor-F1 animals when compared to Con-F1 animals (*p < .001). (B) There was a significant decrease in OPRM1 expression in the hypothalamus of Sal-F1 animals when compared to Con-F1 animals (*p < .05) in the hypothalamus (panel B).
DISCUSSION
The current findings indicate that adolescent experiences, including daily injections of either saline or morphine, result in a number of measurable effects in subsequent offspring. This includes a significant decrease in body weight and reduced numbers of distress calls elicited by separation from the dam when compared to the offspring of non-handled controls. In addition, significant alterations in the expression of both OPRM1 and DA D2 receptor mRNA were appreciated, with Sal-F1 displaying a greater number of alterations than Mor-F1 when compared to non-handled controls. One effect that was specific to Mor-F1 offspring, however, was a significant increase in maternal potentiation during the second postnatal week. Such findings suggest that in these offspring there may be a shift in mechanisms that regulate attachment or a shift in the developmental trajectory of this response. Overall, these data indicate that manipulations occurring during this particular adolescent period can impact the next generation; however, the nature of these effects may be dependent upon the precise nature of that manipulation.
Several common effects were observed as a function of daily injections during adolescence. While all females were similar ages when mated (~60–65 days of age), when body weights were measured on PND1, Con females weighed significantly less than either Sal or Mor dams. Unfortunately, we did not weigh the females prior to mating as all previous studies with these females failed to discern differences between Sal and Mor females when measured as adults (Johnson et al., 2011). Indeed, on PND1 no differences in body weights were observed between these two groups. Thus, it remains to be determined if Sal and Mor females are heavier than controls prior to mating or whether this increased body weight on PND1 is due to an interaction between their adolescent experiences and pregnancy. For example, the effects of this adolescent manipulation could alter systems that regulate pregnancy-induced hyperphagia or energy metabolism. These findings also suggest that even what some may consider a relatively modest manipulation, that is, a daily injection of saline for a total of 10 days, can have significant effects on subsequent development, including, apparently, increased body weight gain. Interestingly, other reports have noted effects on body weight following different types of housing environments during juvenile and adolescent development, with rats reared in more enriched environment demonstrating reduced body weight when compared to rats reared in more impoverished environments (Zaias, Queeney, Kelley, Zakharova, & Izenwasser, 2008). It is possible that the shift in body weight in the Sal and Mor females is due to the stress of the daily injection. Indeed, studies have shown that adolescent isolation, a known stressor (McCormick, Merrick, Secen, & Helmreich, 2007), leads to hyperphagia and increased body weight (Jahng, Yoo, Ryu, & Lee, 2012). Thus, daily injections during adolescence may have prolonged effects for both the female and her subsequent offspring.
While Con females were lighter than both Sal and Mor dams, their offspring were significantly heavier. Thus, the decreased body weights observed in both Sal-F1 and Mor-F1 pups was not a consequence of reduced body weights or hypophagia during pregnancy. Of note, a recent study reported significant transgenerational effects of mild adolescent stress on a number of parameters, including changes in corticotropin releasing hormone mRNA expression at birth and anxiety-like behavior in adult F1 and F2 offspring (Zaidan & Gaisler-Salomon, 2015). Thus, within the current context, it would appear that the repeated injection of either saline or morphine to adolescent females can result in decreased growth in their future offspring.
In addition to changes in body weight, both Sal-F1 and Mor-F1 offspring emitted fewer separation-induced distress calls. These effects appeared more robust during the earlier postnatal days examined, particularly in Mor-F1. Indeed, post hoc analyses on PND3 observed a significant decrease in separation-induced distress calls in Mor-F1 when compared to Sal-F1 subjects (p < .05). However, these findings would need to be examined more carefully in future studies to determine whether any truly significant differences can be discerned between Sal-F1 and Mor-F1 offspring during this early phase of postnatal development. Nonetheless, both Sal-F1 and Mor-F1 animals displayed a reduction in separation-induced distress calls during the early postnatal development when compared to Con-F1. How such changes in distress calls might alter maternal behavior remains unknown.
