Abstract
Background:
Ventilator-associated pneumonia (VAP) is the most frequent Intensive Care Unit acquired infection.
Aims:
The aim is to determine the incidence, bacteriology and factors affecting VAP and to determine the multi-drug resistant (MDR) pathogens.
Settings and Design:
This was a prospective observational study conducted over a period of 1 year from April 1, 2011, to March 31, 2012.
Materials and Methods:
The patients fulfilling criteria of VAP were included in this study.
Statistical Analysis:
This was performed using SPSS trial version 11.0 software (SPSS Inc., Chicago, Illinois, USA) and the values of P < 0.05 were considered statistically significant.
Results:
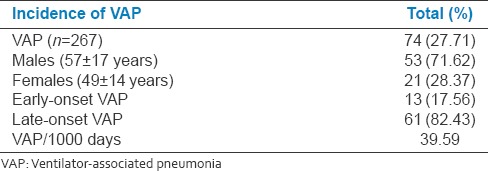
Totally 74 (27.71%) patients were developed VAP. Of total 74 patients with VAP 53 (71.62%) were females and 21 (28.37%) were females (P < 0.0001). Total 13 (17.56%) patients had early-onset VAP and 61 (82.43%) had late-onset VAP (P < 0.0001). The overall incidence of VAP rate per 1000 ventilator days was 39.59. Total 126 bacterial isolates found in 74 patients with VAP. Predominant isolates were Gram-negative 52 (70.27%). Total 41 (55.40%) patients had polymicrobial VAP, and 33 (44.59%) had single isolate. Total 55 (43.65%) isolates were MDR organisms. Total 22 patients with VAP succumbed during treatment with overall case fatality rate of 29.72%. Of total 55 MDR isolates in VAP, 13 (26.63%) were Klebsiella spp., 11(20%) Pseudomonas aeruginosa, 14 (25.45%) Acinetobacter, 8 (14.54%) Escherichia coli, and 9 (16.36%) coagulase positive Staphylococcus aureus. Total 12 (21.41%) patients succumbed among MDR isolates.
Conclusions:
There was a high incidence of MDR pathogens in late-onset VAP. The Gram-negative organisms Klebsiella, Pseudomonas E. coli and Acinetobacter were the most commonly isolated organisms with high mortality rates.
Key words: Bacteriology, Intensive Care Unit, multi-drug resistant organisms, poly-microbial, ventilator-associated pneumonia
INTRODUCTION
Ventilator-associated pneumonia (VAP) is a major cause of hospital morbidity and mortality in Intensive Care Unit (ICU) patients despite recent advances in diagnosis and accuracy of management. VAP is the most frequent ICU acquired infection, occurring in 25% of patients intubated for longer than 48 h. The incidence of VAP ranges from 13 to 51 per 1000 ventilator days.[1] Early-onset VAP is usually less severe, associated with a better prognosis, and is more likely to be caused by antibiotic-sensitive bacteria. Late-onset VAP, is usually caused by multi-drug resistant (MDR) pathogens and is associated with increased morbidity and mortality.[2,3] Many studies from India have investigated the causative organisms of VAP. Pseudomonas spp., Acinetobacter spp., Escherichia coli, Klebsiella pneumoniae, and Staphylococcus aureus were identified as the common VAP pathogens, with varying prevalence. Up to 40% of these infections can be polymicrobial. Pseudomonas spp., Acinetobacter spp. and even Enterobacteriaceae are quite often MDR.[3,4] Therefore, the local microbial flora causing VAP needs to be studied in each setting to guide more effective and rational utilization of antimicrobial agents. So for there is scanty literature about incidence, bacteriology, and antibiotic susceptibility pattern about VAP in India. The initial empirical therapy of VAP modified based on the knowledge of local microbiological data. Although mechanical ventilation (MV) is a life-saving intervention, it has its own potential complications. Newer antibiotics in the past decade have not brought down the mortality in the critical care facilities across the world, associated with VAP. The increasing incidence, mortality, MDR pathogens of VAP in critical care units we did this study to identify the culture sensitivity pattern of microbial isolates from endotracheal aspirates (EA). The objectives of this study were to determine the incidence, bacteriology, antibiotic susceptibility, and resistance pattern including MDR isolates, risk factors, and outcome of VAP at a tertiary care teaching hospital.
MATERIALS AND METHODS
Study design
This was the prospective observational noninterventional study of VAP cohort, conducted in medical ICUs of a tertiary care teaching hospital over a period of 1 year (April 1, 2011–March 31, 2012). This study was approved by the research and ethical committee. Informed consent was obtained from each patient's next of kin.
Aims and objectives
The objectives of this study were to determine the incidence bacteriology, risk factors, and outcome of VAP patients and to determine their antibiotic susceptibility and resistance pattern including MDR isolates.
Setting
The study was conducted in the medicine ICU of a tertiary care teaching hospital. The Departments of Microbiology and medicine were involved in this study. The ICU is comprised well-spaced beds and patients were either admitted directly to the ICU or transferred from other wards, namely medicine, surgery, obstetrics, and neurosurgery. The nurse-patient ratio was as per standard norms.
Definition of ventilator-associated pneumonia
VAP is defined as pneumonia occurring more than 48 h after endotracheal intubation and initiation of MV.
Early-onset ventilator-associated pneumonia
Developed during the first 4 days of MV.
Late onset ventilator-associated pneumonia
Developed five or more days after initiation of MV.
Subject and sample size
During 12 months study, a total of 673 patients who were intubated and were on MV of them only 267 patients who were ventilated for more than 48 h were eligible for inclusion in the study.
Procedure for data collection
All patients included in the study were monitored at frequent intervals (every 2 days) for the development of VAP using clinical and microbiological criteria until either discharge or death. The clinical parameters were recorded from bedside charts. Details of antibiotic therapy, surgery, use of steroids, duration of hospitalization, presence of neurological disorders, and impairment of consciousness were also noted.
