Abstract
Whole-genome sequencing (WGS) provides the highest resolution analysis for comparison of bacterial isolates in public health microbiology. However, although increasingly being used routinely for some pathogens such as Listeria monocytogenes and Salmonella enterica, the use of WGS is still limited for other organisms, such as Neisseria gonorrhoeae. Multi-antigen sequence typing (NG-MAST) is the most widely performed typing method for epidemiological surveillance of gonorrhoea. Here, we present NGMASTER, a command-line software tool for performing in silico NG-MAST on assembled genome data. NGMASTER rapidly and accurately determined the NG-MAST of 630 assembled genomes, facilitating comparisons between WGS and previously published gonorrhoea epidemiological studies. The source code and user documentation are available at https://github.com/MDU-PHL/ngmaster.
Keywords: Neisseria gonorrhoeae, Multi-antigen sequence typing, NG-MAST, Whole-genome sequencing, In silico typing
Data Summary
The Python source code for NGMASTER is available from GitHub under GNU GPL v2. (URL: https://github.com/MDU-PHL/ngmaster)
The software is installable via the Python ‘pip’ package management system. Install using ‘pip install – user git https://github.com/MDU-PHL/ngmaster.git’
Sequencing data used are available for download from the EBI European Nucleotide Archive under BioProject accessions PRJEB2999, PRJNA29335, PRJNA266539, PRJNA298332, and PRJEB14168.
Impact Statement
Whole-genome sequencing (WGS) offers the potential for high-resolution comparative analyses of microbial pathogens. However, there remains a need for backward compatibility with previous molecular typing methods to place genomic studies in context. NG-MAST is currently the most widely used method for epidemiological surveillance of Neisseria gonorrhoeae. We present NGMASTER, a command-line software tool for performing multi-antigen sequence typing (NG-MAST) of Neisseria gonorrhoeae from WGS data. This tool is targeted at clinical and research microbiology laboratories that have performed WGS of N. gonorrhoeae isolates and wish to understand the molecular context of their data in comparison to previously published epidemiological studies. As WGS becomes more routinely performed, NGMASTER has been developed to completely replace PCR-based NG-MAST, reducing time and labour costs.
Introduction
Neisseria gonorrhoeae is one of the most common sexually transmitted bacterial infections worldwide. There is growing concern about the global spread of resistant epidemic clones, with extensively drug-resistant gonorrhoea being listed as an urgent antimicrobial resistance threat (CDC, 2013; WHO, 2014).
Multi-antigen sequence typing of N. gonorrhoeae (NG-MAST) has been important in tracking these resistant clones, such as the NG-MAST 1407 clone associated with decreased susceptibility to third-generation cephalosporins (Unemo & Dillon, 2011). It involves sequence-based typing using established PCR primers of two highly variable and polymorphic outer membrane protein genes, porB and tbpB by comparing the sequences to an open-access database (http://www.ng-mast.net/) (Martin et al., 2004). Although NG-MAST is the most frequently performed molecular typing method for N. gonorrhoeae, it requires multiple PCR amplification and sequencing reactions, making it more laborious than other gonococcal typing methods such as single porB gene sequencing, or fragment analysis methods such as multiple locus variable-number tandem repeat analysis (MLVA) (Heymans et al., 2012).
Whole-genome sequencing (WGS) is increasingly being used for molecular typing and epidemiological investigation of microbial pathogens as it provides considerably higher resolution. A number of studies using genomic data to understand the epidemiology of N. gonorrhoeae have already been published (Grad et al., 2014) (Demczuk et al., 2015) (Ezewudo et al., 2015) (Demczuk et al., 2016). However, the ability to perform retrospective comparisons with previous epidemiological studies is reliant on conducting both traditional typing (such as NG-MAST) as well as more modern WGS analyses on the same isolates.
NGMASTER is a command-line software tool for rapidly determining NG-MAST types in silico from genome assemblies of N. gonorrhoeae.
Description
NGMASTER is an open source tool written in Python and released under a GPLv2 Licence. The source code can be downloaded from Github (https://github.com/MDU-PHL/ngmaster). It has two software dependencies: isPcr (http://hgwdev.cse.ucsc.edu/~kent/src/) and BioPython (Cock et al., 2009), and uses the allele databases publicly available at http://www.ng-mast.net/, which NGMASTER can automatically download and update locally for running.
NGMASTER is based on the laboratory method published by Martin et al. (2004), and uses isPcr to retrieve allele sequences from a user-specified genome assembly in FASTA format by locating the flanking primers. These allele sequences are trimmed to a set length from starting key motifs in conserved gene regions, and then checked against the allele databases. Results are printed in machine readable tab- or comma-separated format.