While the data reveal increasing separation-induced distress calls across the postnatal days tested, significant maternal potentiation did not emerge until the second postnatal week, with the highest levels observed on PND9. The developmental trajectory observed in the current study is similar to previous findings (Hofer et al., 1998; Shair et al., 2003). Typically, maternal potentiation increases with postnatal age, appearing after approximately 7–9 days in the passive model, or 2 days earlier in the active model (active model is used here) and peaking at approximately PND12 (Hofer et al., 1998; Shair, 2007). However, in the current study, this effect on PND9 was largely due to the significant increase in maternal potentiation displayed by Mor-F1 animals. Indeed, the level of maternal potentiation in both Con-F1 and Sal-F1 animals did not appear to vary across the postnatal days examined. In contrast, Mor-F1 animals demonstrated significant maternal potentiation on PND9 and PND12. Post hoc analyses indicate that Mor-F1 animals show significantly increased maternal potentiation when compared to Sal-F1. Thus, while adolescent injection of either saline or morphine decreases the number of separation-induced distress calls in future offspring when compared to non-handled controls, only adolescent morphine exposure results in increased maternal potentiation.
In this study, we did not measure other aspects of the isolation response, such as increased locomotor activity, self-grooming, and micturition. Given the significant effects on body weight and separation-induced distress calls in Sal-F1, it is possible that the magnitude of the difference observed in Mor-F1 animals may be due in part to blunted maternal potentiation in Sal-F1. Indeed, neither group differs significantly from Con-F1 which appear to fall somewhere in between. Nonetheless, these data suggest that adolescent morphine exposure modulates maternal potentiation in future offspring.
While it is clear that adolescent morphine exposure alters maternal potentiation in subsequent offspring, it is unclear if changes in neurodevelopment underlie these behavioral effects or whether differences in maternal care or maternal motivation play a role in these effects. The isolation-induced vocalizations of a rat pup are modulated both by current social interactions and by the pup’s rearing experience (Shair, 2007). It is known that dopamine plays a role in adult as well as infant social behavior and interactions (Muller et al., 2009). Specifically, it was shown that the D2 receptor agonist, quinpirole, disrupts maternal potentiation (Muller et al., 2005) and that the ventral striatum is a critical region involved with this disruption (Muller et al., 2008). Therefore, the increased D2 receptor mRNA expression observed in the nucleus accumbens on PND12 in Sal-F1 animals may partly explain their lower levels of maternal potentiation when compared to Mor-F1. However, it has also been shown that D2 receptor antagonists as well as D2 receptor knock down in mice reduce pup vocalizations (Curry et al., 2013). This mouse knockout model has a more global reduction in D2 receptors. Sal-F1 animals also have significantly reduced D2 receptor mRNA expression in the hypothalamus on PND12, which could relate to the observed effects on both the number of distress-calls and maternal potentiation. Further studies investigating the expression of region specific D2 receptor protein across postnatal development as well as studies examining the effects of direct infusion of dopamine agonists and antagonists in Sal-F1 animals would help better determine the role of these changes in D2 receptor gene expression and the observed effects on vocalizations. The endogenous opioid system has also been implicated in filial bonding and social reward/ attachment (Goodman, Hans, & Cox, 1999; Nelson & Panksepp, 1998; Schino & Troisi, 1992). Mice with the mu opioid receptor knocked out demonstrate fewer separation-induced distress calls and display deficits in attachment behavior, such as a lack of preference for maternal cues (Moles et al., 2004). However, another study found that the administration of opiate agonists or antagonists do not modulate maternal potentiation response (Shair et al., 2005). Here, we report that both Sal-F1 and Mor-F1 animals have decreased expression of OPRM1 in the nucleus accumbens at PND12 when compared to Con-F1 animals. In addition, Sal-F1 animals also have reduced OPRM1 expression in the hypothalamus, an effect that is not observed in Mor-F1 animals. These alterations in OPRM1 may underlie the differences in separation-induced distress calls, although that remains to be determined. As with the D2 receptor data, additional studies will be need to determine the functional relevance of any observed change in gene expression. It would also be useful to examine the effects of cross-fostering on both vocalization and gene expression to begin to parse the contribution of the dam in these effects.