Criteria for diagnosis of ventilator-associated pneumonia
The diagnosis of VAP was based on clinical and microbiological criteria. A clinical suspicion of VAP was made in patients with a modified clinical pulmonary infection score (CPIS) >6; the diagnosis was confirmed by performing a quantitative culture of the EA and observing ≥105 cfu/ml.[4,5,6] We used the following criteria to diagnose VAP: Ventilated for more than 48 h; New and persistent infiltrates shadow developing in the Chest X-ray; presence of fever (temperature <96.8 or >99°F); White cell count >11,000/ml or <4000/ml; declining ratio of partial pressure to inspired fraction of oxygen (PaO2/FiO2 ratio) was found to be the earliest indicator of VAP; Cultures positive from endotracheal secretions.[4] (The CPIS was developed to serve as a surrogate tool to facilitate the diagnosis of VAP. The CPIS is calculated on the basis of points assigned for various signs and symptoms of pneumonia [fever and extent of oxygenation impairment]). Based on these criteria, 74 of 267 enrolled patients were diagnosed with VAP.
Microbiological techniques
The organisms isolated by quantitative culture of the EA from VAP patients were identified based on standard microbiological techniques. The susceptibility of the clinical isolates to some routinely used antibiotics was determined by the Kirby-Bauer disk diffusion method.[7,8]
Multi-drug resistance
MDR pathogens were defined as those resistant to three or more antimicrobial classes.
Exclusion criteria
All patients with clinical and radiological signs suggestive of pneumonia on admission and clinic-radiological evidence with alternative diagnosis other than VAP were excluded from the study.
Statistical analysis
Data entry and analysis were performed using SPSS for windows version SPSS 11.0 software (Trial version SPSS Inc., Chicago, Illinois, USA). Means and standard deviations were calculated for numerical variables. The Chi-square test was calculated and all P < 0.05 were considered statistically significant. Odds ratio and relative risk (RR) was calculated for univariate analysis. The VAP rate per 1000 ventilator days was calculated as total number of VAPs in ICUs/total number of ventilator days in medical ICU × 1000.
RESULTS
Total 267 patients were intubated and were on MV for more than 48 h (fulfilling inclusion and exclusion criteria) for various reasons for respiratory failure during the study period of 1 year (April 1, 2011–March 31, 2012). Of 267 patients on mechanical ventilator, 74(27.71%) patients developed VAP [Figure 1]. The incidence of VAP in present population was 27.71%. Of total 74 patients with VAP, 53(71.62%) were males (mean age 57 ± 17 years) and 21(28.37%) were females (mean age 49 ± 14 years) with male to female ratio of 2.52 (RR: 2.5238 95% confidence interval [CI]: 1.7099–3.7252: z statistic: 4.660 P < 0.0001: Odds ratio: 6.369). Total 13(17.56%) patients had early-onset VAP and 61(82.43%) had late-onset VAP (odds ratio: 0.0454; 95% CI: 0.0195–0.1059; z statistic: 7.157; P < 0.0001; RR: 0.2131). The overall incidence of VAP rate per 1000 ventilator days was 39.59 [Table 1].
Figure 1.
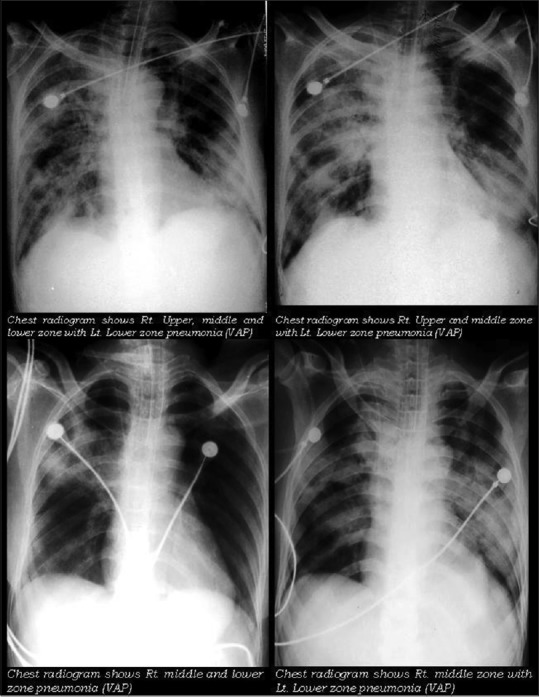
Chest radiogram of patient with ventilator associated pneumonia
Table 1.
Incidence, demographic and clinical profile of ventilator associated pneumonia
Total 126 cultures were positive for pathogenic bacteria among 74 patients with VAP (i.e., more than one organism were present in EA). Total 102(80.95%) isolates were found in male and 24(19.04%) isolates in female population. Total 41(55.40%) patients had polymicrobial VAP and 33(44.59%) had single isolate [Table 2, Figure 2 and Graph 1].
Table 2.
Organisms isolated in patients with ventilator-associated pneumonia

Figure 2.

Culture growth of endotracheal aspirate. MacConkey Agar (Escherichia coli), MacConkey Agar Pseudomonas aeruginosa, Nutrient Agar (Staphylococcus aureus), blood Ager (Staphylococcus aureus), blood Ager (Klebsiella spp.)
Graph 1.