Methods
NGMASTER was validated against 630 publicly available N. gonorrhoeae genome sequences derived from published studies (Table 1). A PubMed search for published studies with N. gonorrhoea whole-genome sequencing data was conducted (on 4 May, 2016) using the search terms ‘Neisseria gonorrhoeae’ and ‘whole-genome sequencing’. We excluded studies with less than 20 isolates, and those that did not publish NG-MAST results or make their raw sequencing data available. Our search identified three studies, contributing 572 sequences for testing that had undergone manual in silico NG-MAST from WGS data (Demczuk et al., 2015; Demczuk et al., 2016; Grad et al., 2014), including the fully assembled reference genome NCCP11945 (Chung et al., 2008). The panel of isolates also included the genome sequencing data for eight well characterised WHO reference genomes with published NG-MAST results. Raw WGS data for these sequences were retrieved from the European Nucleotide Archive (ENA). Average sequencing depth was >30× for all ENA sequences, with a combination of 100 bp, 250 bp and 300 bp paired-end Illumina reads. In addition, we tested an additional 50 local isolates that had undergone ‘traditional’ NG-MAST by PCR and Sanger sequencing (Martin et al., 2004). These isolates underwent WGS on the Illumina MiSeq/NextSeq using Nextera libraries and manufacturer protocols, each with an average sequencing depth >50×. The raw sequencing reads for these local isolates have been uploaded to the ENA (BioProject accession PRJEB14168).
Table 1. Concordance between NGMASTER results from draft genome assemblies using MEGAHIT and SPAdes, and previously published NG-MAST results.
| MEGAHIT | SPAdes | Two-stage¶ | Total | |
|---|---|---|---|---|
| PRJEB2999* | 176 (95 %) | 184 (99 %) | 184 (99 %) | 186 |
| PRJNA29335† | – | – | – | 1 |
| PRJNA266539‡ | 162 (91 %) | 169 (94 %) | 178 (99 %) | 179 |
| PRJNA298332§ | 199 (93 %) | 207 (97 %) | 208 (97 %) | 214 |
| PRJEB14168|| | 50 (100 %) | 50 (100 %) | 50 (100 %) | 50 |
| Total | 587 (93 %) | 610 (97 %) | 620 (98 %) | 630 |
§Closed reference genome NCCP11945 (Genbank accession CP001050.1) – in silico NG-MAST results reported by Demczuk et al. (2015).
||Local isolates with NG-MAST performed by PCR/Sanger sequencing.
¶Two-stage assembly: 1. NGMASTER run using rapid assembly with MEGAHIT; 2. NGMASTER also run using SPAdes if there was no result or a mixed result using MEGAHIT assembly.
Sequencing reads were trimmed to clip Illumina adapters and low-quality sequences (minimum Q20) using Trimmomatic v0.35 (Bolger et al., 2014). Draft genomes were assembled de novo with MEGAHIT v1.0.3 and SPAdes v3.7.1 (Li et al., 2015) (Bankevich et al., 2012) to investigate whether the faster, but approximate genome assembler, MEGAHIT, would be sufficient for NGMASTER. A list of the commands and parameters used is included in Appendix 1.
The de novo assembled draft genomes and the fully assembled NCCP11945 reference genome in FASTA format were used as input to NGMASTER with the overall results shown in Table 1. Complete NGMASTER results with sequencing and assembly metrics are included in Appendix 2. Running NGMASTER on 630 genome assemblies using a single Intel(R) Xeon(R) 2.3GHz CPU core was completed in less than two minutes.
Overall, NGMASTER assigned NG-MAST types that were concordant with published results for 93 % of the tested N. gonorrhoeae genomes using MEGAHIT assemblies, and 97 % using SPAdes assemblies. Notably, comparisons with results from traditional NG-MAST were 100 % concordant (58/58), including 50 local isolates and the eight well-characterised WHO reference isolates. Reasons for discordant results are shown in Table 2. In general, running NGMASTER using SPAdes assemblies resolved more NG-MAST types than when using MEGAHIT assemblies. However, ten genomes assembled with SPAdes v3.7.1 were found to have assembly errors in either por or tbpB introduced at the repeat resolution stage of the SPAdes assembly process, resulting in discordant NG-MAST types for those isolates (major errors). Running NGMASTER on preliminary contigs prior to this process (in particular, on the ‘before_rr.fasta’ intermediate file generated by SPAdes in the assembly output folder) or disabling repeat resolution using the flag ‘--disable-rr’ when running SPAdes alleviated these major errors, and were concordant with MEGAHIT results and the published results (Appendix 2). In contrast, minor errors (due to incomplete NG-MAST types or multiple alleles detected) were more frequent using MEGAHIT assemblies, particularly those with poor assembly metrics (e.g. >500 contigs, N50<10 kbp). When MEGAHIT assemblies successfully produced complete NGMASTER results, these NG-MAST types were highly concordant with the published results.
