If it were possible to develop a treatment for a disease for a few million dollars or less, the implications would be far reaching. There are over 7000 rare diseases, approximately 75% affect children and over 340 currently have treatments. Research for rare diseases has therefore been termed an example of a long-tailed problem 1 in which traditional research has focused on the 20% of genetic conditions (accounting for 80% of all diseases), but at the same time neglecting the other 80% of genetic conditions representing the remaining 20% of all diseases (rare diseases) 2, 3. The irony here is that rare diseases can in general be readily treated by replacing the gene, protein or stabilizing protein folding etc. Many of the rare diseases also have very small numbers of patients (in the low tens or fewer and can be considered ultra-rare), so the cost of clinical trials would be a fraction of the cost of much bigger more common diseases.
A recent example of how a gene therapy treatment can be developed quickly (in approximately 7 years) and cheaply, is for the rare disease called Giant Axonal Neuropathy (GAN, OMIM #256850). GAN is an autosomal recessive inherited condition caused by loss of function of the gigaxonin protein resulting in progressive nerve death 4. In cells this is seen as intermediate filament (IF) aggregation, leading to a progressive and fatal peripheral neuropathy. A rare-disease parent led foundation 5, Hannah's Hope Fund (HHF) raised over $6 million to fund the development of a gene therapy at the University of North Carolina 6. In an in vivo study, GAN knock out mice which received an intracisternal injection of an AAV9/GAN vector to globally deliver the GAN gene to the brainstem and spinal cord, cleared peripherin IF accumulation, suggesting reversal of the pathology 7. The gene therapy treatment for GAN started in the clinic in 2015 and the 5th patient has recently been treated (NCT02362438). This example shows that a gene therapy for an ultra-rare disease can be developed expeditiously and brought to the patients without a large clinical trial.
A second rare disease example can be obtained from efforts with academic collaborators working on an enzyme replacement therapy for a rare disease called Sanfilippo Syndrome D (mucopolysaccharidosis type IIID, MPSIIID, OMIM #252940), for which there are thought to be only two patients in the USA. In this case, a phase I NIH Small Business Technology Transfer grant (STTR, $225,000) can enable the preparation of enough purified protein to do in vitro experiments to show that it can get into cells and perhaps correct the deficiency in fibroblasts from the rare disease patients. A phase II STTR ($1.5M) may provide enough funding to develop more protein, show that it can be delivered and works in the knock out mouse model of the disease. Several million dollars more will be needed to perform the toxicology studies (including large animal immunogenicity testing) and produce clinical grade protein before it is ready for the clinic. It may be possible to bring this this treatment to a clinical trial for less than $5M.
A production line
How can the cost shrink further so that more rare diseases could be addressed for around $1bn, which is the frequently cited approximate cost to develop a single treatment for a major disease? What is needed are economies of scale, emulating what Henry Ford did to mass produce cars, to create a production line for rare disease treatments (Figure 1). The challenges we face in the area of gene therapies are the many competing vectors, patents, and little sharing or open collaboration. Widely known, year long delays in gene therapy vector manufacturing along with delays caused by a lengthy NIH institutional review board regulatory process suggest costly bottlenecks. The same issues will be seen for the development of protein replacements, where purification and expression is still as much of an art as a science with know-how trapped inside companies. When a small company or academic group develops a treatment for a rare disease it is eventually licensed or sold, the company closes and the expertise moves on. The art of purifying a protein, the knowledge of how to make certain compounds, the skills in delivering a gene therapy all tend to go elsewhere. The current system for rare disease research and treatment development is far from a production line. To develop economies of scale there needs to be a focus on generalizing the approaches to develop therapeutics across all types. For example why not identify and develop the best vector and serotype 8 for gene therapy to a particular organ and use it for tens or hundreds of diseases at the same time, rather than just one. Bringing the costs down to $1M per disease to take a treatment to the clinic would require the identification of the best approaches to: make human proteins, to deliver gene therapies to the brain, find molecules to act as chaperones for many diseases, and scale up the process of expression, purification and analysis of multiple protein replacement therapies. One could imagine a factory instead of a lab, with hundreds of skilled experts working on each step of producing treatments for rare diseases (Figure 1) with the infrastructure to retain the talent and share the knowledge in one place. A system to select the diseases would also be important and this could be based on the information in the various databases we have to date (GenBank, PubMed, OMIM, Orphanet etc.) so that those diseases currently without treatments could be addressed. The key factors are likely centralizing these experimental and production capabilities and increasing openness. Such industrialization efforts could help common disease treatment development too and drive down costs.
Figure 1.
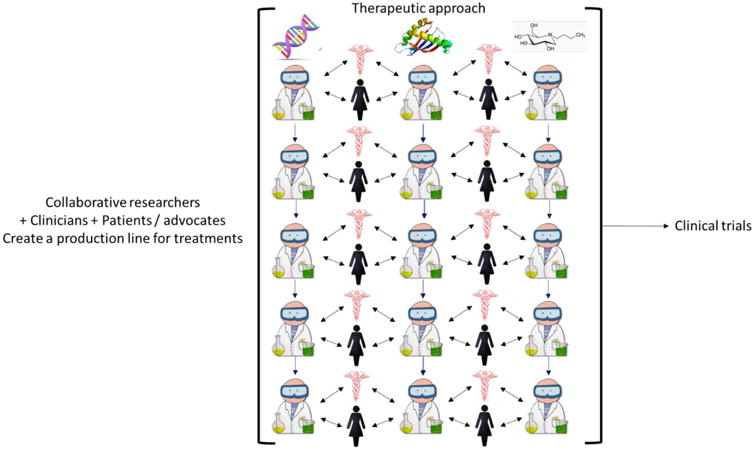
Schematic of a ‘production line’ for developing gene therapies, protein replacement and small molecule approaches for rare diseases in which researchers, clinicians, patients and advocates collaborate to bring different treatments for different rare diseases to the point of clinical trials.