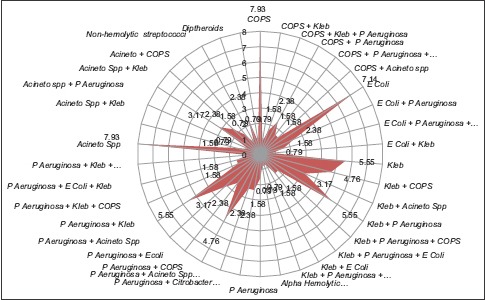
Bacteriological profile of ventilator associated pneumonia isolates
Of total 126 isolates with VAP 55(43.65%) were MDR organisms. Total 39(38.23%) MDR isolates in male patients and 16(66.66%) female patients, predominated by females. Of total 55 patients with MDR isolates 13(23.63%) had early VAP (≤5 days) and 42(76.36%) had late-onset VAP (>5 days) VAP (odds ratio: 0.0958; 95% CI: 0.0397–0.2309; z statistic: 5.225; P < 0.0001; RR: 0.3095; 95% CI: 0.1882–0.5089; z statistic: 4.622; P < 0.0001) [Table 3].
Table 3.
Bacteriological isolate profile of ventilator associated pneumonia
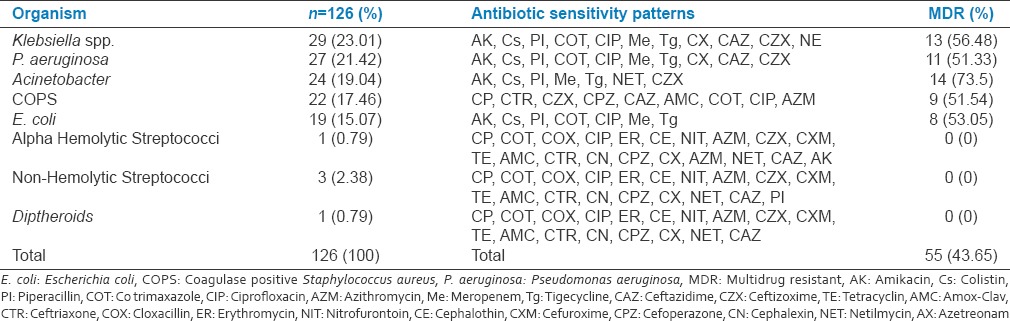
Total Gram-positive cocci (GPC) were 22(17.46%) and 52(70.27%) were Gram-negative bacilli (GNB). The organisms isolated were predominantly GNB Klebsiella 29(23.01587%), Pseudomonas 27(21.42%), Acinetobacter 24(19.04%), and E. coli 19(15.07%) with high mortality rates. The list of antibiotics and sensitivity pattern with various bacterial isolates is shown in Table 3. Total 16(12.69%) isolates were sensitive to meropenem, 19(15.07%) to piperacillin (PI), 20(15.87%) to amikacin, 27(21.42%) to colistin, and 18(14.28%) to tigecycline. Colistin, PI-T, amikacin, and meropenem have found to the better sensitivity to the majority of GNB isolates [Table 4 and Graph 2].
Table 4.
Classification of drug sensitivity for important bacterial isolates causing ventilator associated pneumonia
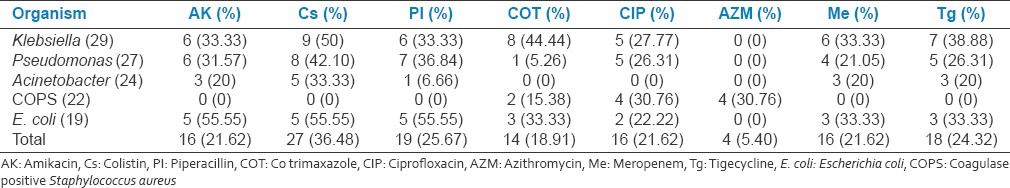
Graph 2.
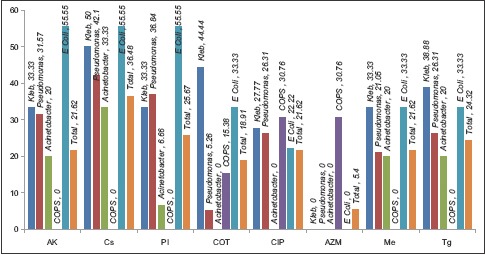
Culture sensitivity and antibiotic suceptibility pattern of important ventilator associated pneumonia isolates
Of total 55 MDR isolates in VAP, 13(26.63%) were Klebsiella, 11(20%) Pseudomonas, 14(25.45%) Acinetobacter, 8(14.54%) E. coli and 9(16.36%) coagulase positive S. aureus (COPS).
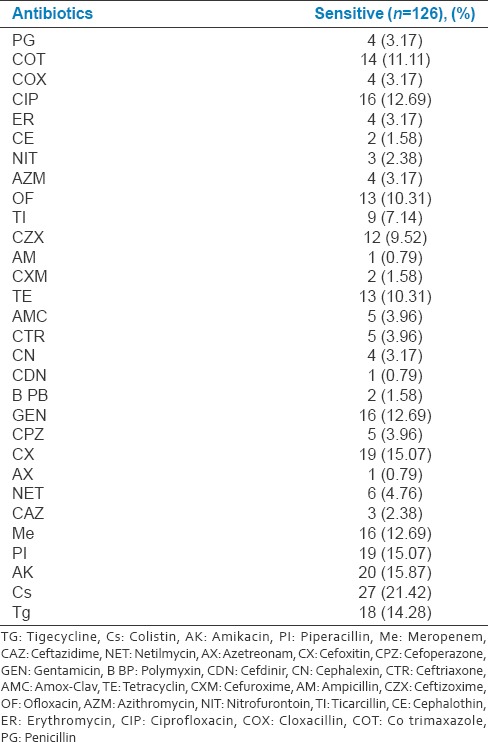
Ceftizoxime was sensitive in 12(9.52%) and cefoxitin 19(15.07%) should be equally considered for treating late-onset VAP, considering their sensitivity pattern [Table 5].
Table 5.
