Abstract
Background:
Oxidative stress is characterized by increased production of reactive oxygen species resulting in the generation of lipid peroxides such as malondialdehyde (MDA). The studies have shown that ischemia-modified albumin (IMA), which has widely been studied as a marker of ischemia, also increases as result of oxidative stress. Hence, the current study was done to evaluate the serum MDA, IMA along with serum uric acid, and albumin, which are important metabolic antioxidants.
Materials and Methods:
Fifty patients with acute ischemic stroke were taken as cases and compared with 50 age- and sex-matched controls. Serum MDA, IMA, uric acid, and albumin were estimated both in cases and controls. Serum MDA was estimated by the method of Satoh and IMA by Bar-Or et al. The results were analyzed statistically.
Results:
Serum MDA and IMA values were significantly increased in cases (P < 0.0001), whereas serum uric acid and albumin values were significantly decreased (P < 0.05) in comparison to controls. There was also highly significant positive correlation between serum IMA and MDA (r = 0.843,P < 0.0001), whereas there were significant negative correlations between serum IMA and uric acid (r = −0.237,P < 0.05), and albumin (r = −0.326,P < 0.05).
Conclusion:
Hence, we conclude the oxidative stress plays a major role in the etiopathogenesis of acute ischemic stroke, and the deranged oxidant-antioxidant balance further contributes to its severity.
Key words: Acute ischemic stroke, ischemia-modified albumin, malondialdehyde, oxidative stress, reactive oxygen species
INTRODUCTION
Stroke is a worldwide health problem. The WHO has defined stroke as “rapidly developed clinical signs of focal and at times global disturbances of cerebral function lasting more than 24 h or leading to death, with no apparent cause other than vascular origin.” It is one of the leading causes of adult disability and the second most common cause of death.[1,2] Stroke is a major cause of morbidity and mortality in an aging population. In the elderly, ischemic stroke accounts for more than 80% of all stroke cases.[3]
The causes of cellular injury following ischemia are multifactorial, but there is increasing evidence suggesting the role of reactive oxygen species (ROS) in its pathogenesis. Oxidative stress resulting from the generation of ROS is involved in the neuronal damage induced by ischemia and reperfusion, and the antioxidant activity of plasma may be an important factor providing protection from neuronal damage caused by stroke-associated oxidative stress.[4] Ischemia-modified albumin (IMA) is a relatively new marker of ischemia. It is widely been studied in different types of ischemic diseases.[5,6,7] It is a metabolic variant of protein generated during acute ischemic conditions due to a decrease in binding capacity of albumin for transition metals, such as cobalt, nickel, and copper.[8,9] During ischemia and reperfusion, modification altering binding capacity of albumin to transition metals also occurs as a result of oxidative stress.[10]
Taking into consideration of the above facts, this study was done to evaluate the serum malondialdehyde (MDA), IMA, uric acid, and albumin in acute ischemic stroke cases.
MATERIALS AND METHODS
In this study was conducted in the Department of Biochemistry, S.C.B. Medical College, Cuttack, in collaboration with the Department of Medicine. Patients with clinical manifestations of stroke within 24 h of onset of symptoms were considered as cases. Diagnoses were supported by computed tomography (CT)/magnetic resonance imaging (MRI). Those who were having stroke other than ischemic stroke were excluded from the study. Patients having any other type of ischemia, abnormal serum albumin, renal, and cardiac insufficiency were also excluded from the study. Age- and sex-matched healthy volunteers were taken as controls.
Blood was collected from both cases and controls. A detailed history was collected from all. Serum MDA was estimated by the method of Satoh.[11] Serum IMA was estimated using the method of Bar-Or et al. 2000.[12] Other parameters such as serum uric acid and albumin were estimated using Flexor-XL autoanalyzer. The study has been approved by the Institutional Ethics Committee S.C.B Medical College, Cuttack, Odisha.
Statistical analysis
Statistical Analysis was done using software SPSS version 20 and MS Excel. The continuous variables represented as a mean ± standard deviation and categorical data as percentages. Student's unpaired t-test was used to analyze differences between two continuous variables and Chi-square test was used to find out the differences between categorical variables. Relationships between the variables were evaluated using Pearson's correlation coefficient. P <0.05 was considered to be statistically significant.
