Abstract
Background:
Acute myocardial infarction (MI) is associated with high mortality and among survivors have high morbidity. Electrocardiogram (ECG), a cost-effective and easily available, has traditionally been used not only just for diagnosis of MI but also for culprit vessel recognition and for prognostication. However, the role of lead augmented vector right (aVR) and leads V7–V9 in acute MI are often neglected in clinical practice. We studied the role of lead aVR and leads V7–V9 in ST-elevation MI (STEMI) patients.
Methods:
A total of 209 patients presenting with STEMI were enrolled in the study. History of comorbid conditions and habits was enquired. Routine blood tests were performed. Full spectrum ECG (including V7–9) and 2D-ECHO was performed on all patients. All the patients underwent revascularization by primary percutaneous coronary intervention. The role of lead aVR, lead V7, and leads V8–9 was analyzed in anterior wall MI (AWMI) and inferior wall MI. All the patients were followed up for 1 month for outcome assessment.
Results:
Of the 209 patients, 85.1% were males and 35.8% were diabetic, 60.2% were smokers, AWMI accounted for 55.5%. Lead aVR ST deviation was noted in 75.1% of patients (elevation in 17.7% and depression in 47.1%). V7 ST elevation occurred in 27.6% and V8–9 elevation occurred in 7.5% of the study population. Total death was 11.9% in the study (including the in-hospital mortality), all these patients had lead aVR ST segment deviation (P < 0.001).
Conclusion:
Lead aVR ST deviation and Lead V7 ST deviation helps to prognosticate the STEMI patients as high risk and those with aVR ST depression had higher mortality compared to aVR ST elevation because of larger myocardial involvement.
Key words: Lead augmented vector right, leads V7–9, outcome, primary percutaneous coronary intervention, ST deviation, ST-elevation myocardial infarction
INTRODUCTION
In the standard 12-lead electrocardiogram (ECG) some parts of the heart are under-represented. Present lead positions only represent the anterior surface and the inferior surface of heart but not the posterior and the apical segments. The vector of lead augmented vector right (aVR) is orientated approximately from the cardiac apex to the outflow tract of the right ventricle. Directionally, lead aVR is as much reciprocal to V5 (facing the antero-apical region) as it is to V7 (facing the inferior-apical region).[1] Transmural infarction in the basal interventricular septum may produce ST elevation in aVR when the injury current dipole has a component in the direction of the vector of lead aVR. This injury current dipole induces ST depression in left lateral chest leads V5–V7 and in leads I and II because these leads are reciprocal to aVR, in that their lead vectors have components in a direction opposite to that of aVR. Likewise, a transmural apical infarction can produce aVR ST depression. Since the lead V7 records changes from the inferior cardiac apex, which is not available on the standard 12-lead ECG, ST segment changes in the often neglected lead aVR becomes particularly relevant.[2] The unique nonanterior and noninferior direction of lead aVR means that the prognostic implications of ST deviations (elevation or depression) could be different depending on the infarct location. In particular, aVR ST depression can be reflective of ST elevation in V7 that the standard 12-lead ECG fails to capture.[3] The standard chest leads cover the anterior part of the chest only up to the midaxillary line (lead V6), whereas V7 lead placed at the posterior axillary line will also record ST elevation from a larger apical infarction due to an occlusion of a left anterior descending artery with a larger distribution to the inferior portion of the cardiac apex. Deeper aVR ST depression thus reflects higher STGJ elevation not only in leads V5 and V6 but also in lead V7 and signifies a bigger apical infarction. However, V7 is not a “standard” ECG lead and is not commonly recorded. The literature on its utility is also relatively nonuniform.[4] The major obstacle in the ECG diagnosis of posterior infarction lies in the absence of standard leads facing the posterior left ventricular wall.[5] The chest leads V7–V9 identifies those patients with concomitant posterior wall involvement,[6] as the ST depression in the precordial chest leads are neither specific nor sensitive for the diagnosis of posterior infarction.[7,8] However, these leads (V7–V9) are rarely used in clinical practice.
METHODS
Study design
A prospective study conducted in Sri Jayadeva Institute of Cardiovascular Sciences and Research, Bengaluru, India.
Study population
Includes patients both male and female aged 18 years and above presenting with ischemic chest pain with ECG diagnosis of ST-elevation acute myocardial infarction (MI).
