Abstract
AIM
To elucidate how high diet-induced endoplasmic reticulum-stress upregulates thioredoxin interacting protein expression in Müller cells leading to retinal inflammation.
METHODS
Male C57Bl/J mice were fed either normal diet or 60% high fat diet for 4-8 wk. During the 4 wk study, mice received phenyl-butyric acid (PBA); endoplasmic reticulum-stress inhibitor; for 2 wk. Insulin resistance was assessed by oral glucose tolerance. Effects of palmitate-bovine serum albumin (BSA) (400 μmol/L) were examined in retinal Müller glial cell line and primary Müller cells isolated from wild type and thioredoxin interacting protein knock-out mice. Expression of thioredoxin interacting protein, endoplasmic reticulum-stress markers, miR-17-5p mRNA, as well as nucleotide-binding oligomerization domain-like receptor protein (NLRP3) and IL1β protein was determined.
RESULTS
High fat diet for 8 wk induced obesity and insulin resistance evident by increases in body weight and impaired glucose tolerance. By performing quantitative real-time polymerase chain reaction, we found that high fat diet triggered the expression of retinal endoplasmic reticulum-stress markers (P < 0.05). These effects were associated with increased thioredoxin interacting protein and decreased miR-17-5p expression, which were restored by inhibiting endoplasmic reticulum-stress with PBA (P < 0.05). In vitro, palmitate-BSA triggered endoplasmic reticulum-stress markers, which was accompanied with reduced miR-17-5p and induced thioredoxin interacting protein mRNA in retinal Müller glial cell line (P < 0.05). Palmitate upregulated NLRP3 and IL1β expression in primary Müller cells isolated from wild type. However, using primary Müller cells isolated from thioredoxin interacting protein knock-out mice abolished palmitate-mediated increase in NLRP3 and IL1β.
CONCLUSION
Our work suggests that targeting endoplasmic reticulum-stress or thioredoxin interacting protein are potential therapeutic strategies for early intervention of obesity-induced retinal inflammation.
Keywords: High fat diet, Palmitate, Endoplasmic-reticulum-stress, Inflammation, Thioredoxin-interacting protein, Micro-RNA 17-5p
Core tip: We previously showed that high fat diets (HFD) induced retinal inflammation and vascular dysfunction. These results were associated with an increase in thioredoxin interacting protein (TXNIP) at the mRNA and protein level. Here, we examined the mechanisms by which HFD triggers retinal TXNIP. Interestingly, we found that HFD/palmitate triggers ER-stress mediators including the inositol requiring enzyme 1, an RNAse that can degrade number of mRNAs including the microRNA; miR-17-5p and sustains TXNIP expression. Inhibiting ER-stress prevented the increase in TXNIP in vivo and in Müller cells, the main glia in the retina. Deletion of TXNIP blunted NLRP-3 inflammasome and IL-1β release in Müller cells.
INTRODUCTION
Obesity, recently upgraded from a mere risk factor to a disease state, is affecting one third of United States population[1]. Clinical evidence showed that obesity not only can accelerate developing type-2 diabetes and cardiovascular complications, but also induce retinal microvascular abnormalities, which eventually leads to visual impairments[2,3]. High fat diets (HFD) together with the improper physical activity are the culprit in the obesity-induced pre-diabetes. Therefore, there is an urgent need to unravel the mechanisms involved in HFD-mediated neurovascular abnormalities. Our lab has previously shown that consumption of high caloric diet saturated fatty acids induced retinal inflammation and microvascular dysfunction via upregulating the expression of thioredoxin interacting protein (TXNIP); a regulator of the antioxidant thioredoxin; and activating NOD (NOD)-like receptor protein (NLRP3)-inflammasome[4]. Similar observations showed the contribution of TXNIP/NLRP3-inflammasome signaling pathway to the development of various disorders in other organs[5-7]. However, molecular mechanisms by which HFD triggers early TXNIP expression in the retina are still unclear.
MicroRNAs are small non-coding RNAs that control the translation and transcription of various genes via annealing to the complementary sequences in the 3′ untranslated region of their target gene[8]. To date, several miR classes have been identified to be involved in development of obesity, diabetes and diabetic complications[9]. Bioinformatic analysis of the TXNIP 3′ UTR identified 11 possible miRNAs that can regulate its expression including miR-130/301, miR-128, miR-148/152, miR-135, miR-106/302, miR-17-5p/20/93.mr/106/519.d, miR-128, miR-15/16/195/424/497, miR-106/302, miR-148/152. Nevertheless, levels of miR-17-5p have been reported to rapidly decline under stress condition resulting in enhancing TXNIP expression[10,11].
Unfolded protein response (UPR) is an adaptive response, which prevents the accumulation of misfolded proteins in the lumen of the endoplasmic reticulum (ER). The UPR is transduced by three major ER-resident stress sensors, namely Protein Kinase RNA-like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol requiring enzyme 1 (IRE1). However, when protein misfolding exceeds the capacity of the UPR an ER-stress will result that triggers programmed cell death. So far, ER-stress has been shown to play a critical role in the pathogenic progression of various chronic diseases including diabetic retinopathy (reviewed in[12-14]). Among UPR pathways, IRE1α, an ER bifunctional kinase/RNase has been shown to destabilize number of RNA and microRNA including miR-17-5p in pancreatic beta-cells[10,11]. Several studies reported the impact of HFD and its related metabolite such as free fatty acid in inducing ER-stress[15-17]. In the current study we were trying to decipher the underlying mechanisms that link HFD-mediated ER-stress to retinal inflammation. Here, we tested the hypothesis that HFD-mediated ER-stress upregulates TXNIP mRNA expression via dysregulating miR-17-5p resulting in retinal inflammation.