To what extent the specific effects on maternal potentiation observed in Mor-F1 indicate an alteration in social motivation, attachment, and/or a more general shift in neurodevelopment remains unknown. Moreover, the significant effects observed in Sal-F1 animals when compared to Con-F1 animals demonstrate the potential impact that even moderately stressful experiences during adolescence can have on the organism, including effects that are transferred to future generations. What should be clear from the current findings is the importance of using a carefully matched control group when examining potential transgenerational effects of exogenously administered substances. Thus, when compared to Sal-F1 animals, the most robust transgenerational effect of adolescent morphine exposure was an increase in maternal potentiation. These effects could be related to the differential effects observed with regard to D2 receptor expression, perhaps in concert with the overall effects on OPRM1 observed in both Sal-F1 and Mor-F1 animals. Future studies will be needed to determine the mechanism(s) of transmission, the role of the maternal-pup dyad in mediating some of these effects, as well as the neural modifications that play a critical role in mediating these effects.
Acknowledgments
NOTES
This work was supported by grants from NIH (5T35RR029724 and R01DA25674). Funding source had no involvement in the experimental design or interpretation of the results.
Contract grant sponsor: NIH
Contract grant numbers: 5T35RR029724, R01DA25674
REFERENCES
- Barr GA, Wang S, Carden S. Aversive properties of the kappa opioid agonist U50,488 in the week-old rat pup. Psychopharmacology (Berl) 1994;113(3–4):422–428. doi: 10.1007/BF02245218. [DOI] [PubMed] [Google Scholar]
- Brunelli SA, Shair HN, Hofer MA. Hypothermic vocalizations of rat pups (Rattus norvegicus) elicit and direct maternal search behavior. Journal of Comparative Psychology. 1994;108(3):298–303. doi: 10.1037/0735-7036.108.3.298. [DOI] [PubMed] [Google Scholar]
- Byrnes EM. Transgenerational consequences of adolescent morphine exposure in female rats: Effects on anxiety-like behaviors and morphine sensitization in adult offspring. Psychopharmacology (Berl) 2005;182(4):537–544. doi: 10.1007/s00213-005-0122-4. [DOI] [PubMed] [Google Scholar]
- Byrnes EM. Chronic morphine exposure during puberty induces long-lasting changes in opioid-related mRNA expression in the mediobasal hypothalamus. Brain Research. 2008;1190:186–192. doi: 10.1016/j.brainres.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Byrnes JJ, Babb JA, Scanlan VF, Byrnes EM. Adolescent opioid exposure in female rats: Transgenerational effects on morphine analgesia and anxiety-like behavior in adult offspring. Behavioural Brain Research. 2011;218(1):200–205. doi: 10.1016/j.bbr.2010.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes JJ, Johnson NL, Carini LM, Byrnes EM. Multigenerational effects of adolescent morphine exposure on dopamine D2 receptor function. Psychopharmacology (Berl) 2013;227(2):263–272. doi: 10.1007/s00213-012-2960-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carden SE, Davachi L, Hofer MA. U50,488 increases ultrasonic vocalizations in 3-, 10-, and 18-day-old rat pups in isolation and the home cage. Developmental Psychobiology. 1994;27(1):65–83. doi: 10.1002/dev.420270107. [DOI] [PubMed] [Google Scholar]
- Chen HD, Simar D, Morris MJ. Hypothalamic neuroendocrine circuitry is programmed by maternal obesity: Interaction with postnatal nutritional environment. PLoS ONE. 2009;4(7):e6259. doi: 10.1371/journal.pone.0006259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SM, Makhlouf M, Hankins GD, Anderson GD, Saade GR, Longo M. The effect of the postnatal environment on altered fetal programming of adult vascular function in mice that lack endothelial nitric oxide synthase. American Journal of Obstetrics Gynecology. 2007;196(4):354.e1–354.e7. doi: 10.1016/j.ajog.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutellier L, Friedrich AC, Failing K, Würbel H. Variations in the postnatal maternal environment in mice: Effects on maternal behaviour and behavioural and endocrine responses in the adult offspring. Physiology & Behavior. 2008;93(1–2):395–407. doi: 10.1016/j.physbeh.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Crews D, Fuller T, Mirasol EG, Pfaff DW, Ogawa S. Postnatal environment affects behavior of adult transgenic mice. Experimental Biology and Medicine (Maywood) 2004;229(9):935–939. doi: 10.1177/153537020422900910. [DOI] [PubMed] [Google Scholar]