List of antibiotics and sensitivity pattern with various bacterial isolates
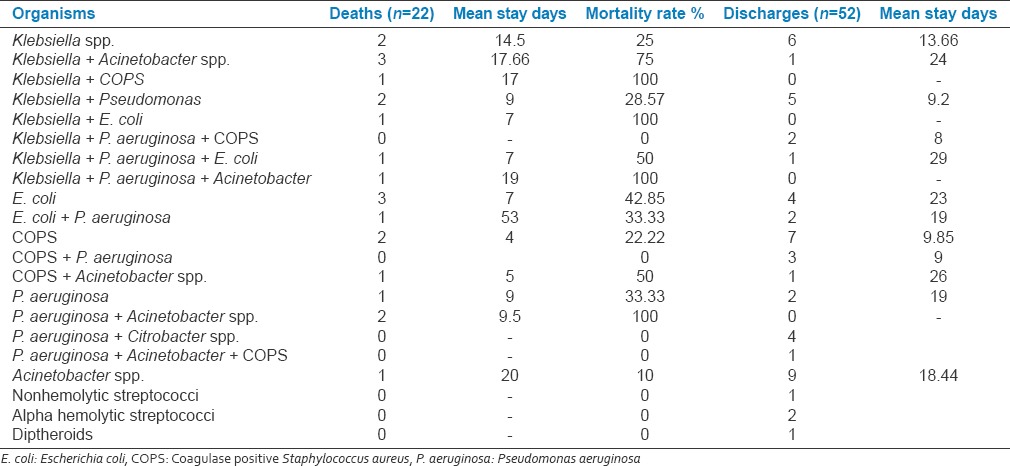
Total 52(70.27%) patients (mean age 58 ± 13) with VAP were discharged after successful treatment (males: 38 [71.69%], females: 14 [66.66%]) (RR: 0.8491; 95% CI: 0.4046–1.7818; odds ratio: 0.7895; 95% CI: 0.2664–2.3398; z statistic: 0.426; P = 0.6698; z statistic: 0.433; P = 0.6653). Total 22 patients with VAP succumbed during treatment with overall case fatality rate of 29.72% (mean age [58 ± 13]). Of total 22 death due to VAP 15(71.62%) were male and 7(28.37%) were female patients. Total 5(22.72%) had early-onset VAP and 17(77.27%) patients had late-onset VAP (odds ratio: 0.0865; 95% CI: 0.0211–0.3544; z statistic: 3.402; P = 0.0007). The mean age of patients with death was significantly more than discharge patients (58 ± 13: 45 ± 15). Total 2(25%) patients died and six discharged with Klebsiella. spp. isolates. Total 3(75%) patients died and one discharged with Klebsiella + Acineto spp. isolates. Total 3(42.85%) patients died and four discharged with E. coli isolates. Total 2(28.57%) patients died and five discharged with Klebsiella + pseudomonas isolates. Total 1(33.33%) patients died and two discharged with E. coli + Pseudomonas aeruginosa isolates. Total 2(22.22%) patients died and seven discharged with COPS isolates. Total 1(100%) patient died with Klebsiella + P. aeruginosa + Acineto spp. isolates. Total 2(100%) patient died with P. aeruginosa + Acineto spp. isolates. Total 1(10%) patients died and 9 discharged with Acineto spp. isolates. Total 1(50%) patients died and one discharged with COPS + Acineto spp. isolates. Total 1(100%) patients died with Klebsiella + E. coli isolates. Total 2(22.22%) patients died and seven discharged with COPS isolates. Total 1(50%) patients died and one discharged with Klebsiella + P. aeruginosa + E. coli isolates. Total 1(33.33%) patients died with P. aeruginosa isolates. Total 1(100%) patients died with Klebsiella + COPS isolates. The multiple and GNB isolates had higher mortality rate compared to single isolate (P < 0.001). The comparison of mean duration of stay in death and discharged group by ANOVA was P = 0.657 (Degree of freedom: 1) [Table 6].
Table 6.
Single and multiple isolates associated with mortality in ventilator associated pneumonia
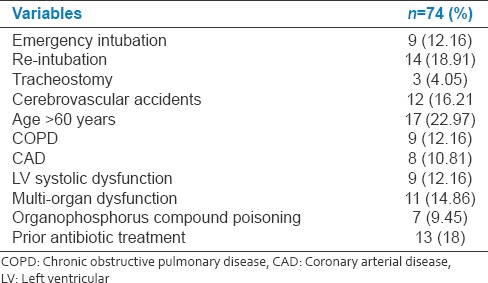
Total 12(21.81%) succumbed among MDR isolates (RR: 1.20; 95% CI: 0.6613–2.1775; z statistic 0.600; P = 0.5487). Total 4(33.33%) isolates of MDR Klebsiella, 3(37.5%) E. coli, 1(8.33%) Pseudomonas, 2(14.28%) Acinetobacter, 2(22.22%) COPS succumbed during treatment. The emergency intubation, re-intubation, tracheostomy, cerebrovascular accidents and longer duration of ventilation, advancing age, preexisting comorbidities (chronic obstructive pulmonary disease [COPD], and coronary artery disease), multi-organ dysfunction, prior use of antibiotics and LV systolic dysfunction were the risk factors for developing VAP (P < 0.02) [Table 7].
Table 7.