MATERIALS AND METHODS
Animals
All animal experiments were conducted in agreement with Association for Research in Vision and Ophthalmology statement for use of animals in ophthalmic and vision research, and Charlie Norwood VA Medical Center Animal Care and Use Committee (ACORP#15-04-080). 6-8 wk old male C57BL6/J mice (Stock 000664, Jackson Laboratory, ME, United States) were used in the in vivo studies. For the long term study, mice were fed ad libitum with normal rat chow (7% fat) or HFD [36 g %, 251 kJ (60 kcal) %fat] (F2685 Bioserv, Frenchtown, NJ, United States) for 8 wk. For the short term study, mice were fed either normal diet (ND) or 60% HFD for 2 wk. Mice were then kept on HFD for additional 2 wk while receiving an ER-stress inhibitor [Phenyl-butyric acid (PBA), 100 mg/kg] or vehicle. PBA was dissolved in DMSO/PBS and administered via oral gavage 5 d/wk. Mice were weighed weekly to track the increase in the body weight.
Intra-peritoneal glucose tolerance test
Mice went overnight fasting, and their fasting plasma blood glucose was measured as the baseline. Then all mice received an intraperitoneal injection of glucose (2 g/kg). Blood glucose levels were measured at different time points till 120 min after the glucose injection using a glucometer.
In-vitro studies
The rat retinal Müller glial cell line (rMC-1) was obtained originally from V. Sarthy (Department of Ophthalmology, Northwestern University, Chicago, IL, United States)[18]. Primary mouse Müller Cells from WT and TKO mice were isolated and cultured as described previously[19]. Cells were grown to confluency in complete media (DMEM, 10% vol/vol. FBS, 1% vol/vol. penicillin/streptomycin). Sodium palmitate (Cat.# P9767; Sigma-Aldrich, St. Louis,MO, United States) was dissolved in 50% ethyl alcohol, then added drop-wise to preheated 10% endotoxin- and fatty acid-free BSA (Cat.# 22070017; Bioworld, Dublin, OH) in DMEM at 50 °C to create an intermediate stock solution of palmitate coupled to BSA (Pal-BSA). Confluent cells were switched to serum-free medium for overnight then were treated for 6 h with Pal-BSA solutions (400 μmol/L final concentration). Equal volumes of 50% ethyl alcohol with BSA alone served as control. In another set of rMC-1, cells were serum starved for 4 h then treated with PBA (1 mmol/L, Cat.#P21005, Sigma-Aldrich) or IRE1α inhibitor (STF-083010, 50 μmol/L) for 2 h then palmitate was added and kept overnight.
Quantitative real-time PCR
A one-step quantitative RT-PCR kit (Invitrogen) was used to amplify 10 ng retinal mRNA as described previously[4]. PCR primers (Table 1) were obtained from Integrated DNA Technologies (Coralville, IA, United States). Quantitative PCR was conducted using StepOnePlus qPCR system (Applied BioSystems, Life Technologies). The percent expression of various genes was normalized to 18S.
Table 1.
The sequence of the polymerase chain reaction primers used in the experiments
| Gene | Forward | Reverse |
| 18 S | CGCGGTTCTATTTTGTTGGT | AGTCGGCATCGTTTATGGTC |
| XBP1 | ACACGCTTGGGAATGGACAC | CCATGGGAAGATGTTCTGGG |
| XBP1-SPLICED | GAGTCCGCAGCAGGTG | GTGTCAGAGTCCATGGGA |
| PERK | AGTCCCTGCTCGAATCTTCCT | TCCCAAGGCAGAACAGATATACC |
| IRE1α | GGGTTGCTGTCGTGCCTCGAG | TGGGGGCCTTCCAGCAAAGGA |
| ATF6 | TGCCTTGGGAGTCAGACCTAT | GCTGAGTTGAAGAACACGAGTC |
| CHOP | CTGGAAGCCTGGTATGAGGAT | CAGGGTCAAGAGTAGTGAAGGT |
| TXNIP | AAGCTGTCCTCAGTCAGAGGCAAT | ATGACTTTCTTGGAGCCAGGGACA |
Micro-RNA detection
MirVana PARIS kit (Cat.# AM1556, Invitrogen) was used for miRNA isolation according to manufacturer’s protocol. Reverse transcriptase reactions; including samples and no-template controls; were run using TaqMan® MicroRNA Reverse Transcription Kit (Cat.# 4366596, Applied Biosystems) as described previously[20]. PCR amplification was performed using TaqMan® Universal PCR Master Mix (Cat.# 4324018, Applied Biosystems) according to manufacturer’s protocol. The percent expression of miR-17-5p was normalized to U6.
Western blot analysis
Retinas were isolated and homogenized in cell disruption buffer as described previously[21]. Müller cells were harvested by scraping thoroughly with cell scraper after the addition of cell disruption buffer. Samples (25 μg protein) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Membranes were probed with the primary antibodies; anti-TXNIP (Cat.# K0205-3 MBL Abacus ALS Australia and Cat.# 403700, Invitrogen, Grand Island, NY), anti-NLRP-3 (Cat.# LS-B4321, LifeSpan Biosciences, Inc, Seatle, WA), anti-IL1β (Cat.# ab9722, Abcam, Cambridge, MA, United States) then reprobed with housekeeping gene; anti-GAPDH (Cat.# 5174, Cell Signaling, Danvers, MA, United States), anti-tubulin (Cat.# ab4074, Abcam, Cambridge, MA, United States) or anti-actin (Cat.# a5060, Sigma-Aldrich) to confirm equal loading. The primary antibody was detected using a horseradish peroxidase (HRP) and enhanced chemiluminescence. The films were scanned and the band intensity was quantified using densitometry software version 6.0.0 Software from alphaEaseFC (Santa Clara, CA) and expressed as relative optical density (OD).