RESULTS
Fifty acute ischemic stroke cases and 50 age- and sex-matched healthy controls were included in this study. The demographic profiles and risk factors were shown in Table 1. Serum IMA, MDA, uric acid, and albumin values were shown in Table 2. There was a highly significant rise in serum IMA (P < 0.0001) and MDA (P < 0.0001) in acute ischemic stroke cases in comparison to controls. There was also a significant decrease in endogenous antioxidants such as uric acid (P < 0.05) and albumin (P < 0.05) in acute ischemic stroke cases in comparison to controls. The serum IMA values demonstrated a highly significant positive correlation with serum MDA (r = 0.843, P < 0.0001) as shown in Figure 1. Whereas serum IMA demonstrated a significant negative correlation with serum uric acid (r = 0.237, P < 0.05) and albumin (r = −0.326, P < 0.05) as shown in Figures 2 and 3, respectively.
Table 1.
Distribution of demographic profiles and risk factors

Table 2.
Distribution of malondialdehyde, ischemia-modified albumin, uric acid, and albumin
Figure 1.
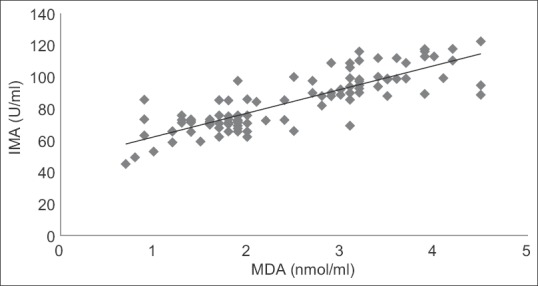
Correlation graph between serum ischemia-modified albumin and malondialdehyde in the study population. r = 0.843, P < 0.0001
Figure 2.

Correlation graph between serum ischemia-modified albumin and uric acid in the study population. r = −0.237, P < 0.05
Figure 3.
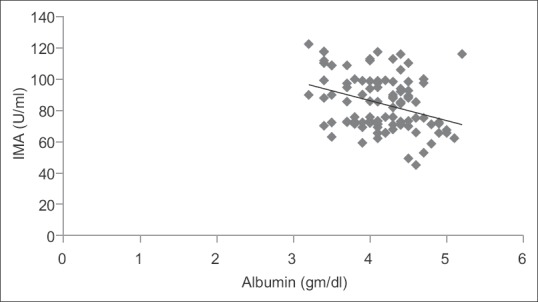
Correlation graph between serum ischemia-modified albumin and albumin in the study population. r = −0.326, P < 0.05
DISCUSSION
There are many studies which indicate that the generation of free radicals leading to oxidative stress plays an important role in the pathogenesis of ischemic brain injury. Brain tissues are prone to the deleterious effects of free radicals for a number of reasons. The brain cellular membrane lipids are very rich in polyunsaturated fatty acid side chains, which are especially sensitive to free radical attacks.[13,14,15] IMA is a nonspecific marker of tissue ischemia, which has been previously, mostly studied in patient with acute chest pain and shown to be increased in patients of myocardial ischemia either spontaneously or subsequent to percutaneous coronary intervention.[5,16] There are also studies showing the association of IMA with ischemic brain injury.[6,17,18] Our study documented highly significant rise (P < 0.0001) in serum IMA and serum MDA, which is a well-known marker for lipid peroxidation and there is also a highly significant positive correlation (r = 0.843, P < 0.0001) between the two parameters (serum IMA and MDA). This finding supports the findings of Behera et al.[5] and Jena et al.[6] Behera et al. estimated the serum IMA and MDA in acute myocardial infarction patients and also found a significant positive correlation between the two. Jena et al. documented a significant rise in serum IMA and MDA in acute cerebral infarction patients with a significant positive correlation between the two.