Risk factors associated with ventilator associated pneumonia
DISCUSSION
VAP accounts for one-fourth of the infections occurring in critically ill patients and is the reason for half of antibiotic prescriptions in mechanically ventilated patients. Several countries have reported mortality rates ranging from 24% to 76%. Total 22 patients were GP and 52 were with GNB. Klebsiella, Pseudomonas, Acinetobacter, COPS and E. coli were the most commonly isolated organisms with high mortality rates in this study. The prevalence of MDR organisms were 43.65%. About 55.40% of patients had poly-microbial VAP and 44.59% had single isolate. We compared our results with various studied from India and overseas [Table 8]. Gadani et al. reported that the most common organism isolated was Pseudomonas with early-onset VAP was found in 27% and late-onset in 73%. Late-onset VAP had poor prognosis regarding mortality (66%) as compared to the early-onset type (20%).[9] These findings are comparable with our study were Klebsiella 29(23.01587%), Pseudomonas 27(21.42%), Acinetobacter 24(19.04%), and E. coli 19(15.07%).
Table 8.
Comparison of various studies
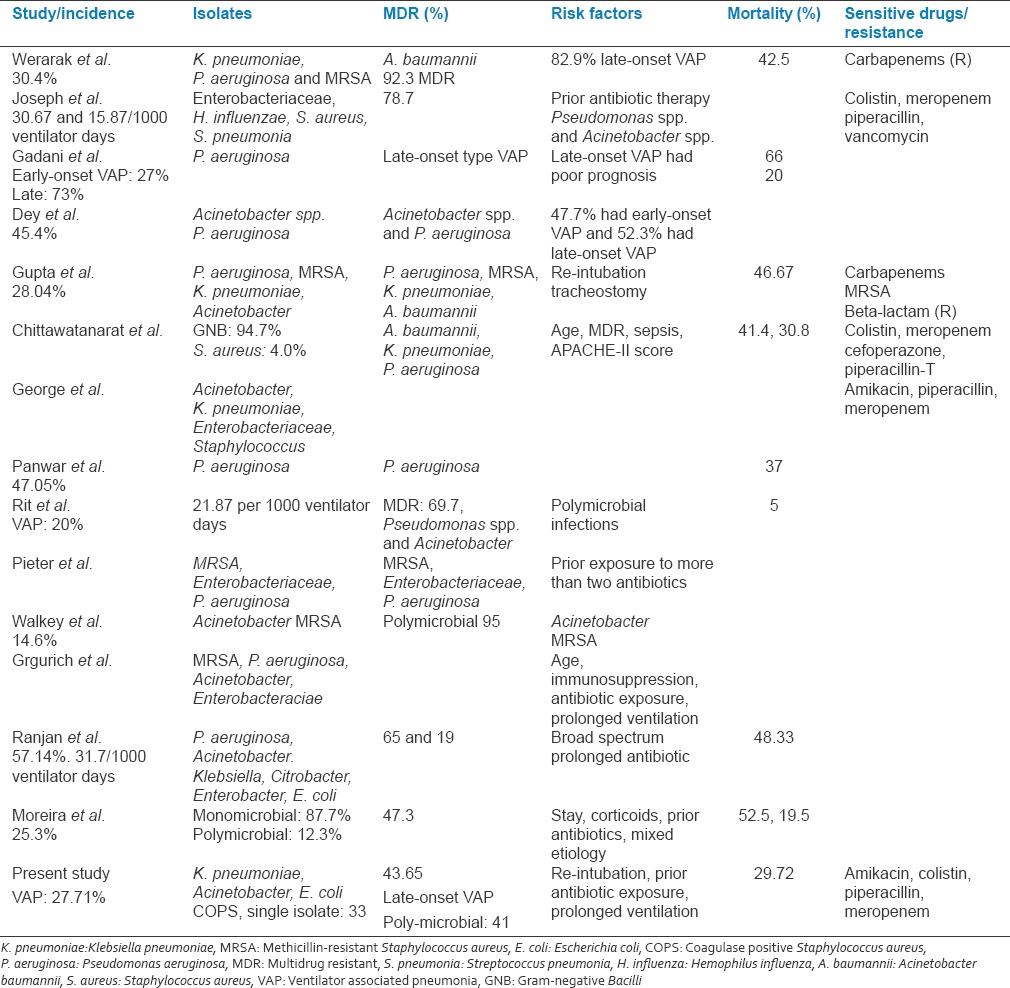
Were isolated in patients with VAP with 23.63% had early VAP and 76.36% had late-onset VAP. In this study, the case fatality rate was 29.72% (early-onset VAP: 22.72%, late-onset VAP: 77.27%). Similarly, Rakshit et al. reported that half of the cases developed VAP with P. aeruginosa.[10] Pawar et al. reported that significant risk factors for VAP were COPD, reintubation, coma, steroid treatment, intra-aortic balloon counter pulsation, enteral feedings, and tracheostomy. The most common pathogens isolated were P. aeruginosa, E. coli, K. pneumoniae, Staphylococcus species, and Acinetobacter species with mortality rate of 16%.[11] Similarly, in our study, most common organisms were Klebsiella, Pseudomonas, Acinetobacter, E. coli and COPS with overall mortality of 29.72%. In this study, impaired consciousness, re-intubation, emergency intubation, and preexisting co-morbidities were the significant risk factors for developing VAP. Joseph et al. reported the incidence of VAP 30.67 and 15.87 per 1000 ventilator days in the two different ICUs.[12] Similarly, in the current study, the incidence of VAP was 27.71% with the rate per 1000 ventilator days was 39.59 predominated by late-onset VAP. Joseph et al. and Combes et al. reported poly-microbial VAP in 30–70% and 48%, respectively.[3,13] Similarly, in our study, 55.40% patients had poly-microbial VAP. The VAP is significant public health issues all over the world including Asian countries.[14] Dey and Bairy quoted 45.4% incidence of VAP, (early-onset: 47.7%, late-onset VAP: 52.3%) with Acinetobacter spp. and P. aeruginosa were the most commonly isolated multiresistant pathogens.[15] These findings are comparable with our results in which 43.65% isolates were MDR predominated by Pseudomonas, E. coli, Klebsiella spp., Acinetobacter and COPS. Chung et al. quoted major bacterial isolates in VAP were Acinetobacter spp., P. aeruginosa, Staphylococcus aureus, and K. pneumoniae with MDR isolates with mortality rate was 38.9%.[16] Rello et al. stated that the Acinetobacter spp. prevalence > 10% in pneumonia episodes requiring carbapenems and colistin.[17] Similarly, in our study, 33.33% were Acinetobacter spp. sensitive to colistin. Arabi et al. stated that the rates of VAP varied from 10 to 41.7 per 1000 ventilator days. GNB were the most common pathogens (41–92%), followed by GPC (6–58%). VAP was associated with a crude mortality that ranged from 16% to 94%.[18] These findings are comparable with our study with incidence of VAP rate per 1000 ventilator days was 39.59 predominated by GNB (70.27%) with case fatality rate of 29.72%. Werarak et al. reported VAP predominated by elderly males with and late onset caused by A. baumanni (92.3%) with MDR or pandrug-resistant. The other common isolated pathogens were K. pneumoniae, P. aeruginosa and methicillin-resistant S. aureus (MRSA) with mortality of 42.5%, these findings are comparable with our results.[19] Combes et al. reported mortality of VAP was 18% which is relatively less compared to our results (29.72%).[13] Joseph et al. reported that Enterobacteriaceae, Haemophilus influenzae, Staphylococcus aureus, and Streptococcus pneumoniae were more common in early-onset VAP and Pseudomonas spp. and Acinetobacter spp. were significantly associated with late-onset VAP (P = 0.0267) with three fourth of them were MDR. Prior antibiotic therapy and hospitalization of 5 days or more were independent risk factors for VAP by MDR pathogens. These findings are comparable with our results in which 43.65% isolates were MDR and 18% of patients with VAP received prior antibiotics.