Statistical analysis
All the data are expressed as mean ± SD or SEM. Differences between ND vs HFD and control vs palmitate were tested using two-sample t tests. One-way ANOVA followed by Bonferroni post-hoc multiple comparisons to assess significant differences between 3 or more groups (Graphpad-Ver.6). For body weight and blood glucose measurements, area under the curve (AUC) across all the time points was calculated. A series of 2 gene (WT vs KO) × 2 treatment (TRT) (no vs yes) ANOVAs with interaction were used to determine the effect of palmitate on NLRP3 and IL1β. A Bonferroni post-hoc multiple comparison test was used for significant interactions. Significance for all tests was determined at alpha = 0.05.
RESULTS
HFD/palmitate triggered ER-stress markers in retina and Müller cells
Several studies showed that HFD or palmitate triggers ER-stress in different organs and cell types[17,22-24]. Therefore, we checked the levels of various ER-stress markers in the retina isolated from mice fed with HFD, and rMC1 treated with palmitate. HFD for 8 wk induced obesity and impaired glucose tolerance indicated by an increase in body weights (Figure 1A) and glucose levels (Figure 1B) across the different time points compared to ND. We also found that HFD induced an increase in XBPS1 and ATF6 mRNA levels only, while, there was no change in XBP1, PERK, CHOP and IRE1α (Figure 1C). In order to study the role of Müller cells in HFD-induced inflammation, rMC-1 were treated with 400 μmol/L palmitate coupled to bovine serum albumin (Pal-BSA) for 6hr. Palmitate; a saturated fatty acid that is increased in plasma following a HFD[25]; significantly upregulated IRE1α, PERK, ATF6 and CHOP (Figure 1D).
Figure 1.
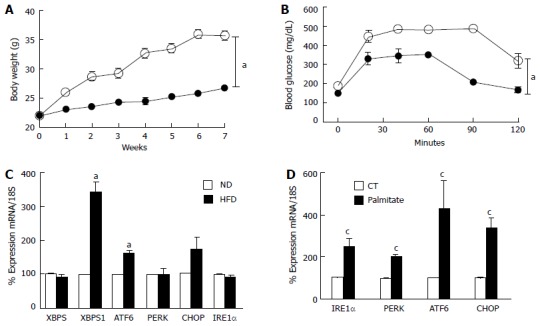
High fat diet/palmitate triggered endoplasmic-reticulum-stress markers in retina and Müller cells. A: Total body weight (grams) recorded weekly was significantly higher in mice fed with HFD for 8 wk compared to ND; B: Glucose tolerance was impaired after 8 wk of HFD compared to ND; C: Realtime PCR showing increases in mRNA levels of XBP1S and ATF6, while no change in XBP1, PERK, CHOP and IRE1α mRNA in retina after 8 wk of HFD compared to ND; D: Realtime PCR showing significant increases in IRE1α, PERK, ATF6 and CHOP mRNA levels in rMC1 treated with palmitate compared to control (CT) (aP < 0.05 vs ND, n = 3-4 and cP < 0.05 vs CT, area under the curve across all the time points was calculated, n = 3-4). HFD: High fat diet; PERK: Protein Kinase RNA-like endoplasmic-reticulum kinase; XBP: Xbox binding protein; ATF6: Activating transcription factor 6; CHOP: CCAAT-enhancer-binding protein homologous protein; IRE1: Inositol requiring enzyme 1.
HFD/palmitate induced TXNIP upregulation and miR-17-5p dysregulation in retina and Müller cells
Our lab has previously reported that HFD and palmitate can induce TXNIP mRNA expression in whole retina and retina endothelial cells respectively[4]. However, the upstream events by which HFD/palmitate trigger TXNIP expression are still unclear. In agreement with the previous study, we found that 8 wk of HFD and palmitate led to an upregulation of TXNIP mRNA levels in whole retina and Müller cells (Figure 2). These results were associated with miR-17-5p dysregulation in both whole retina and Müller cells (Figure 2).
Figure 2.
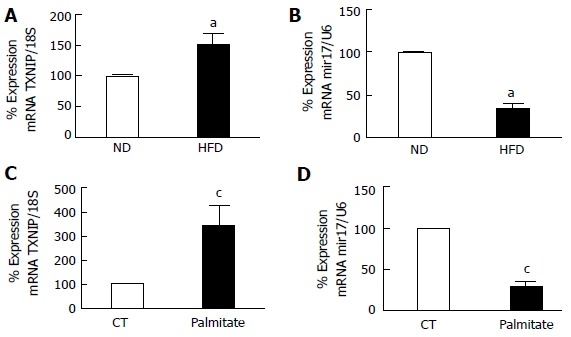
Realtime polymerase chain reaction. It shows significant (A) increase in TXNIP mRNA and (B) miR-17-5p dysregulation in retina after 8 wk of HFD compared to ND. Realtime PCR showing significant (C) increase in TXNIP mRNA levels (D) reduction in miR-17-5p in rMC1 treated with palmitate compared to control (CT) (aP < 0.05 vs ND, n = 3-4 and cP < 0.05 vs CT, n = 3). ND: Normal diet; HFD: High fat diet.