Inclusion criteria
Patients both male and female age of 18 years and above
Chest pain with ST elevation MI <12 h duration
New onset Left bundle branch Block
On-going chest pain with ST elevation MI >12 h duration
Patients undergoing primary percutaneous coronary intervention (PCI).
Exclusion criteria
Non-ST-elevation MI
History of cerebrovascular accident (hemorrhagic within 3 months)
Patients thrombolyzed elsewhere.
Study method
Patients baseline characteristics involving age, sex, body mass index, diabetes (on treatment or fasting blood sugar (FBS) >120), hypertension (on treatment/two readings >140/90 mm of Hg), smoking (current/ex-smoker), alcohol are taken into consideration. The onset of symptoms to first medical contact, first medical contact to referral to tertiary center for further management, door to balloon time was noted. All the patients underwent primary PCI.
INVESTIGATIONS AND PROCEDURES
Blood investigations
Blood investigations including a complete hemogram, renal function test, FBS and fasting lipid profile (12 h overnight fasting), serology were performed.
Electrocardiogram
All the patients will undergo 12 lead ECG with additional leads of V7, V8, V9 as per the protocol for lead placement. The amount of ST-segment change was measured to the nearest 0.5 mm at 60 ms after the J-point on all 12 leads including aVR and V7, V8, V9 (0.5 mm of ST deviation representing a change of 50 μV). Any aVR ST depression/elevation of 0.5 mm or more and V7, V8, V9 ST elevation of 0.5 mm was considered as significant.
Echocardiogram
To assess for LV function, regional wall motion abnormality, chamber enlargement, pericardial effusion and any other structural abnormality.
Coronary angiogram and angioplasty
All patients who gave consent for primary PCI underwent coronary angiography followed by percutaneous transluminal coronary angioplasty/stenting of the culprit vessel. The stent used was either DES or BMS (as per the consent). Revascularization was considered successful if TIMI III flow is established, resolution of symptoms and resolution of ST segment by >50%.
Premedications
All the patients received aspirin 325 mg bolus dose and a P2Y12 inhibitor either 600 mg of clopidogrel or 60 mg of prasugrel or 180 mg of ticagrelor and 80 mg of atorvastatin as per the ACC/AHA guidelines.[9] Heparin and GP2b3a inhibitor (eptifibatide) as per the cardiologist's discretion.
Medications postprocedure
All the patients received maintenance dose of aspirin, clopidogrel/prasugrel/ticagrelor, 12 h infusion of GP2b3a inhibitor. Other medications such as angiotensin-converting enzyme inhibitor, angiotensin receptor blocker's, diuretics, and beta-blockers were added if there were no contraindications.
Follow-up
All the patients were followed up for 30 days for outcome assessment.
Outcome/end point
Primary endpoint: All cause 30 days mortality. Secondary endpoint: Rehospitalization within 30 days either for heart failure, reinfarction, or other morbidity.
RESULTS
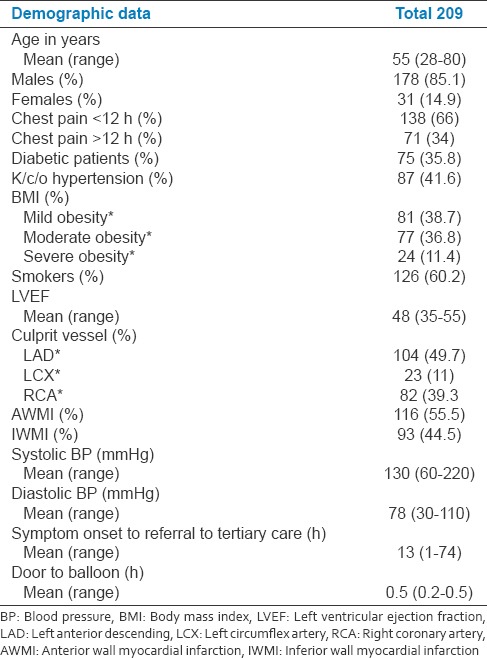
The baseline demographic profile of the patients studied is represented in Table 1. The mean age was 55 years with the range of 28–80 years. Of the total 209 patients, 85.1% of patients were male. About 66% of patients had chest pain <12 h, diabetic accounted for 35.8% and hypertensive patients were 41.6% of the total study population. Anterior wall MI (AWMI) accounted for 55.5% and 45.5% were inferior wall MI (IWMI). LAD was the culprit vessel in 49.7% of patients.