[20] Werarak et al. quoted A. baumannii as most common cause of VAP 30.4%, of them 80% had late-onset Niemann-pick with the median duration of 10 days after admission and were resistant to many antibiotics including carbapenems.[21] Similarly, in our study, 19.04% were A. Baumannii prevalent in late-onset VAP with MDR isolates of A. Baumannii were 73.5%. Panwar et al. reported incidence of 47.05% VAP predominantly caused by P. aeruginosa with mortality of 37%.[22] Similarly, in our study, the incidence of VAP in present population was 27.71% (VAP rate per 1000 ventilator days was 39.59). Total 55(43.65%) were MDR organisms. Klebsiella, P. aeruginosa, Acinetobacter and E. coli were the most commonly isolated organisms. The overall case fatality rate was 29.72%. Park reported common pathogens for VAP include Pseudomonas species, resistant GNB, Staphylococci, Enterobacteriaceae, streptococci and Haemophilus species.[23] These findings are comparable with our results. Similar to our study, Chittawatanarat et al. reported that Gram-negative organisms were the major pathogens (94.7%). The first three most common organisms were Acinetobacter baumannii, K. pneumoniae, and P. aeruginosa with significant case fatality rate. The most common GP organism was Staphylococcus aureus. Half of all VAP cases were caused by susceptible organisms. Antibiotic resistance was demonstrated by 49.3% of the GNB and 62.5% of the GPCs.[24] Walkey et al. reported VAP rate of 14.6% frequently caused by poly-microbial bacteria including Acinetobacter spp., GNB and MRSA (MDR). Patients with VAP were more likely to have a neurological reason for ventilator dependence, (69.6%) with mortality rate of 5%.[25] The rate of VAP was relatively low compared to our study (27.71%), which could be because of different clinical setting, duration of ventilation, organism resistance pattern, antibiotic policy and indication for MV. Ranjit and Bhattarai reported VAP rate of 31.88%, which is comparable with our rate of 27.71%.[26] Gupta et al. quoted incidence of 28.04% VAP with mortality of 46.67%, caused by P. aeruginosa, MRSA, K. pneumoniae and A. baumannii. About 50% isolates of Acinetobacter were resistant to carbapenems. Mortality was highest for infections caused by A. baumannii (83.33%) and K. pneumoniae (71.42%).[27] Comparable to our study, Rit et al. quoted incidence of VAP of 20% with 21.875 per 1000 ventilator days with late-onset VAP in 60.7% caused by Pseudomonas spp. and Acinetobacter spp. and poly-microbial infections with about three fourth were MDR.[28] George and Sequiera reported Acinetobacter were isolated in 37.5% (12), Pseudomonas in 21.87% (7), Klebsiella in 15.6% (5), Enterobacter in 12.5% (4), and Staphylococcus in 6.25% (2) in patient with VAP. Acinetobacter were sensitive to amikacin (44.66%), meropenem (25%). Pseudomonas was sensitive to amikacin, PI, (85.71%) and meropenem (57.14%). The most common organism isolated was Acinetobacter.[29] These findings are comparable with our results. Similarly, Moreira and Gontijo Filho carried out a case–control using patients with VAP by MDR pathogens (case) and non-MDR pathogens (control). They found that 25.3% developed VAP and 47.3% due to MDR pathogens. The risk factors for MDR group for VAP were length of hospital stay, use of corticoids and prior use of antibiotics, inappropriate empirical antimicrobial therapy, and mixed/poly-microbial etiology and were significantly associated mortality.[30] In the present study, 18% of patients with VAP have received prior antibiotics for other source of infection developed VAP. The administration of broad-spectrum antibiotics is a risk factor for developing MDR VAP. Ranjan et al. observed that the prior use of antibiotics increases the risk of acquiring drug resistant pathogens (P. aeruginosa and Acinetobacter spp.).[31] Similarly, Joseph et al. stated that prior antibiotic therapy was independent risk factors for VAP by MDR pathogens.[20] Similarly Depuydt et al. and Grgurich et al. stated that the risk of MDR pathogens causing VAP was mainly determined by prior exposure to antibiotics.[32,33] The organisms isolated in the present study were predominantly Gram-negative. The antibiotics such as PI-T, amikacin, and meropenem have been found to the good antibiotic options for VAP to start with till culture reports are available.