PBA mitigated HFD-mediated ER-stress
To verify the role of ER-stress in HFD-induced TXNIP upregulation, mice were fed either ND or HFD for 2 wk. Then mice were kept on HFD for additional 2 wk while receiving PBA; an ER-stress inhibitor. Body weights were not changed by the HFD or PBA treatment (Figure 3A). However, blood glucose tolerance was significantly less in mice fed with HFD compared to ND after intra-peritoneal glucose tolerance test (Figure 3B). HFD-induced insulin resistance suggested by marked increase in the area under the curve remained unaffected by inhibiting ER-stress with PBA (Figure 3C). HFD for 4 wk induced expression of retinal ER-stress markers mRNA including the RNAse IRE1α, ATF6 and PERK which were restored by PBA treatment to control level (Figure 3D).
Figure 3.
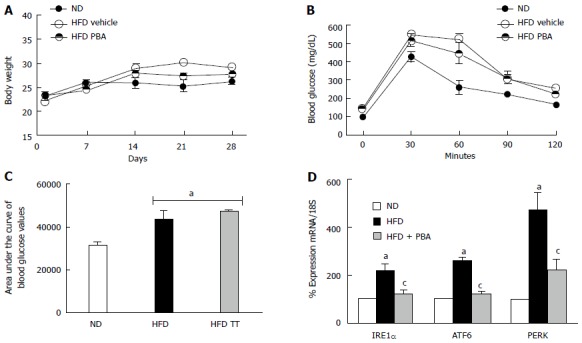
PBA mitigated high fat diet-mediated endoplasmic-reticulum-stress. A: Total body weight (g) recorded weekly for 4 wk was not changed among the different groups; B: Glucose tolerance was impaired after 4 wk of HFD compared to ND, and was not restored with PBA treatment; C: Statistical analysis of area under the curve showing an increase in blood glucose levels in HFD compared to ND, which was not reversed by the treatment; D: Realtime PCR showing significant increases in IRE1α, ATF6, and PERK mRNA levels in mice kept on HFD for 4 wk compared to ND, which were nullified with PBA treatment (aP < 0.05 vs ND, cP < 0.05 vs HFD, n = 3-4). ND: Normal diet; HFD: High fat diet; PBA: Phenyl-butyric acid; PERK: Protein Kinase RNA-like endoplasmic-reticulum kinase; IRE: Inositol requiring enzyme.
ER-stress inhibition prevented HFD-induced TXNIP upregulation and miR-17-5p dysregulation
To establish a causal relationship of the role of ER-stress miR-17-5p and TXNIP expression, we assessed their expression in animals that were treated with ER-stress inhibitor PBA. As shown in Figure 4A, intervention with PBA treatment in HFD partially but significantly increased retinal miR-17-5p compared to untreated HFD. HFD triggered TXNIP mRNA and protein expression compared to ND, which were significantly inhibited in HFD-animals treated with PBA (Figure 4B-D). To establish a causal relationship of the role of ER-stress and activation of IRE1α in palmitate-induced TXNIP expression, rMC1 were treated for 2 h with PBA or IRE1α inhibitor prior to the addition of palmitate. As shown in Figure 4, inhibiting ER-stress or IRE1α markedly reduced the increase in TXNIP protein expression in palmitate-treated cells.
Figure 4.
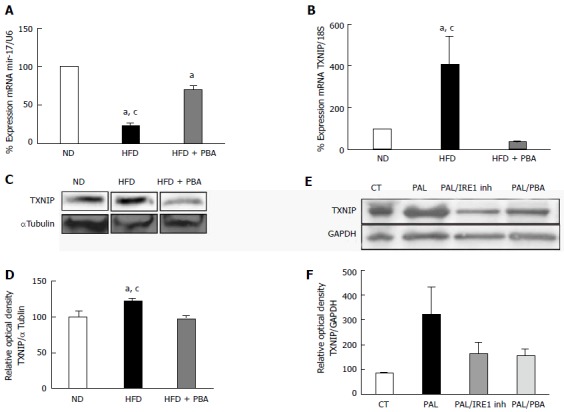
Dysregulation of realtime polymerase chain reaction. It shows significant (A) reduction in miR-17-5p and (B) increase in TXNIP mRNA levels in mice kept on HFD for 4 wk compared to ND, which were reversed with PBA treatment (n = 3-4); C: Representative western blot were cut from the same membrane for TXNIP and αtubulin from retina (D) Statistical analysis showed an upregulation in TXNIP expression in HFD mice compared to ND, PBA treatment nullified this effect (n = 4-5) (aP < 0.05 vs ND, cP < 0.05 vs HFD + PBA) (E) Representative western blot of TXNIP and GAPDH from rMC1 treated with palmitate (pal), after the addition of IRE inhibitor or PBA; D: Statistical analysis showed a trend increase in TXNIP expression, which is reversed by IRE inhibitor or PBA (P = 0.076, n = 3). HFD: High fat diet; ND: Normal diet; PBA: Phenyl-butyric acid; TXNIP: Thioredoxin-interacting protein; CT: Control; PAL: Palmitate; PAL/IRE1: Palmitate + inositol requiring enzyme 1; PAL/PBA: Palmitate + phenyl-butyric acid.
Knocking out TXNIP abolished palmitate induced inflammation in Müller cells
We recently showed that HFD induced expression of TXNIP in Müller cells, which was associated with increased TXNIP-NLRP3 inflammasome interaction as well as the expression of cleaved caspase-1 and IL-1β[4]. Therefore, to dissect the role of TXNIP in palmitate-mediated inflammation in Müller cells, primary Müller cells from both WT and TKO mice were used. Primary Müller cells were serum starved overnight then treated with 400 μmol/L palmitate coupled to bovine serum albumin (Pal-BSA) for 6 h. We found that palmitate led to an increase in NLRP3 and IL1β protein expression in cells isolated from WT but has no effect on cells isolated from TKO mice (Figure 5).
Figure 5.