- Curry T, Egeto P, Wang H, Podnos A, Wasserman D, Yeomans J. Dopamine receptor D2 deficiency reduces mouse pup ultrasonic vocalizations and maternal responsiveness. Genes, Brain, and Behavior. 2013;12(4):397–404. doi: 10.1111/gbb.12037. [DOI] [PubMed] [Google Scholar]
- D’Amato FR, Scalera E, Sarli C, Moles A. Pups call, mothers rush: Does maternal responsiveness affect the amount of ultrasonic vocalizations in mouse pups? Behavior Genetics. 2005;35(1):103–112. doi: 10.1007/s10519-004-0860-9. [DOI] [PubMed] [Google Scholar]
- Ehret G. Infant rodent ultrasounds—A gate to the understanding of sound communication. Behavior Genetics. 2005;35(1):19–29. doi: 10.1007/s10519-004-0853-8. [DOI] [PubMed] [Google Scholar]
- Goodman G, Hans SL, Cox SM. Attachment behavior and its antecedents in offspring born to methadone-maintained women. Journal of Clinical Child Psychology. 1999;28(1):58–69. doi: 10.1207/s15374424jccp2801_5. [DOI] [PubMed] [Google Scholar]
- Gorski JN, Dunn-Meynell AA, Hartman TG, Levin BE. Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistance. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2006;291(3):R768–R778. doi: 10.1152/ajpregu.00138.2006. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Saito TR, Furudate S, Takahashi KW. Prolactin levels and maternal behavior induced by ultrasonic vocalizations of the rat pup. Experimental Animals. 2001;50(4):307–312. doi: 10.1538/expanim.50.307. [DOI] [PubMed] [Google Scholar]
- Hofer MA, Brunelli SA, Shair HN. Potentiation of isolation-induced vocalization by brief exposure of rat pups to maternal cues. Developmental Psychobiology. 1994;27(8):503–517. doi: 10.1002/dev.420270804. [DOI] [PubMed] [Google Scholar]
- Hofer MA, Brunelli SA, Masmela J, Shair HN. Maternal interactions prior to separation potentiate isolation-induced calling in rat pups. Behavioral Neuroscience. 1996;110(5):1158–1167. doi: 10.1037//0735-7044.110.5.1158. [DOI] [PubMed] [Google Scholar]
- Hofer MA, Masmela JR, Brunelli SA, Shair HN. The ontogeny of maternal potentiation of the infant rats’ isolation call. Developmental Psychobiology. 1998;33(3):189–201. doi: 10.1002/(sici)1098-2302(199811)33:3<189::aid-dev1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Jahng JW, Yoo SB, Ryu V, Lee JH. Hyperphagia and depression-like behavior by adolescence social isolation in female rats. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience. 2012;30(1):47–53. doi: 10.1016/j.ijdevneu.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Johnson NL, Carini L, Schenk ME, Stewart M, Byrnes EM. Adolescent opiate exposure in the female rat induces subtle alterations in maternal care and transgenerational effects on play behavior. Front Psychiatry. 2011;2:29. doi: 10.3389/fpsyt.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Park CH, Choi SH, Kim NJ, Choe JC, Suh YH. The postnatal environment can counteract prenatal effects on cognitive ability, cell proliferation, and synaptic protein expression. The FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2003;17(11):1556–1558. doi: 10.1096/fj.02-1032fje. [DOI] [PubMed] [Google Scholar]
- Kraebel KS, Brasser SM, Campbell JO, Spear LP, Spear NE. Developmental differences in temporal patterns and potentiation of isolation-induced ultrasonic vocalizations: Influence of temperature variables. Developmental Psychobiology. 2002;40(2):147–159. doi: 10.1002/dev.10022. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Merrick A, Secen J, Helmreich DL. Social instability in adolescence alters the central and peripheral hypothalamic-pituitary-adrenal responses to a repeated homotypic stressor in male and female rats. Journal of Neuroendocrinology. 2007;19(2):116–126. doi: 10.1111/j.1365-2826.2006.01515.x. [DOI] [PubMed] [Google Scholar]