CONCLUSIONS
This study highlights the burden of VAP. The incidence of VAP in present population was 27.71%. VAP is associated with 43.65% MDR pathogens. The overall case fatality rate of VAP was 29.72%. Late-onset VAP was predominated, caused by MDR pathogens (77.27%) and was associated with increased morbidity and mortality. GNB isolates were more prevalent in this study (Pseudomonas, Klebsiella, E. coli and Acinetobacter) with high mortality rates. The emergency intubation, re-intubation, tracheostomy, and longer duration of ventilators were the risk factors for developing VAP. The present study highlight burden of MDR pathogens in VAP. Knowledge of the susceptibility pattern of the local pathogens will guide for de-escalation strategy (switching from a broad-spectrum empiric antimicrobial therapy to a narrower spectrum) depending on the microbiological data. Colistin and PI-tazobactam along with Amikacin and Meropenem may be used for successful treatment Acinetobacter spp. and Pseudomonas spp. as they showed good in vitro activity against these pathogens. Prior antibiotics have no role in prevention of VAP. Previous use of antibiotic will leads to MDR organisms. Newer therapy like, inhalation antibiotics and infusion should be investigated. An appropriate and judicious use of antibiotic is recommended to treat VAP, empirically. Knowledge of risk factors for VAP may be useful in implementing simple and effective preventive measures. Evidence-based protocol based strategies is suggested to prevent VAP, associated healthcare costs and to reduce the mortality. Future studies should attempt to determine whether specific diagnostic or therapeutic strategies could markedly improve VAP outcomes.
Limitations of study
Our results cannot be applied to other institute, as various factors and bacteriological agents causing VAP may vary from institution to institution. This is single center study and included patients from medical ICU, findings and interpretation of our result cannot be generalized to other institute or other ICU.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to acknowledge the staff of ICU, Medicine and Microbiology Department.
REFERENCES
- 1.Torres A, Ferrer M, Badia JR. Treatment guidelines and outcomes of hospital-acquired and ventilator-associated pneumonia. Clin Infect Dis. 2010;51(Suppl 1):S48–53. doi: 10.1086/653049. [DOI] [PubMed] [Google Scholar]
- 2.Niederman MS, Craven DE. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 3.Joseph NM, Sistla S, Dutta TK, Badhe AS, Parija SC. Ventilator-associated pneumonia in a tertiary care hospital in India: Incidence and risk factors. J Infect Dev Ctries. 2009;3:771–7. doi: 10.3855/jidc.396. [DOI] [PubMed] [Google Scholar]
- 4.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 5.Fartoukh M, Maitre B, Honoré S, Cerf C, Zahar JR, Brun-Buisson C. Diagnosing pneumonia during mechanical ventilation: The clinical pulmonary infection score revisited. Am J Respir Crit Care Med. 2003;168:173–9. doi: 10.1164/rccm.200212-1449OC. [DOI] [PubMed] [Google Scholar]
- 6.Porzecanski I, Bowton DL. Diagnosis and treatment of ventilator-associated pneumonia. Chest. 2006;130:597–604. doi: 10.1378/chest.130.2.597. [DOI] [PubMed] [Google Scholar]
- 7.Collee JG, Duguid JP, Fraser AG, Marmion BP, Simmons A. Laboratory strategy in diagnosis of infective syndromes. In: Collee JG, Fraser AG, Marmion BP, Simmons AC, editors. Mackie and McCartney Practical Medical Microbiology. 14th ed. New York: Churchill Livingstone; 1996. pp. 53–94. [Google Scholar]
- 8.Clinical and Laboratory Standards Institute (CLSI) 17th Informational Supplement M100-S22. Vol. 32. Wayne, Pennsylvania, USA: Clinical and Laboratory Standards Institute; 2012. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard – Eleventh Edition. CLSI Document M02-A11. [Google Scholar]
- 9.Gadani H, Vyas A, Kar AK. A study of ventilator-associated pneumonia: Incidence, outcome, risk factors and measures to be taken for prevention. Indian J Anaesth. 2010;54:535–40. doi: 10.4103/0019-5049.72643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rakshit P, Nagar VS, Deshpande AK. Incidence, clinical outcome, and risk stratification of ventilator-associated pneumonia – A prospective cohort study. Indian J Crit Care Med. 2005;9:211–6. [Google Scholar]
- 11.Pawar M, Mehta Y, Khurana P, Chaudhary A, Kulkarni V, Trehan N. Ventilator-associated pneumonia: Incidence, risk factors, outcome, and microbiology. J Cardiothorac Vasc Anesth. 2003;17:22–8. doi: 10.1053/jcan.2003.4. [DOI] [PubMed] [Google Scholar]
- 12.Joseph NM, Sistla S, Dutta TK, Badhe AD, Parija SC. Ventilator-associated pneumonia in a tertiary care hospital in India: Incidence and risk factors. Eur J Inter Med. 2010;21:360–8. doi: 10.3855/jidc.396. [DOI] [PubMed] [Google Scholar]
- 13.Combes A, Luyt CE, Fagon JY, Wolff M, Trouillet JL, Chastre J. Early predictors for infection recurrence and death in patients with ventilator-associated pneumonia. Crit Care Med. 2007;35:146–54. doi: 10.1097/01.CCM.0000249826.81273.E4. [DOI] [PubMed] [Google Scholar]