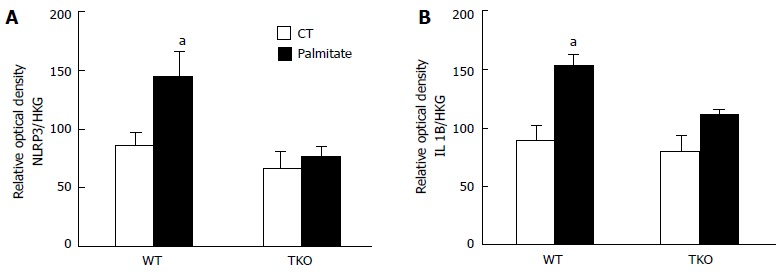
Statistical analysis showed an increase in. (A) NLRP3 and (B) IL1β expression following palmitate treatment in primary Müller cells isolated from WT but no effect on TKO. Actin and α-tubulin were used as housekeeping genes (HKG), to which NLRP3 and IL1β expression was normalized (aP < 0.05 vs ND, n = 3).
DISCUSSION
Central obesity and insulin resistance are hallmarks of metabolic syndrome that comprises dyslipidemia, hypertriglyceridemia, hyperinsulinemia, hypertension, and reduced HDL cholesterol. Changes in lipid profile and accumulation of free fatty acids are highly significant in all forms of diabetes pointing to its possible link with inflammation and vascular complications (reviewed in[26]). Several studies showed the role of free fatty acids mainly palmitate in inducing pro-inflammatory response[27,28]. It should be noted that thorough understanding of the interaction between vascular and non-vascular cells is crucial for the management of retinal dysfunction. Müller cells are the principal glial cell found in the retina, which span the entire retinal layers and considered as resident innate immune cells (reviewed in[29]). Because of their unique morphology, Müller cells are considered a signaling hub that senses minute changes in retinal milieu, connecting retinal neuronal with retinal endothelial cells. In the current study we were interested in unraveling the mechanisms through which HFD leads to retinal inflammation. We also highlighted the critical role of Müller cells after the insult with the free fatty acid palmitate, which hasn’t been reported so far. The main findings of this study are that (1) HFD or palmitate induced ER-stress dysregulates miR-17-5p in retina and Müller cells; (2) ER-stress triggers TXNIP expression in retina and Müller cells and (3) amplified TXNIP levels activate NLRP3, which contributes to inflammation.
Müller cells are considered major sources of inflammatory mediators, which become activated in response to various insults[19,30-32]. We and others have shown the increase of TXNIP expression in glial Müller cells due to chronic hyperglycemia[33-35] or HFD[4]. TXNIP is a physiological inhibitor of the thioredoxin system, which is one of the main antioxidant defense mechanisms in our body. TXNIP acts via binding to thioredoxin, making it unable to bind with other proteins (reviewed in[36]). In addition to the ability of TXNIP in inducing inflammatory cytokines via activating nuclear factor κB, it can act as a direct activator of NOD-like receptor protein (NLRP3)[34,37]. NLRP3-inflammasome is a component of the innate immune system responsible for initiating obesity-induced inflammation[38]. TXNIP-NLRP3 interaction results in NLRP3 complex assembly and auto-activation of caspase-1, which eventually processes pro-IL1β into its mature form leading to inflammation[38,39]. Recent studies showed that HFD and palmitate trigger ER-stress in various organs and cell types[17,22-24]. However, the link between HFD/palmitate-induced ER-stress and TXNIP expression in Müller cells is yet to be determined. Here, we observed significant activation of the unfolded protein response ER-stress chaperons in retinas from 8-wk HFD mice (Figure 1). We also observed no difference in mRNA level of IRE1α an ER-stress marker and a bifunctional kinase/Rnase in HFD. However, there was an increase in the splicing of XBP1; IRE1α downstream target; evident by 3.5-fold increase in spliced XBP-1 in HFD compared to ND, which suggests IRE1α activation. Interestingly, treatment of Müller cells with palmitate; one of the most abundant saturated fatty acids in plasma that is significantly increased following HFD[25]; led to an increase in all ER-stress markers at the mRNA level including IRE1α (Figure 1). Among UPR pathways, IRE1α has been shown to degrade key cell regulators such as the neuronal cue, netrin in the retina[39,40] and miR-17-5p in pancreatic beta-cells[10,11]. MiR-17-5p is a small noncoding RNAs that binds predominantly to the 3′-UTR of TXNIP leading to down-regulation of its expression[10]. Indeed, HFD and palmitate resulted in a significant decrease in miR-17-5p in the total retina and Müller cells, respectively, an effect that coincided with TXNIP upregulation (Figure 2). These findings support the link between HFD, ER-stress and TXNIP upregulation in Müller cells.
Epidemiological studies showed a significant reduction in miR-17-5p in omental fat and blood from obese non-diabetic subjects compared to lean subjects[41,42]. In the current study, we showed that HFD or palmitate dysregulated miR-17-5p in retina and Müller cells (Figure 2). Interestingly, retinal miR-17-5p expression is not affected by hyperglycemia or diabetes compared to normal glycemic controls (data not shown). In agreement, Lerner et al[10] reported similar insensitivity of miR-17-5p to high glucose treatment in pancreatic beta cells. These findings shed light on the selective sensitivity of miR-17-5p to degrade in response to HFD and palmitate. Taken together, our findings suggest that HFD-induced ER-stress uniquely triggers TXNIP expression via dysregulating miR-17-5p.