Table 1.
Basic demographic data
DISCUSSION
Acute MI is associated with high mortality and among the survivors have high morbidity. These events have been related to the time duration from symptom onset to recanalization of the occluded vessel. Higher the time elapsed, worse the outcome. Delay in revascularization may lead to greater myocardial loss and more significant ventricular dysfunction and recurrent hospitalizations for heart failure. Because of varying symptoms of acute MI ranging from classical symptoms of MI to atypical symptoms to silent MI, clinical recognition of the acute event is important and challenging. Misdiagnosis of the clinical symptoms or neglecting the subtle complaints without, proper investigation can be fatal to the patients. Myocardial survival depends on the timely recognition and effective management. One of the simplest easily available and cost effective investigations is an electrocardiogram. ECG has traditionally been used not just for the diagnosis for the acute MI but also for the recognition of the culprit vessel involved as well as, for the prognosis (e.g., presence of QRBBB pattern, the presence of complete heart block in AWMI, etc., are associated with higher mortality). However, the technical difficulty is the recognition of the ECG changes by the primary health care providers and timely initiation of treatment and early referral to tertiary care hospital for further management. There are certain pitfalls in the 12 lead ECG such as the role of lead aVR ST-segment deviation without ST elevation in precordial leads and role of ST depression in V1 and V2 leads with tall R wave and upright T wave (indicative of posterior wall MI). Lead V1 and V2 represents mirror image of the posterior leads (V7–V9) which do not represent in the regular 12 lead ECG and are infrequently ordered. This may result in failure to recognize an isolated posterior wall MI. Prior studies have evaluated the role of Lead aVR and ST deviation in patient presenting with acute coronary syndrome (ACS). There are also studies which have evaluated the role of V7–V9 leads ST deviation in patients presenting with ACS. In some of these studies, patient received only thrombolytic therapy and few received primary PCI. In this study, we combined both aVR lead and lead V7–V9 ST segment deviation in patient presenting with acute MI [Tables 2a–c]. The study population who underwent primary PCI were included and evaluated. In this study, we found that lead aVR and V7 correlate with each other more often. Lead V8 and V9 correlated more with the posterior wall involvement rather than lead V7. We first evaluated the association of aVR ST deviation with ST-elevation MI (STEMI) [Table 3] and found that aVR ST deviation was associated with higher mortality [Table 4] compared with normal aVR in STEMI, which was statistically significant. We further evaluated aVR ST elevation versus aVR ST depression and found that aVR ST depression was associated with higher mortality than aVR ST elevation. We then assessed the importance of ECG leads V7–V9 in the study population mainly patients with IWMI. There was a statistically significant correlation of V7–V9 leads with STEMI. V7 is more associated with AWMI [Tables 5 and 6], whereas V8–V9 showed more significance with IWMI [Tables 7 and 8]. Two patients who had V8–V9 ST elevation had mortality. Of total 209 patients recruited for the study, the overall mortality was 25 (11.96%). 17 (68%) were in-hospital mortality (IHM) and 8 (32%) occurred within 30 days (reinfarction-3, LVF-2, VT/VF-3). Among the 17 patients who had IHM [Table 9], it was seen that.
Table 2a.
Of the total 209 patients, 17.7% patients had aVR ST elevation and 47.4% of patients had aVR ST depression while 34.9% had no aVR ST deviation. In AWMI group, 25.8% patients had ST elevation and 66.3% patients has ST depression in lead aVR
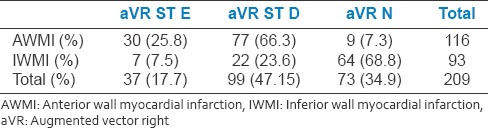
Table 2c.
9.5% of the study population had V8-V9 ST elevation. In IWMI group, 13.9% of patients had associated V8-V9 ST elevation
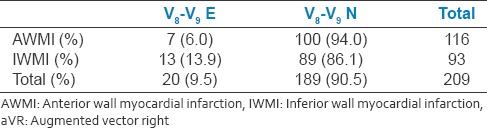
Table 3.