- 14.Chawla R. Epidemiology, etiology, and diagnosis of hospital-acquired pneumonia and ventilator-associated pneumonia in Asian countries. Am J Infect Control. 2008;36(4 Suppl):S93–100. doi: 10.1016/j.ajic.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Dey A, Bairy I. Incidence of multidrug-resistant organisms causing ventilator-associated pneumonia in a tertiary care hospital: A nine months’ prospective study. Ann Thorac Med. 2007;2:52–7. doi: 10.4103/1817-1737.32230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung DR, Song JH, Kim SH, Thamlikitkul V, Huang SG, Wang H, et al. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am J Respir Crit Care Med. 2011;184:1409–17. doi: 10.1164/rccm.201102-0349OC. [DOI] [PubMed] [Google Scholar]
- 17.Rello J, Ulldemolins M, Lisboa T, Koulenti D, Mañez R, Martin-Loeches I, et al. Determinants of prescription and choice of empirical therapy for hospital-acquired and ventilator-associated pneumonia. Eur Respir J. 2011;37:1332–9. doi: 10.1183/09031936.00093010. [DOI] [PubMed] [Google Scholar]
- 18.Arabi Y, Al-Shirawi N, Memish Z, Anzueto A. Ventilator-associated pneumonia in adults in developing countries: A systematic review. Int J Infect Dis. 2008;12:505–12. doi: 10.1016/j.ijid.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Werarak P, Kiratisin P, Thamlikitkul V. Hospital-acquired pneumonia and ventilator-associated pneumonia in adults at Siriraj Hospital: Etiology, clinical outcomes, and impact of antimicrobial resistance. J Med Assoc Thai. 2010;93(Suppl 1):S126–38. [PubMed] [Google Scholar]
- 20.Joseph NM, Sistla S, Dutta TK, Badhe AS, Rasitha D, Parija SC. Ventilator-associated pneumonia in a tertiary care hospital in India: Role of multi-drug resistant pathogens. J Infect Dev Ctries. 2010;4:218–25. doi: 10.3855/jidc.634. [DOI] [PubMed] [Google Scholar]
- 21.Werarak P, Waiwarawut J, Tharavichitkul P, Pothirat C, Rungruanghiranya S, Geater SL, et al. Acinetobacter baumannii nosocomial pneumonia in tertiary care hospitals in Thailand. J Med Assoc Thai. 2012;95(Suppl 2):S23–33. [PubMed] [Google Scholar]
- 22.Panwar R, Vidya SN, Alka KD. Incidence, clinical outcome and risk stratification of ventilator-associated pneumonia: A prospective cohort study. Indian J Crit Care Med. 2005;9:211–6. [Google Scholar]
- 23.Park DR. The microbiology of ventilator-associated pneumonia. Respir Care. 2005;50:742–63. [PubMed] [Google Scholar]
- 24.Chittawatanarat K, Jaipakdee W, Chotirosniramit N, Chandacham K, Jirapongcharoenlap T. Microbiology, resistance patterns, and risk factors of mortality in ventilator-associated bacterial pneumonia in a Northern Thai tertiary-care university based general surgical intensive care unit. Infect Drug Resist. 2014;7:203–10. doi: 10.2147/IDR.S67267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walkey AJ, Reardon CC, Sulis CA, Nace RN, Joyce-Brady M. Epidemiology of ventilator-associated pneumonia in a long-term acute care hospital. Infect Control Hosp Epidemiol. 2009;30:319–24. doi: 10.1086/596103. [DOI] [PubMed] [Google Scholar]
- 26.Ranjit S, Bhattarai B. Incidence and risk factor for ventilator-associated pneumonia in Kathmandu University Hospital. Kathmandu Univ Med J (KUMJ) 2011;9:28–31. doi: 10.3126/kumj.v9i1.6258. [DOI] [PubMed] [Google Scholar]
- 27.Gupta A, Agrawal A, Mehrotra S, Singh A, Malik S, Khanna A. Incidence, risk stratification, antibiogram of pathogens isolated and clinical outcome of ventilator associated pneumonia. Indian J Crit Care Med. 2011;15:96–101. doi: 10.4103/0972-5229.83015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rit K, Chakraborty B, Saha R, Majumder U. Ventilator associated pneumonia in a tertiary care hospital in India: Incidence, etiology, risk factors, role of multidrug resistant pathogens. Int J Med Public Health. 2014;4:51–6. [Google Scholar]
- 29.George P, Sequiera A. Antimicrobial sensitivity pattern among organisms which were isolated from the endotracheal aspirates of patients with ventilator associated pneumonia. J Clin Diagn Res. 2010;4:3397–401. [Google Scholar]
- 30.Moreira MR, Gontijo Filho PP. Multidrug-resistant pathogens causing ventilator associated pneumonia: Risk factors, empirical antimicrobial therapy and outcome of patients in an intensive care unit (ICU) of a Brazilian university hospital. Int J Med Med Sci. 2012;4:204–10. [Google Scholar]
- 31.Ranjan N, Chaudhary U, Chaudhry D, Ranjan KP. Ventilator-associated pneumonia in a tertiary care intensive care unit: Analysis of incidence, risk factors and mortality. Indian J Crit Care Med. 2014;18:200–4. doi: 10.4103/0972-5229.130570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Depuydt PO, Vandijck DM, Bekaert MA, Decruyenaere JM, Blot SI, Vogelaers DP, et al. Determinants and impact of multidrug antibiotic resistance in pathogens causing ventilator-associated-pneumonia. Crit Care. 2008;12:R142. doi: 10.1186/cc7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grgurich PE, Hudcova J, Lei Y, Sarwar A, Craven DE. Management and prevention of ventilator-associated pneumonia caused by multidrug-resistant pathogens. Expert Rev Respir Med. 2012;6:533–55. doi: 10.1586/ers.12.45. [DOI] [PubMed] [Google Scholar]