To dissect the role of ER-stress in regulating TXNIP expression, PBA was added to cultured rMC1 prior to palmitate treatment. PBA is an FDA approved drug for the clinical management of urea cycle disorder. PBA is a chemical chaperone that stabilizes protein conformation and in turns ER-folding (reviewed in[43,44]). Indeed, treating the cells with PBA a general ER-stress inhibitor showed a trend decrease in TXNIP expression. Similar findings were obtained by the use of a selective IRE1α inhibitor (Figure 4). However, the observed reduction didn’t reach significance, which could be due to the small sample size. We overcame this limitation, by treating mice kept on HFD with PBA for 2 wk. We showed that inhibiting ER-stress significantly blunted the increase in TXNIP observed in HFD group (Figure 4), without altering insulin resistance (Figure 3). Next step we tried to verify the role of TXNIP in inflammatory response in Müller cells. Building on our previous findings that silencing TXNIP reversed palmitate-induced IL1β release and eventually cell death in endothelial cells[4], we isolated primary Müller cells from WT and TKO mice then exposed them to palmitate. We demonstrated that palmitate led to an increase in NLRP3 and IL1β expression in WT and has no effect on TKO (Figure 5), which indicates that TXNIP is responsible for inflammation in Müller cells. These results lend further support to prior findings that manifest the critical role of IL1β in mediating vascular injury in the pathogenesis of diabetic retinopathy. Kowluru et al[45] showed that injecting IL-1β into the vitreous of normal rats increased cell apoptosis similar to what is observed in diabetes. Deletion of IL1β receptor prevented autocrine loop of inflammation[46] and protected retinas from diabetes-induced development of acellular capillaries[47].
In summary, clinical and experimental studies have repeatedly reported the contribution of inflammation to the pathogenesis of diabetic retinopathy (reviewed in[48,49]). Similarly, suppression of inflammation has shown protective effects via decreasing leukostasis, blood-retinal barrier breakdown and the acellular capillaries formation[50]. Here, we provide preliminary evidence that exposure to high fat diet and palmitate trigger retinal ER-stress and glial TXNIP expression and render the retina vulnerable to inflammation. Early intervention of ER-stress or TXNIP presents potential therapeutic strategy in obesity-induced inflammation in diabetic retinopathy.
COMMENTS
Background
The authors have previously shown that high fat diet (HFD) induced retinal inflammation and vascular dysfunction. These results were associated with an increase in the thioredoxin interacting protein (TXNIP) at the mRNA and protein level. Here, they examined the mechanisms by which HFD triggers retinal TXNIP and regulates inflammation.
Research frontiers
Currently, there is a great interest to understand how microRNA, the endogenous regulators of transcription can contribute to metabolic disorders. Here, they examined the impact of HFD or the free fatty acid palmitate on microRNA; miR-17-5p as it has been shown to regulate TXNIP mRNA expression. This study demonstrates the effect of HFD-induced obesity on degradation of miR-17-5p via activation of the ER-stress mediators including the inositol requiring enzyme 1α (IRE1α). The authors also demonstrate that inhibiting ER-stress can restore miR-17-5p and TXNIP levels and hence inflammation back to comparable levels seen in normal controls.
Innovations and breakthroughs
The results of their study delineate the contribution of Müller cells, main glia in the retina in palmitate-mediated retinal inflammation. They identify ER-stress as new therapeutic target that is involved in obesity-induced inflammation in pre-diabetic retinopathy.
Applications
Their results suggest that inhibitors of ER-stress reversed the increase in TXNIP in vivo and in Müller cells, the main glia in the retina. The findings of their short-term study support the interventional use of the ER-Stress inhibitor PBA, FDA approved drug with high safety profile. This report should open the door for its future studies in diseases associated with TXNIP-NLRP3 inflammation.
Terminology
MicroRNAs are small non-coding RNAs that contribute to the post-transcriptional regulation of various genes expressions. Inflammasome is a multiprotein oligomer responsible for the induction of inflammatory process. Unfolded protein response (UPR) is unfolded protein response, an adaptive mechanism to resolve and slow down protein processing. ER-stress is when the endoplasmic reticulum capacity to deal with UPR is overwhelmed then stress markers such as ATF6, PERK and IRE1α are expressed.
Peer-review
The paper is interesting.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Charlie Norwood VA Medical Center Institutional Review Board.
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Association for Research in Vision and Ophthalmology statement for use of animals in ophthalmic and vision research, and Charlie Norwood VA Medical Center Animal Care and Use Committee (ACORP#15-04-080).
Conflict-of-interest statement: There is no conflict of interest.