Modifications within the N-terminus end of albumin, which is an important site for binding heavy metals such as cobalt, can occur either due to acetylation or deletion of amino acid leading to the formation of IMA. With increasing ischemia following acute stroke, there occurs anaerobic metabolism of glucose leading to excess production of lactic acid causing acidosis, which may also lead to IMA formation. Ischemia also produces necrosis by starving neurons of glucose, which in turn leads to failure of mitochondria to produce ATP, which leading to failure of energy-dependant functions of cells such as ion pumps. This energy-dependant pump failure may be reason behind the generation of IMA. During ischemia and reperfusion modification hampering, the binding capacity of albumin for cobalt may occur due to acidosis, decreased oxygen tension, and generation of free radicals, leading to the formation of IMA.[6,12,19,20]
Our study also documented a significant decrease in serum uric acid (P < 0.05) and albumin (P < 0.05) in cerebral ischemia patients in comparison to controls. There is also a significant negative correlation between serum uric acid (P < 0.05) and albumin (P < 0.05) with serum IMA of the study population. The reason behind the decrease in serum uric acid and albumin in acute ischemic stroke in comparison to control is that both of them play an important role as an endogenous metabolic antioxidant in our body. Cherubini et al.[21] in their study demonstrated that majority of antioxidants including uric acid were reduced immediately after an acute ischemic stroke, possibly as a consequence of increased oxidative stress. Kumar et al.[22] in their study ‘Oxidative stress, endogenous antioxidant, and IMA in normolipidemic acute myocardial infarction patients’ had shown decrease in endogenous antioxidants like uric acid, albumin, and bilirubin due to oxidative stress with a simultaneous increase in IMA. Albumin is the most abundant serum protein. However, it may give antioxidant protection by playing a role as serum peroxidase in the presence of reduced glutathione, which is an important intracellular antioxidant.[23] Uric acid, is an end product of purine metabolism, bears significant antioxidant property. It contributes to almost two-thirds of free radical scavenging action in plasma. It is effective in quenching hydroxyl, superoxide, and peroxynitrite radicals and also prevents lipid peroxidation.[24] The negative correlation between serum uric acid and albumin with IMA of study population further supports the fact that imbalance in oxidant and antioxidant status plays an important role in pathophysiology and generation of IMA in ischemic brain injury.
CONCLUSION
Hence, we conclude the oxidative stress plays a major role in the etiopathogenesis of acute ischemic stroke, and the deranged oxidant-antioxidant balance further contributes to its severity. Thus, if the findings of our study are further supported with a wide range of studies in future; then, this IMA may be utilized as a cost-effective biomarker of acute ischemic stroke, particularly in low socioeconomic areas where CT scan is not available.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mukherjee D, Patil CG. Epidemiology and the global burden of stroke. World Neurosurg. 2011;76(6 Suppl):S85–90. doi: 10.1016/j.wneu.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 2.Kim AS, Johnston SC. Temporal and geographic trends in the global stroke epidemic. Stroke. 2013;44(6 Suppl 1):S123–5. doi: 10.1161/STROKEAHA.111.000067. [DOI] [PubMed] [Google Scholar]
- 3.Milionis HJ, Liberopoulos E, Goudevenos J, Bairaktari ET, Seferiadis K, Elisaf MS. Risk factors for first-ever acute ischemic non-embolic stroke in elderly individuals. Int J Cardiol. 2005;99:269–75. doi: 10.1016/j.ijcard.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Leinonen JS, Ahonen JP, Lönnrot K, Jehkonen M, Dastidar P, Molnár G, et al. Low plasma antioxidant activity is associated with high lesion volume and neurological impairment in stroke. Stroke. 2000;31:33–9. doi: 10.1161/01.str.31.1.33. [DOI] [PubMed] [Google Scholar]
- 5.Behera S, Mangaraj M, Mohapatra PC. Diagnostic efficacy of ischemia modified albumin and its correlation with lipid profile, oxidative stress in acute myocardial infarct patients on admission. Asian Pac J Trop Dis. 2012;2:62–5. [Google Scholar]