- Moles A, Kieffer BL, D’Amato FR. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004;304(5679):1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- Muller JM, Brunelli SA, Moore H, Myers MM, Shair HN. Maternally modulated infant separation responses are regulated by D2-family dopamine receptors. Behavior Neuroscience. 2005;119(5):1384–1388. doi: 10.1037/0735-7044.119.5.1384. [DOI] [PubMed] [Google Scholar]
- Muller JM, Moore H, Myers MM, Shair HN. Ventral striatum dopamine D2 receptor activity inhibits rat pups’ vocalization response to loss of maternal contact. Behavioral Neuroscience. 2008;122(1):119–128. doi: 10.1037/0735-7044.122.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JM, Moore H, Myers MM, Shair HN. Dopamine’s role in social modulation of infant isolation-induced vocalization: II. Maternally modulated infant separation responses are regulated by D1- and D2-family dopamine receptors. Developmental Psychobiology. 2009;51(2):158–172. doi: 10.1002/dev.20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Panksepp J. Brain substrates of infant-mother attachment: Contributions of opioids, oxytocin, and norepinephrine. Neuroscience and Biobehavioral Reviews. 1998;22(3):437–452. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; New York: 1997. [DOI] [PubMed] [Google Scholar]
- Pickens RW, Svikis DS. Genetic influences in human substance abuse. Journal of Addictive Diseases: The Official Journal of the ASAM, American Society Of Addiction Medicine. 1991;10(1–2):205–213. doi: 10.1300/J069v10n01_14. [DOI] [PubMed] [Google Scholar]
- Schino G, Troisi A. Opiate receptor blockade in juvenile macaques: Effect on affiliative interactions with their mothers and group companions. Brain Research. 1992;576(1):125–130. doi: 10.1016/0006-8993(92)90617-i. [DOI] [PubMed] [Google Scholar]
- Shair HN, Brunelli SA, Masmela JR, Boone E, Hofer MA. Social, thermal, and temporal influences on isolation-induced and maternally potentiated ultrasonic vocalizations of rat pups. Developmental Psychobiology. 2003;42(2):206–222. doi: 10.1002/dev.10087. [DOI] [PubMed] [Google Scholar]
- Shair HN, Brunelli SA, Hofer MA. Lack of evidence for mu-opioid regulation of a socially mediated separation response. Physiological Behavior. 2005;83(5):767–777. doi: 10.1016/j.physbeh.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Shair HN. Acquisition and expression of a socially mediated separation response. Behavioral Brain Research. 2007;182(2):180–192. doi: 10.1016/j.bbr.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizonenko PC. Normal sexual maturation. Pediatrician. 1987;14(4):191–201. [PubMed] [Google Scholar]
- Sizonenko PC. Physiology of puberty. Journal of Endocrinological Investigation. 1989;12(8 Suppl 3):59–63. [PubMed] [Google Scholar]
- Vassoler FM, Johnson-Collins NL, Carini LM, Byrnes EM. Next generation effects of female adolescent morphine exposure: Sex-specific alterations in response to acute morphine emerge before puberty. Behavioural Pharmacology. 2014;25(2):173–181. doi: 10.1097/FBP.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaias J, Queeney TJ, Kelley JB, Zakharova ES, Izenwasser S. Social and physical environmental enrichment differentially affect growth and activity of preadolescent and adolescent male rats. Journal of the American Association for Laboratory Animal Science: JAALAS. 2008;47(2):30–34. [PMC free article] [PubMed] [Google Scholar]
- Zaidan H, Gaisler-Salomon I. Prereproductive stress in adolescent female rats affects behavior and corticosterone levels in second-generation offspring. Psychoneuroendocrinology. 2015;58:120–129. doi: 10.1016/j.psyneuen.2015.04.013. [DOI] [PubMed] [Google Scholar]