Table 2. Reasons for discordant results between NGMASTER and published data using SPAdes assemblies.
| Reason for discordant result | MEGAHIT | SPAdes |
|---|---|---|
| Major errors (incorrect result) | ||
| Assembly error | 0 | 10 |
| Minor errors (incomplete/missing result) | ||
| Alternate conserved key motif | 1 | 1 |
| Multiple alleles detected | 6 | 2 |
| Allele not detected | 29 | 0 |
| Errors in published data | ||
| Possible sequence mix-up in published data | 4 | 4 |
| Probable transcription error in published data | 1 | 1 |
| Error in published data | 1 | 1 |
To overcome this issue, a two-stage assembly approach was also tested, where a draft genome was first assembled using MEGAHIT for initial testing. If a complete NG-MAST result was obtained, this was recorded as the final result for that isolate. If the result was incomplete or suggested multiple alleles were present, the genome was also assembled using SPAdes. Using this combined approach, 620 out of 630 (98 %) NG-MAST types derived from NGMASTER were concordant with the published results, with only 42 genomes requiring additional assembly with the slower SPAdes assembler.
For the remaining ten discordant results, seven of these were likely to be due to errors in the published data, including for NCCP11945. A further two isolates were found to have multiple tbpB alleles in both SPAdes and MEGAHIT assemblies, with the dominant allele (indicated by higher read coverage and better flanking assembly) matching the published result. The tbpB allele for the final isolate was not able to be determined by NGMASTER due to a mutation in the conserved starting key motif required for sequence trimming to a standard size.
Issues with implementation
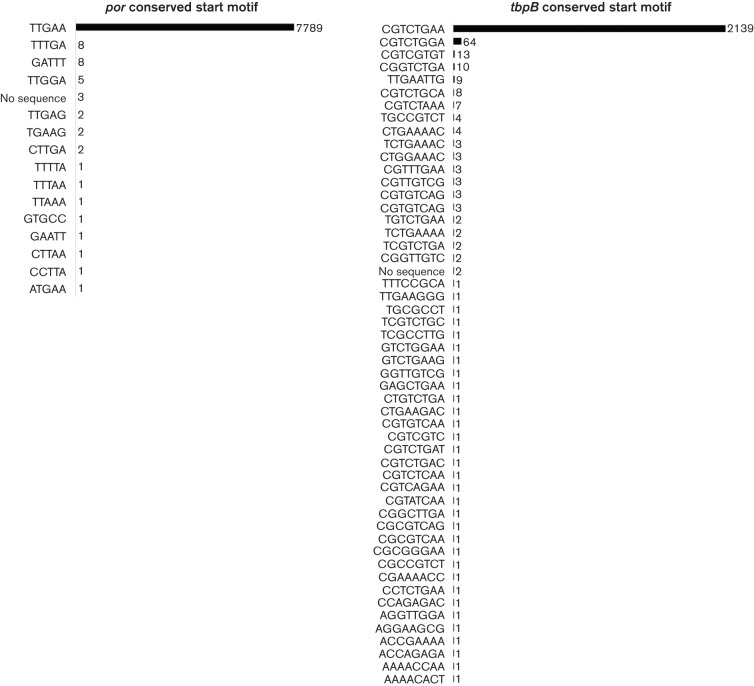
The NG-MAST procedure involves sequencing the internal regions of por and tbpB that encode two variable outer membrane proteins. The sequences are trimmed to a standard length from a starting key motif in conserved regions of each gene. However, despite being relatively conserved, a number of variations of this starting motif appear in the NG-MAST database (Fig. 1), causing one discordant result (Table 2). Some sequences appeared to lack a tbpB gene due to the presence of non-typeable tbpB genes acquired from N. meningitidis, though this was also noted in the published data. Another source of discordant results was genomes that appeared to have multiple alleles, suggesting isolate contamination or polyclonal infection.
Fig. 1.
Number and frequency of alternate starting key motifs within ‘conserved’ gene regions for trimming allele sequences.
A number of isolates were found to have novel alleles or allele combinations that were not in the most recent version of the database available at http://www.ng-mast.net. For convenience, NGMASTER includes an option to save these allele sequences in FASTA format for manual submission to the database and allele type assignment.
