Abstract
Aims:
There are variable reports on the reversibility of type 2 diabetes mellitus (type 2 DM) with higher rates among younger patients with short duration of diabetes. Hence, we studied the reversibility of diabetes among young adults with newly diagnosed type 2 DM.
Methods:
This prospective study included 32 patients with newly diagnosed type 2 DM. All type 2 DM patients were initially treated with intensive lifestyle therapy (ILT) (low-calorie diet [1500 kcal/day] and brisk walking for 1 h/day]). Four patients who with HbA1C <9.0% were treated with ILT alone. Except for three patients with concomitant infections who were treated with insulin, remaining 25 patients with HbA1C ≥9.0% were treated with metformin (1000–2000 g) in addition to ILT. When fasting plasma glucose was <126 mg/dl or HbA1C was <6.5% antidiabetic drug dose was reduced or stopped. The patients were followed for a minimum period of 2 years.
Results:
Reversal/remission rates at 3 months, 1 year, and 2 years were 24 (75%), 24 (75%), and 22 (68.75%), respectively. Seventeen (53.1%) patients achieved complete reversal and seven (21.9%) patients achieved partial reversal at 3 months. Rates of complete and partial remission at 1 year were 50% and 25% and at 2 years were 46.9% and 21.9%, respectively.
Conclusion:
Young adults with newly diagnosed type 2 DM have high rates of diabetes reversal and should receive ILT to achieve reversal of diabetes.
Key words: Intensive lifestyle therapy, remission, reversal, type 2 diabetes
INTRODUCTION
The prevalence of diabetes mellitus is increasing in India. According to the International Diabetes Federation 2015, there are around 69.2 million people with diabetes mellitus in India.[1] The age of presentation in Asian Indian diabetics is a decade lesser than that in Caucasians.[2] The prevalence of obesity is also increasing in India, especially in children, leading to an increased prevalence of type 2 diabetes mellitus (type 2 DM).[3] Recently, there is a shift in the age of onset of type 2 DM to a younger age in India.[1]
Initial definitive evidence for reversal of diabetes was demonstrated with bariatric surgery. Biliopancreatic diversion confers the highest rates of remission, followed by Roux-en-Y gastric bypass (RYGB), vertical sleeve gastrectomy, and adjusted gastric banding.[4] In an Indian study, all type 2 DM patients with body mass index (BMI) <35 kg/m2 achieved reversal of diabetes at 3 months after the performance of RYGB procedure.[5]
In 2011, the counterpoint study by Lim et al. demonstrated that very low-calorie diet (600 kcal/day) leads to rapid (1 week) reversal of fasting hyperglycemia.[6] Outside a research setting, motivated patients who followed very low-calorie diet achieved reversal of diabetes in 61% with highest rates of reversal (73%) in those with a short duration of diabetes (<4 years).[7] Remission of diabetes for as long as 8 years has been reported.[8] Hence, we have studied the rates of reversibility among young adults with newly diagnosed type 2 DM with intensive lifestyle therapy (ILT).
METHODS
This prospective study was conducted at Vydehi Institute of Medical Sciences and Research Center, Bengaluru, India. Institutional Ethics Committee permission was obtained, and a written informed consent was obtained from all participants. Diabetes mellitus was defined according to American Diabetes Association (ADA) criteria.[9] All newly diagnosed patients with type 2 DM who consulted the corresponding author between November 2012 and October 2013 were included in the study. Patients with gestational diabetes mellitus were excluded from the study.
At recruitment, data on family history of diabetes, symptoms at presentation, anthropometric data (height, weight, BMI, waist circumference), calorie intake, and glycemic parameters (fasting plasma glucose [FPG], postprandial plasma glucose, HbA1c) at diagnosis were collected. All patients were counseled regarding the possibility of type 2 DM reversal with ILT. ILT included daily calorie intake of 1500 kcal (60% as carbohydrates, 15% as proteins, and 25% as fat) and brisk walking for 1 h every day. All patients were advised to follow ILT. In addition, subjects with HbA1c ≥9.0% were started on metformin 500–2000 mg. Only those patients with comorbid conditions requiring early glycemic control were started on insulin followed by oral antidiabetic agents (metformin ± DPP4 inhibitors). The patients were followed-up every month for first 3 months and then once in every 2 months. At each visit glycemic parameters, weight, and calorie intake were assessed, and all patients were counseled for the continued need for adherence to ILT to sustain remission. The doses of oral antidiabetic agents were adjusted depending on the glycemic parameters and tolerance. When FPG was <126 mg/dl or HbA1C was <6.5%, antidiabetic drug dose was reduced or stopped. Those with HbA1c ≥7.0% were started on additional oral antidiabetic agents preferably, DPP4 inhibitors. All except three patients were followed up for a minimum period of 2 years. For the three patients relocated out of Bengaluru, the glycemic parameters, and anthropometric data at last follow-up was obtained by communication through e-mail.
Complete reversal at 3 months was defined as normalization of FPG (<100 mg/dl) and partial reversal at 3 months was defined as FPG between 100 and 125 mg/dl. Partial remission was defined as FPG of 100–125 mg/dl and HbA1C of 5.7–6.4% in the prediabetic range at 1 year or later and complete remission as normal FPG <100 mg/dl and HbA1C <5.7% at 1 year or later.
Statistical analysis
Statistical analyses were performed using SPSS software version 21.0 (SPSS, IL, Chicago, USA). Data are presented as mean ± standard deviation. Comparison of glycemic parameters, weight, and calorie intake between patients who achieved reversal at 3 months and those who did not perform using a two-tailed Student's t-test. P < 0.05 was considered as statistically significant.