Correlation of lead augmented vector right changes (elevation or depression) with myocardial infarction
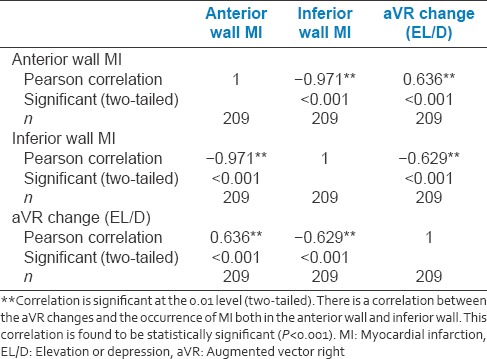
Table 4.
Association between augmented vector right changes and the outcome

Table 5.
Relation between V7 changes and anterior wall myocardial infarction
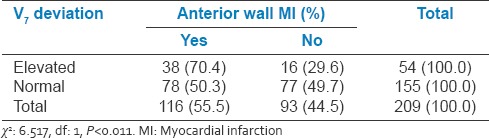
Table 6.
Relation between V7 changes and inferior wall myocardial infarction
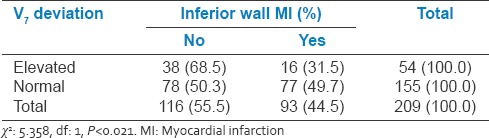
Table 7.
Relation between V8 and V9 changes and anterior wall myocardial infarction
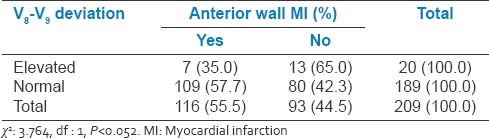
Table 8.
Relation between V8 and V9 changes and inferior wall myocardial infarction
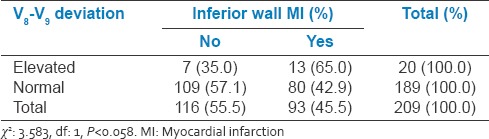
Table 9.
Relation between lead changes and outcome
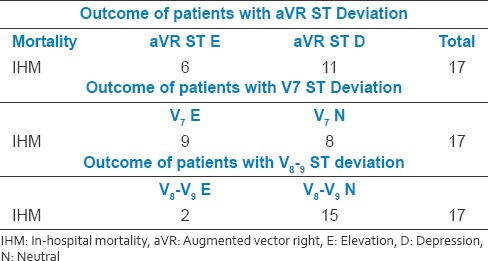
Table 2b.
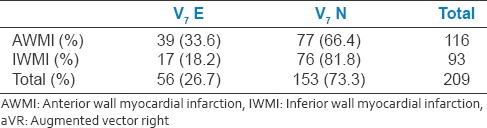
V7 elevation has seen in 26.7% of the study population. In AWMI group, 33.6% of patients had V7 elevation
Lead augmented vector right and in hospital mortality
6 (35.3%) had aVR ST elevation
11 (64.7%) had aVR ST depression.
Lead V7 and coronary angiography
9 (52.94%) had V7 elevation
8 (47.05%) had V7 depression.
Lead V8–V9 and coronary angiography
2 (11.76%) had V8–V9 elevation
15 (88.23%) had V8–V9 depression.
Our study showed that patients with aVR ST depression had higher mortality 11 (64.7%) than patients with aVR STelevation6 (35.3%). This is also true with lead V7 which is facing reciprocally to lead aVR.
Our study correlated with the study conducted by Wong et al.,[1] supports the above showing aVR ST depression was associated with higher 30 days mortality for anterior but not for inferior acute MI.
The create registry by Dewis Xvier et al.[10] which was a multicenter study carried out in Indian patients during 2001–2005. We correlated our study results with that of create registry [Table 10] and is shown below:
Table 10.
Comparison of our study with create registry
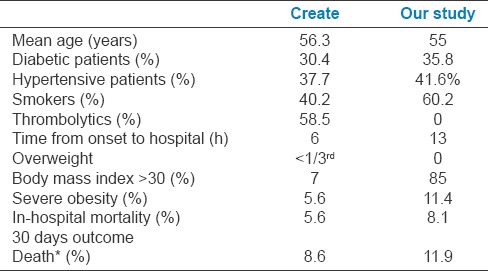
We found that in our study the IHM was higher compared to create registry. This may be due to various reasons such as higher diabetic patients, hypertensive patients, and higher number of smokers. Gross increases in obesity with 11.1% severe obesity and also added to these is the delay in arrival to the hospital. These may be attributed to the changing lifestyle and food habits of the population over the past one decade since the create registry to our study.