Notably, results were dependent on the accuracy and quality of the de novo draft genome assembly. It should be noted that for this study, draft genomes were assembled de novo using relatively standard parameters for MEGAHIT and SPAdes without post-assembly error checking (see Appendix 1). We were alerted to the presence of SPAdes assembly errors after finding the corresponding MEGAHIT assemblies produced different NGMASTER results. Concordant results were obtained for each of these genomes after identifying and correcting assembly errors through re-mapping each isolate’s sequencing reads back to the respective draft SPAdes assembly (see Appendix 1). These errors introduced during the SPAdes assembly process can also be corrected using an assembly polishing tool such as Pilon (Walker et al., 2014). Assuming accurate closed genome assemblies are used with an accurate and well curated database, based on our testing, we anticipate that NGMASTER would produce NG-MAST results that were >99 % if not 100 % accurate.
Conclusion
NGMASTER rapidly and accurately performs in silico NG-MAST typing of N. gonorrhoeae from assembled WGS data, and may be a useful command-line tool to help contextualise genomic epidemiological studies of N. gonorrhoeae.
Acknowledgements
We wish to acknowledge the contributions of Helen Heffernan at the Institute of Environmental Science and Research, New Zealand, and Kerrie Stevens and the Molecular Diagnostics section staff at the Microbiological Diagnostic Unit Public Health Laboratory, Australia who assisted in providing NG-MAST data. This project was supported by the National Health and Medical Research Council, Australia with a postgraduate scholarship to JCK (GNT1074824), and fellowships to BPH (GNT1105905) and TPS (GNT1008549). Doherty Applied Microbial Genomics is funded by the Department of Microbiology and Immunology at The University of Melbourne. NG-MAST performed at the Institute of Environmental Science and Research was funded by the New Zealand Ministry of Health.
Supplementary Data
Supplementary File 1
Abbreviations:
- WGS
whole-genome sequencing
- NG-MAST
Neisseria gonorrhoeae multi-antigen sequence typing
References
- Bankevich A., Nurk S., Antipov D., Gurevich A. A., Dvorkin M., Kulikov A. S., Lesin V. M., Nikolenko S. I., Pham S., et al. (2012). SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19455–477. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A. M., Lohse M., Usadel B.(2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 302114–2120. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2013). Antibiotic Resistance Threats in the United States, 2013 Atlanta, GA, USA: Centers for Disease Control and Prevention, US Department of Health and Human Services. [Google Scholar]
- Chung G. T., Yoo J. S., Oh H. B., Lee Y. S., Cha S. H., Kim S. J., Yoo C. K.(2008). Complete genome sequence of Neisseria gonorrhoeae NCCP11945. J Bacteriol 1906035–6036. 10.1128/JB.00566-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cock P. J., Antao T., Chang J. T., Chapman B. A., Cox C. J., Dalke A., Friedberg I., Hamelryck T., Kauff F., et al. (2009). Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 251422–1423. 10.1093/bioinformatics/btp163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demczuk W., Lynch T., Martin I., Van Domselaar G., Graham M., Bharat A., Allen V., Hoang L., Lefebvre B., et al. (2015). Whole-genome phylogenomic heterogeneity of Neisseria gonorrhoeae isolates with decreased cephalosporin susceptibility collected in Canada between 1989 and 2013. J Clin Microbiol 53191–200. 10.1128/JCM.02589-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demczuk W., Martin I., Peterson S., Bharat A., Van Domselaar G., Graham M., Lefebvre B., Allen V., Hoang L., et al. (2016). Genomic epidemiology and molecular resistance mechanisms of Azithromycin-resistant Neisseria gonorrhoeae in Canada from 1997 to 2014. J Clin Microbiol 541304–1313. 10.1128/JCM.03195-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezewudo M. N., Joseph S. J., Castillo-Ramirez S., Dean D., Del Rio C., Didelot X., Dillon J. A., Selden R. F., Shafer W. M., et al. (2015). Population structure of Neisseria gonorrhoeae based on whole genome data and its relationship with antibiotic resistance. Peer J 3e806. 