A rare disease center
At the time of writing, clinicaltrials.gov has well over 100 open gene therapy clinical trials and many of these are for rare diseases. This represents just a tiny fraction of the number of known rare diseases. From the previously mentioned bottlenecks it is clear there needs to be a gene therapy center of excellence which could be run by the NIH that incorporates vector, immunology, animal models and cell morphology cores. The capacity needs to be sufficient to cope with growth in gene therapy trials likely in the years ahead. The USA has seen a surge in interest in gene therapy over the last few years and this has lead to numerous academic centers developing their expertise and competing start-ups formed (e.g SPARK, Voyager Therapeutics, Dimension Therapeutics, Bluebird bio, Bamboo etc.) which all put pressure on the available manufacturing facilities. But why stop at just gene therapies, the center should cover all rare disease treatment approaches. It has been recently proposed that patients are key partners in rare disease drug development and it is indeed important that they are engaged for understanding disease burden, establishing disease end-points and assessing benefit-risk profiles 9. We would also suggest that patients and more likely their families can do so much more than this. For example, families are actively partnering with academics to push and fund their ideas for treatments 5 but this has to happen on a much larger scale (Figure 1). A rare disease center would perhaps need to hire tens to hundreds of the world's best scientists to work on those diseases needing a treatment, which would not come cheaply. Public and private funding would therefore be needed to tackle the hundreds to thousands of rare diseases in this way. Learning from the parent-lead successes to date that have brought treatments to the clinic like GAN and others, provides convincing evidence to governments and philanthropists to invest in such an effort. There would also need to be close interactions between these scientists, clinicians, rare disease patients and their families and the many rare disease foundations that could perhaps help fund such a concentrated effort (Figure 1).
Most companies working on rare diseases address a small number of them. Such rare disease efforts are focused on creating a treatment for a single disease rather than finding a way that all diseases can benefit. What is proposed here is a fundamentally different approach. Build the scale that could handle orders of magnitude more diseases would require the use computational, automation and informatics tools to help identify, create and manufacture treatments and facilitate the collaborations necessary to create a pipeline to bring them to the clinic. Centralizing efforts on rare diseases would also need to bring with it the relevant expertise necessary to go from the preclinical to clinical stages.
Treatment discovery in the pharmaceutical and biotech industry has been suggested as needing disruption 10. Industrialization of rare disease treatment development may lead the way to a more sustainable future which will have considerable support if it ultimately drives down the cost of rare disease treatments.
Acknowledgments
I kindly acknowledge extensive discussions with the rare disease parents and foundations led by Lori Sames, Allison Moore and Jill Wood and from discussions with researchers Dr. Patricia Dickson, Dr. Tsui-Fen Chou, and Dr. Coy Heldermon. The Sanfilippo type D enzyme replacement study is funded by NIH NINDS 2R42NS089061-02.
Footnotes
Competing Financial Interests: SE is a co-founder and employee of Collaborations Pharmaceuticals, Inc., Phoenix Nest and consults for the Hereditary Neuropathy Foundation.
References
- 1.Shen T, Lee A, Shen C, Lin CJ. Genet Res (Camb) 2015;97:e15. doi: 10.1017/S0016672315000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swinney DC, Xia S. Future Med Chem. 2014;6:987–1002. doi: 10.4155/fmc.14.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melnikova I. Nat Rev Drug Discov. 2012;11:267–268. doi: 10.1038/nrd3654. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y, Allen E, Ding J, Wang W. Cell Mol Life Sci. 2007;64:601–609. doi: 10.1007/s00018-007-6396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood J, Sames L, Moore A, Ekins S. Drug Discov Today. 2013;18:1043–1051. doi: 10.1016/j.drudis.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Vandendriessche T, et al. J Thromb Haemost. 2007;5:16–24. doi: 10.1111/j.1538-7836.2006.02220.x. [DOI] [PubMed] [Google Scholar]
- 7.Mussche S, et al. Hum Gene Ther. 2013;24:209–219. doi: 10.1089/hum.2012.107. [DOI] [PubMed] [Google Scholar]
- 8.Gilkes JA, Bloom MD, Heldermon CD. Gene Ther. 2016;23:263–271. doi: 10.1038/gt.2015.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bronstein MG, Kakkis ED. Nat Rev Drug Discov. 2016;34:380–383. doi: 10.1038/nrd.2016.133. [DOI] [PubMed] [Google Scholar]
- 10.Munos BH, Orloff JJ. National Academy of Medicine; 2016. https://nam.edu/disruptive-innovation-and-transformation-of-the-drug-discovery-and-development-enterprise/ [Google Scholar]