With acute AWMI, different mechanisms may explain the bad prognosis associated with aVR ST elevation (basal septal infraction from a culprit left coronary descending artery stenosis before the first septal artery potentially involving the left main artery) and that associated with aVR ST depression (large apical area involvement) when compared with patient with a neutral aVR ST level.
Limitations of the study
Small, single-center study population
Patients undergoing primary PCI only were evaluated
Postprocedure ECG and 30 days ECG for the resolution of aVR ST elevations and its correlation with outcome was not evaluated
Different/varying levels of ST elevation and its correlation/impact on mortality was not evaluated.
CONCLUSION
Acute MI is an emergency condition associated with high mortality and morbidity. Early identification and timely intervention can be lifesaving. Lead aVR ST deviation and also Lead V7 (reciprocal to lead aVR) helps to prognosticate the patients as high risk (aVR ST depression had higher risk compared to aVR ST elevation) due to the involvement of large area of myocardium. Posterior leads (V7–V9) should be taken in all ACS patients as to not miss the isolated posterior wall MI. Further larger studies evaluating the role of these posterior leads and its association with the IRA outcome is needed as in aVR lead with lead V7.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wong CK, Gao W, Stewart RA, French JK, Aylward PE, White HD. HERO-Investigators. The prognostic meaning of the full spectrum of aVR ST-segment changes in acute myocardial infarction. Eur Heart J. 2012;33:384–92. doi: 10.1093/eurheartj/ehr301. [DOI] [PubMed] [Google Scholar]
- 2.Pahlm US, Pahlm O, Wagner GS. The standard 11-lead ECG. Neglect of lead aVR in the classical limb lead display. J Electrocardiol. 1996;29(Suppl):270–4. doi: 10.1016/s0022-0736(96)80074-4. [DOI] [PubMed] [Google Scholar]
- 3.Wong CK, Gao W, Stewart RA, Benatar J, French JK, Aylward PE, et al. aVR ST elevation: An important but neglected sign in ST elevation acute myocardial infarction. Eur Heart J. 2010;31:1845–53. doi: 10.1093/eurheartj/ehq161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong CK. Usefulness of leads V7, V8, and V9 ST elevation to diagnose isolated posterior myocardial infarction. Int J Cardiol. 2011;146:467–9. doi: 10.1016/j.ijcard.2010.10.137. [DOI] [PubMed] [Google Scholar]
- 5.Huey BL, Beller GA, Kaiser DL, Gibson RS. A comprehensive analysis of myocardial infarction due to left circumflex artery occlusion: Comparison with infarction due to right coronary artery and left anterior descending artery occlusion. J Am Coll Cardiol. 1988;12:1156–66. doi: 10.1016/0735-1097(88)92594-6. [DOI] [PubMed] [Google Scholar]
- 6.Matetzky S, Freimark D, Chouraqui P, Rabinowitz B, Rath S, Kaplinsky E, et al. Significance of ST segment elevations in posterior chest leads (V7 to V9) in patients with acute inferior myocardial infarction: Application for thrombolytic therapy. J Am Coll Cardiol. 1998;31:506–11. doi: 10.1016/s0735-1097(97)00538-x. [DOI] [PubMed] [Google Scholar]
- 7.Bough EW, Korr KS. Prevalence and severity of circumflex coronary artery disease in electrocardiographic posterior myocardial infarction. J Am Coll Cardiol. 1986;7:990–6. doi: 10.1016/s0735-1097(86)80216-9. [DOI] [PubMed] [Google Scholar]
- 8.Eisenstein I, Sanmarco ME, Madrid WL, Selvester RH. Electrocardiographic and vectorcardiographic diagnosis of posterior wall myocardial infarction. Significance of the T wave. Chest. 1985;88:409–16. doi: 10.1378/chest.88.3.409. [DOI] [PubMed] [Google Scholar]
- 9.Jeffrey L, Anderson, Alice K, Jacobs, Jonathan L, Halperin, et al. ACCF/AHA Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Xavier D, Pais P, Devereaux PJ, Xie C, Prabhakaran D, Reddy KS, et al. Treatment and outcomes of acute coronary syndromes in India (CREATE): A prospective analysis of registry data. Lancet. 2008;371:1435–42. doi: 10.1016/S0140-6736(08)60623-6. [DOI] [PubMed] [Google Scholar]