10.7717/peerj.806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grad Y. H., Kirkcaldy R. D., Trees D., Dordel J., Harris S. R., Goldstein E., Weinstock H., Parkhill J., Hanage W. P., et al. (2014). Genomic epidemiology of Neisseria gonorrhoeae with reduced susceptibility to cefixime in the USA: a retrospective observational study. Lancet Infect Dis 14220–226. 10.1016/S1473-3099(13)70693-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymans R., Golparian D., Bruisten S. M., Schouls L. M., Unemo M.(2012). Evaluation of Neisseria gonorrhoeae multiple-locus variable-number tandem-repeat analysis, N. gonorrhoeae multiantigen sequence typing, and full-length porB gene sequence analysis for molecular epidemiological typing. J Clin Microbiol 50180–183. 10.1128/JCM.05386-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Liu C. M., Luo R., Sadakane K., Lam T. W.(2015). MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 311674–1676. 10.1093/bioinformatics/btv033 [DOI] [PubMed] [Google Scholar]
- Martin I. M., Ison C. A., Aanensen D. M., Fenton K. A., Spratt B. G.(2004). Rapid sequence-based identification of gonococcal transmission clusters in a large metropolitan area. J Infect Dis 1891497–1505. 10.1086/383047 [DOI] [PubMed] [Google Scholar]
- Unemo M., Dillon J. A.(2011). Review and international recommendation of methods for typing Neisseria gonorrhoeae isolates and their implications for improved knowledge of gonococcal epidemiology, treatment, and biology. Clin Microbiol Rev 24447–458. 10.1128/CMR.00040-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2014). Antimicrobial resistance: global report on surveillance 2014. Geneva, Switzerland: : World Health Organization. [Google Scholar]
- Walker B. J., Abeel T., Shea T., Priest M., Abouelliel A., Sakthikumar S., Cuomo C. A., Zeng Q., Wortman J., et al. (2014). Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One 9e112963. 10.1371/journal.pone.0112963 [DOI] [PMC free article] [PubMed] [Google Scholar]
Data Bibliography
- 1.Chung, G. T., Yoo, J. S., Oh, H. B., Lee, Y. S., Cha, S. H., Kim, S. J. & Yoo, C. K. ENA BioProject accession PRJNA29335(2008).
- 2.Demczuk, W., Lynch, T., Martin, I., Van Domselaar, G., Graham, M., Bharat, A., Allen, V., Hoang, L., Lefebvre, B., Tyrrell, G., Horsman, G., Haldane, D., Garceau, R., Wylie, J., Wong, T. & Mulvey, M. R. ENA BioProject accession PRJNA266539 (2015).
- 3.Demczuk, W., Martin, I., Peterson, S., Bharat, A., Van Domselaar, G., Graham, M., Lefebvre, B., Allen, V., Hoang, L., Tyrrell, G., Horsman, G., Wylie, J., Haldane, D., Archibald, C., Wong, T., Unemo, M. & Mulvey, M. R. ENA BioProject accession PRJNA298332 (2016).
- 4.Grad, Y. H., Kirkcaldy, R. D., Trees, D., Dordel, J., Harris, S. R., Goldstein, E., Weinstock, H., Parkhill, J., Hanage, W. P., Bentley, S. & Lipsitch, M. ENA BioProject accession PRJEB2999 (2014). [DOI] [PMC free article] [PubMed]
- 5.Kwong, J. C., Gonçalves da Silva, A., Dyet, K., Williamson, D. W., Stinear, T. P., Howden, B. P. & Seemann, T. ENA BioProject PRJEB14168 (2016). [DOI] [PMC free article] [PubMed]
- 6.Kent, J. isPcr. http://hgwdev.cse.ucsc.edu/~kent/src/ (2005).
- 7.Cock, P. J., Antao, T., Chang, J. T., Chapman, B. A., Cox, C. J., Dalke, A., Friedberg, I., Hamelryck, T., Kauff, F., Wilczynski, B. & de Hoon, M. J. Biopython. http://biopython.org/ (2009). [DOI] [PMC free article] [PubMed]
- 8.Aanensen, D. NG-MAST database. http://www.ng-mast.net/ (2004).
- 9.Kwong, J. C., Gonçalves da Silva, A., & Seemann, T. NGMASTER (v0.3). https://github.com/MDU-PHL/ngmaster (2016).
- 10.Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic (v0.35). http://www.usadellab.org/cms/?page=trimmomatic (2014). [DOI] [PMC free article] [PubMed]
- 11.Li, D., Liu, C. M., Luo, R., Sadakane, K. & Lam, T. W. MEGAHIT (v1.0.3). https://github.com/voutcn/megahit (2015). [DOI] [PubMed]
- 12.Bankevich, A., Nurk, S., Antipov, D., Gurevich, A. A., Dvorkin, M., Kulikov, A. S., Lesin, V. M., Nikolenko, S. I., Pham, S., Prjibelski, A. D., Pyshkin, A. V., Sirotkin, A. V., Vyahhi, N., Tesler, G., Alekseyev, M. A. & Pevzner, P. A. SPAdes (vvvvvvvv3.7.1). http://bioinf.spbau.ru/spades (2012).
- 13.Seemann, T. Snippy (v3.1). https://github.com/tseemann/snippy (2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File 1