Data sharing statement: Data will be available upon request.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: September 6, 2016
First decision: September 29, 2016
Article in press: December 14, 2016
P- Reviewer: Liou GI, Mungrue IN, Sychrova H S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong TY, Duncan BB, Golden SH, Klein R, Couper DJ, Klein BE, Hubbard LD, Sharrett AR, Schmidt MI. Associations between the metabolic syndrome and retinal microvascular signs: the Atherosclerosis Risk In Communities study. Invest Ophthalmol Vis Sci. 2004;45:2949–2954. doi: 10.1167/iovs.04-0069. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen TT, Wong TY. Retinal vascular manifestations of metabolic disorders. Trends Endocrinol Metab. 2006;17:262–268. doi: 10.1016/j.tem.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Mohamed IN, Hafez SS, Fairaq A, Ergul A, Imig JD, El-Remessy AB. Thioredoxin-interacting protein is required for endothelial NLRP3 inflammasome activation and cell death in a rat model of high-fat diet. Diabetologia. 2014;57:413–423. doi: 10.1007/s00125-013-3101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng H, Gu J, Gou F, Huang W, Gao C, Chen G, Long Y, Zhou X, Yang M, Liu S, et al. High Glucose and Lipopolysaccharide Prime NLRP3 Inflammasome via ROS/TXNIP Pathway in Mesangial Cells. J Diabetes Res. 2016;2016:6973175. doi: 10.1155/2016/6973175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao P, He FF, Tang H, Lei CT, Chen S, Meng XF, Su H, Zhang C. NADPH oxidase-induced NALP3 inflammasome activation is driven by thioredoxin-interacting protein which contributes to podocyte injury in hyperglycemia. J Diabetes Res. 2015;2015:504761. doi: 10.1155/2015/504761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Zhang JH, Chen XY, Hu QH, Wang MX, Jin R, Zhang QY, Wang W, Wang R, Kang LL, et al. Reactive oxygen species-induced TXNIP drives fructose-mediated hepatic inflammation and lipid accumulation through NLRP3 inflammasome activation. Antioxid Redox Signal. 2015;22:848–870. doi: 10.1089/ars.2014.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 9.McClelland AD, Kantharidis P. microRNA in the development of diabetic complications. Clin Sci (Lond) 2014;126:95–110. doi: 10.1042/CS20130079. [DOI] [PubMed] [Google Scholar]
- 10.Lerner AG, Upton JP, Praveen PV, Ghosh R, Nakagawa Y, Igbaria A, Shen S, Nguyen V, Backes BJ, Heiman M, et al. IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 2012;16:250–264. doi: 10.1016/j.cmet.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Upton JP, Wang L, Han D, Wang ES, Huskey NE, Lim L, Truitt M, McManus MT, Ruggero D, Goga A, et al. IRE1α cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase-2. Science. 2012;338:818–822. doi: 10.1126/science.1226191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chistiakov DA, Sobenin IA, Orekhov AN, Bobryshev YV. Role of endoplasmic reticulum stress in atherosclerosis and diabetic macrovascular complications. Biomed Res Int. 2014;2014:610140. doi: 10.1155/2014/610140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunys J, Duplan E, Checler F. The transcription factor X-box binding protein-1 in neurodegenerative diseases. Mol Neurodegener. 2014;9:35. doi: 10.1186/1750-1326-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma JH, Wang JJ, Zhang SX. The unfolded protein response and diabetic retinopathy. J Diabetes Res. 2014;2014:160140. doi: 10.1155/2014/160140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139–143. doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu Y, Qian L, Zhang Q, Chen B, Gui L, Huang D, Chen G, Chen L. Palmitate induces apoptosis in mouse aortic endothelial cells and endothelial dysfunction in mice fed high-calorie and high-cholesterol diets. Life Sci. 2013;92:1165–1173. doi: 10.1016/j.lfs.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Dai F, Jiang T, Bao YY, Chen GJ, Chen L, Zhang Q, Lu YX. Fenofibrate improves high-fat diet-induced and palmitate-induced endoplasmic reticulum stress and inflammation in skeletal muscle. Life Sci. 2016;157:158–167. doi: 10.1016/j.lfs.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Sarthy VP, Brodjian SJ, Dutt K, Kennedy BN, French RP, Crabb JW. Establishment and characterization of a retinal Müller cell line. Invest Ophthalmol Vis Sci. 1998;39:212–216. [PubMed] [Google Scholar]
- 19.Mysona BA, Al-Gayyar MM, Matragoon S, Abdelsaid MA, El-Azab MF, Saragovi HU, El-Remessy AB. Modulation of p75(NTR) prevents diabetes- and proNGF-induced retinal inflammation and blood-retina barrier breakdown in mice and rats. Diabetologia. 2013;56:2329–2339. doi: 10.1007/s00125-013-2998-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali TK, Matragoon S, Pillai BA, Liou GI, El-Remessy AB. Peroxynitrite mediates retinal neurodegeneration by inhibiting nerve growth factor survival signaling in experimental and human diabetes. Diabetes. 2008;57:889–898. doi: 10.2337/db07-1669. [DOI] [PubMed] [Google Scholar]
- 22.Gulhane M, Murray L, Lourie R, Tong H, Sheng YH, Wang R, Kang A, Schreiber V, Wong KY, Magor G, et al. High Fat Diets Induce Colonic Epithelial Cell Stress and Inflammation that is Reversed by IL-22. Sci Rep. 2016;6:28990. doi: 10.1038/srep28990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li SJ, Liu CH, Chu HP, Mersmann HJ, Ding ST, Chu CH, Wang CY, Chen CY. The high-fat diet induces myocardial fibrosis in the metabolically healthy obese minipigs-The role of ER stress and oxidative stress. Clin Nutr. 2016 doi: 10.1016/j.clnu.2016.06.002. Jun 16; Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Gwiazda KS, Yang TL, Lin Y, Johnson JD. Effects of palmitate on ER and cytosolic Ca2+ homeostasis in beta-cells. Am J Physiol Endocrinol Metab. 2009;296:E690–E701. doi: 10.1152/ajpendo.90525.2008. [DOI] [PubMed] [Google Scholar]