- 6.Jena I, Mohapatra PC, Mohanty NR. Ischemia modified albumin: A biochemical marker of acute stroke. Int J Pharm Bio Sci. 2016;7:15–9. [Google Scholar]
- 7.Gunduz A, Turedi S, Mentese A, Karahan SC, Hos G, Tatli O, et al. Ischemia-modified albumin in the diagnosis of acute mesenteric ischemia: A preliminary study. Am J Emerg Med. 2008;26:202–5. doi: 10.1016/j.ajem.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 8.Lippi G, Montagnana M, Guidi GC. Albumin cobalt binding and ischemia modified albumin generation: An endogenous response to ischemia? Int J Cardiol. 2006;108:410–1. doi: 10.1016/j.ijcard.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 9.Eom JE, Lee E, Jeon KH, Sim J, Suh M, Jhon GJ, et al. Development of an albumin copper binding (ACuB) assay to detect ischemia modified albumin. Anal Sci. 2014;30:985–90. doi: 10.2116/analsci.30.985. [DOI] [PubMed] [Google Scholar]
- 10.Roy D, Quiles J, Gaze DC, Collinson P, Kaski JC, Baxter GF. Role of reactive oxygen species on the formation of the novel diagnostic marker ischaemia modified albumin. Heart. 2006;92:113–4. doi: 10.1136/hrt.2004.049643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 12.Bar-Or D, Lau E, Winkler JV. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia-a preliminary report. J Emerg Med. 2000;19:311–5. doi: 10.1016/s0736-4679(00)00255-9. [DOI] [PubMed] [Google Scholar]
- 13.Polidori MC, Frei B, Cherubini A, Nelles G, Rordorf G, Keaney JF, Jr, et al. Increased plasma levels of lipid hydroperoxides in patients with ischemic stroke. Free Radic Biol Med. 1998;25:561–7. doi: 10.1016/s0891-5849(98)00085-9. [DOI] [PubMed] [Google Scholar]
- 14.Halliwell B, Gutteridge JM. Oxygen radicals and the nervous system. Trends Neurosci. 1985;8:22–6. [Google Scholar]
- 15.LeBel CP, Bondy SC. Oxygen radicals: Common mediators of neurotoxicity. Neurotoxicol Teratol. 1991;13:341–6. doi: 10.1016/0892-0362(91)90081-7. [DOI] [PubMed] [Google Scholar]
- 16.Van Belle E, Dallongeville J, Vicaut E, Degrandsart A, Baulac C, Montalescot G. OPERA Investigators. Ischemia-modified albumin levels predict long-term outcome in patients with acute myocardial infarction. The French nationwide OPERA study. Am Heart J. 2010;159:570–6. doi: 10.1016/j.ahj.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 17.Abboud H, Labreuche J, Meseguer E, Lavallee PC, Simon O, Olivot JM, et al. Ischemia-modified albumin in acute stroke. Cerebrovasc Dis. 2007;23:216–20. doi: 10.1159/000097644. [DOI] [PubMed] [Google Scholar]
- 18.Gunduz A, Turedi S, Mentese A, Altunayoglu V, Turan I, Karahan SC, et al. Ischemia-modified albumin levels in cerebrovascular accidents. Am J Emerg Med. 2008;26:874–8. doi: 10.1016/j.ajem.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Smith WS, English JD, Johnston SC. Cerebrovascular diseases. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, et al., editors. Harrison's Principle of Internal Medicine. 17th ed. Vol. II. New York: McGraw Hill Companies; 2008. pp. 2513–36. [Google Scholar]
- 20.Rothwell P. Cerebrovascular diseases. In: Donaghy M, editor. Brain's Diseases of the Nervous System. 12th ed. Oxford: Oxford University Press; 2009. pp. 1004–92. [Google Scholar]
- 21.Cherubini A, Polidori MC, Bregnocchi M, Pezzuto S, Cecchetti R, Ingegni T, et al. Antioxidant profile and early outcome in stroke patients. Stroke. 2000;31:2295–300. doi: 10.1161/01.str.31.10.2295. [DOI] [PubMed] [Google Scholar]
- 22.Kumar A, Sivakanesan R, Singh S. Oxidative stress, endogenous antioxidant and ischemia modified albumin in normolipidemic acute myocardial infarction patients. J Health Sci. 2008;54:482–7. [Google Scholar]
- 23.Cha MK, Kim IH. Glutathione-linked thiol peroxidase activity of human serum albumin: A possible antioxidant role of serum albumin in blood plasma. Biochem Biophys Res Commun. 1996;222:619–25. doi: 10.1006/bbrc.1996.0793. [DOI] [PubMed] [Google Scholar]
- 24.Squadrito GL, Cueto R, Splenser AE, Valavanidis A, Zhang H, Uppu RM, et al. Reaction of uric acid with peroxynitrite and implications for the mechanism of neuroprotection by uric acid. Arch Biochem Biophys. 2000;376:333–7. doi: 10.1006/abbi.2000.1721. [DOI] [PubMed] [Google Scholar]