- 25.Paik JS, Cho WK, Oh EH, Lee SB, Yang SW. Palmitate induced secretion of IL-6 and MCP-1 in orbital fibroblasts derived from patients with thyroid-associated ophthalmopathy. Mol Vis. 2012;18:1467–1477. [PMC free article] [PubMed] [Google Scholar]
- 26.Jaiswal M, Schinske A, Pop-Busui R. Lipids and lipid management in diabetes. Best Pract Res Clin Endocrinol Metab. 2014;28:325–338. doi: 10.1016/j.beem.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Staiger H, Staiger K, Stefan N, Wahl HG, Machicao F, Kellerer M, Häring HU. Palmitate-induced interleukin-6 expression in human coronary artery endothelial cells. Diabetes. 2004;53:3209–3216. doi: 10.2337/diabetes.53.12.3209. [DOI] [PubMed] [Google Scholar]
- 28.Krogmann A, Staiger K, Haas C, Gommer N, Peter A, Heni M, Machicao F, Häring HU, Staiger H. Inflammatory response of human coronary artery endothelial cells to saturated long-chain fatty acids. Microvasc Res. 2011;81:52–59. doi: 10.1016/j.mvr.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Goldman D. Müller glial cell reprogramming and retina regeneration. Nat Rev Neurosci. 2014;15:431–442. doi: 10.1038/nrn3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali TK, Al-Gayyar MM, Matragoon S, Pillai BA, Abdelsaid MA, Nussbaum JJ, El-Remessy AB. Diabetes-induced peroxynitrite impairs the balance of pro-nerve growth factor and nerve growth factor, and causes neurovascular injury. Diabetologia. 2011;54:657–668. doi: 10.1007/s00125-010-1935-1. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Ye F, Xiong H, Hu DN, Limb GA, Xie T, Peng L, Zhang P, Wei Y, Zhang W, et al. IL-1β induces IL-6 production in retinal Müller cells predominantly through the activation of p38 MAPK/NF-κB signaling pathway. Exp Cell Res. 2015;331:223–231. doi: 10.1016/j.yexcr.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 32.Mizutani M, Gerhardinger C, Lorenzi M. Müller cell changes in human diabetic retinopathy. Diabetes. 1998;47:445–449. doi: 10.2337/diabetes.47.3.445. [DOI] [PubMed] [Google Scholar]
- 33.Devi TS, Hosoya K, Terasaki T, Singh LP. Critical role of TXNIP in oxidative stress, DNA damage and retinal pericyte apoptosis under high glucose: implications for diabetic retinopathy. Exp Cell Res. 2013;319:1001–1012. doi: 10.1016/j.yexcr.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perrone L, Devi TS, Hosoya KI, Terasaki T, Singh LP. Inhibition of TXNIP expression in vivo blocks early pathologies of diabetic retinopathy. Cell Death Dis. 2010;1:e65. doi: 10.1038/cddis.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devi TS, Lee I, Hüttemann M, Kumar A, Nantwi KD, Singh LP. TXNIP links innate host defense mechanisms to oxidative stress and inflammation in retinal Muller glia under chronic hyperglycemia: implications for diabetic retinopathy. Exp Diabetes Res. 2012;2012:438238. doi: 10.1155/2012/438238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chong CR, Chan WP, Nguyen TH, Liu S, Procter NE, Ngo DT, Sverdlov AL, Chirkov YY, Horowitz JD. Thioredoxin-interacting protein: pathophysiology and emerging pharmacotherapeutics in cardiovascular disease and diabetes. Cardiovasc Drugs Ther. 2014;28:347–360. doi: 10.1007/s10557-014-6538-5. [DOI] [PubMed] [Google Scholar]
- 37.Al-Gayyar MM, Abdelsaid MA, Matragoon S, Pillai BA, El-Remessy AB. Thioredoxin interacting protein is a novel mediator of retinal inflammation and neurotoxicity. Br J Pharmacol. 2011;164:170–180. doi: 10.1111/j.1476-5381.2011.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 40.Binet F, Mawambo G, Sitaras N, Tetreault N, Lapalme E, Favret S, Cerani A, Leboeuf D, Tremblay S, Rezende F, et al. Neuronal ER stress impedes myeloid-cell-induced vascular regeneration through IRE1α degradation of netrin-1. Cell Metab. 2013;17:353–371. doi: 10.1016/j.cmet.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Klöting N, Berthold S, Kovacs P, Schön MR, Fasshauer M, Ruschke K, Stumvoll M, Blüher M. MicroRNA expression in human omental and subcutaneous adipose tissue. PLoS One. 2009;4:e4699. doi: 10.1371/journal.pone.0004699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heneghan HM, Miller N, McAnena OJ, O’Brien T, Kerin MJ. Differential miRNA expression in omental adipose tissue and in the circulation of obese patients identifies novel metabolic biomarkers. J Clin Endocrinol Metab. 2011;96:E846–E850. doi: 10.1210/jc.2010-2701. [DOI] [PubMed] [Google Scholar]
- 43.Welch WJ, Brown CR. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones. 1996;1:109–115. doi: 10.1379/1466-1268(1996)001<0109:iomacc>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolb PS, Ayaub EA, Zhou W, Yum V, Dickhout JG, Ask K. The therapeutic effects of 4-phenylbutyric acid in maintaining proteostasis. Int J Biochem Cell Biol. 2015;61:45–52. doi: 10.1016/j.biocel.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 45.Kowluru RA, Odenbach S. Role of interleukin-1beta in the development of retinopathy in rats: effect of antioxidants. Invest Ophthalmol Vis Sci. 2004;45:4161–4166. doi: 10.1167/iovs.04-0633. [DOI] [PubMed] [Google Scholar]
- 46.Yego EC, Vincent JA, Sarthy V, Busik JV, Mohr S. Differential regulation of high glucose-induced glyceraldehyde-3-phosphate dehydrogenase nuclear accumulation in Müller cells by IL-1beta and IL-6. Invest Ophthalmol Vis Sci. 2009;50:1920–1928. doi: 10.1167/iovs.08-2082. [DOI] [PubMed] [Google Scholar]
- 47.Vincent JA, Mohr S. Inhibition of caspase-1/interleukin-1beta signaling prevents degeneration of retinal capillaries in diabetes and galactosemia. Diabetes. 2007;56:224–230. doi: 10.2337/db06-0427. [DOI] [PubMed] [Google Scholar]
- 48.Adamis AP. Is diabetic retinopathy an inflammatory disease? Br J Ophthalmol. 2002;86:363–365. doi: 10.1136/bjo.86.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30:343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]