RESULTS
The study included 32 subjects. Mean age at diagnosis of diabetes was 24.97 ± 3.81 years. Parental history of diabetes was present in 78.1% patients with maternal history in 13 (40.6%) patients and paternal history in 12 (37.5%) with five (15.6%) having a history of diabetes in both parents. Acanthosis and/or skin tags were present in 12 (37.5%) patients.
Twenty-eight patients with type 2 DM presented with osmotic symptoms and weight loss had an HbA1C 9.0% at the time of diagnosis. Except three patients with concomitant infections (acute paronychia in one patient, hidradenitis suppurativa in one, and urinary tract infection in one), all 25 patients were treated with medical nutrition therapy, regular physical exercise, and metformin (1000–2000 g). All four patients who presented with HbA1C <9.0% were treated with low-calorie diet and regular physical exercise. Of the total 32 patients with type 2 DM, the remission rates at 3 months, 1 year, and 2 years were 24 (75%), 24 (75%), and 22 (68.75%), respectively. Seventeen (53.1%) patients achieved complete reversal and seven (21.9%) patients achieved partial reversal at 3 months. Rates of complete and partial remission at 1 year were 50% and 25% and at 2 years were 46.9% and 21.9%, respectively. Glycemic parameters, drugs required and weight of subjects at baseline and follow-up visits are summarized in Table 1.
Table 1.
Glycemic parameters, drugs required, and weight of patients with type 2 diabetes at baseline and follow-up visits
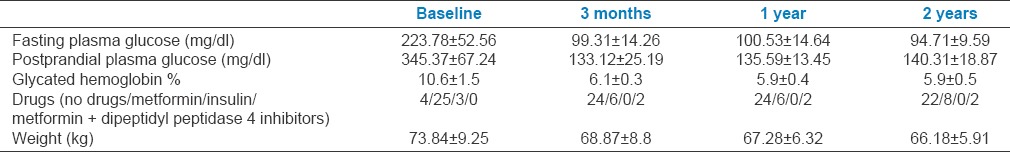
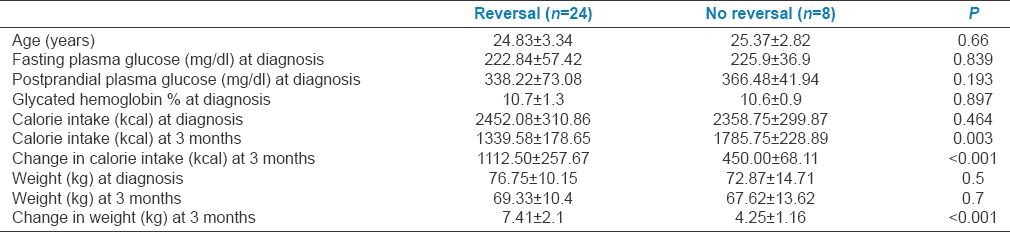
Comparison of glycemic parameters, weight, and calorie intake between patients who achieved reversal and those who did not is summarized in Table 2. Patients who achieved reversal of diabetes at 3 months were consuming significantly lesser calories and had significantly higher weight loss than those who did not. None of the patients who maintained or further decreased the weight at 3 months had a recurrence of DM at 2 years whereas two patients who had regained 3–4 kg weight between 1 year and 2 years of follow-up had a recurrence of DM.
Table 2.
Comparison of glycemic parameters, weight, and calorie intake between patients who achieved reversal and those who did not
DISCUSSION
Majority (75%) of the type 2 DM patients in our cohort achieved partial/complete reversal of diabetes at 3 months. All patients were compliant to therapy and followed the dietary and exercise advice regularly.
Lim et al. reported normalization of FPG as early as 1 week after administration of a very low-calorie diet in diabetics.[6] The reversal was associated with reduction in hepatic triglycerides content by 30% with concomitant reduction in insulin resistance. Over 8 week's period, very low-calorie diet lead to decrease in pancreatic fat and normalization of the first phase insulin secretion.[6] In our study, majority achieved reversal at 3 months after diagnosis. This is probably due to relatively liberal calorie intake in our cohort. The mechanisms of reversal of diabetes in our cohort are probably due to same mechanisms as described above, but occurring at a slower rate.
In a recent study, Steven et al. reported higher rates (87%) of reversal in type 2 DM with short-duration group than that (50%) in the long-duration group after 8 weeks of very low-calorie diet. Younger age, lower fasting glucose and less treatment requirement at baseline were the other predictors of response. There was no effect of weight on degree of response.[10] In our study, none of the baseline glycemic parameters, weight, or calorie intake were different between patients who achieved reversal and those who did not. However, weight reduction and decrease in calorie intake at 3 months were significantly higher among those who achieved remission suggesting that restriction of calories and weight loss predict reversal irrespective of baseline glycemic parameters. Two patients who had regained 3–4 kg weight between 1 year and 2 years of follow-up had recurrence of DM; hence, avoidance of weight regain is essential to prevent recurrence of DM. This would require continued supervision by the clinical practitioner and the nutritionist.
Although small, reversal of diabetes is also reported in routine clinic settings. In diabetes and aging study, partial and complete remission was reported in 1.47% and 0.14% of type 2 DM patients with high rates (4.6%) of remission in the subgroup with new-onset diabetes (<2 years since diagnosis).[11] Nearly, 11–50% of youth with type 2 DM are reported to be managed with lifestyle modification alone.[12,13,14]
A high rate (53%) of dropout was observed among youth who were managed with lifestyle changes alone.[11] Even in those patients who regularly follow-up, greater self-care adherence predicts long-term remission of diabetes.[15] In our study, the follow-up and adherence to therapy was almost 100%. This is probably because of intensive counseling at each follow-up.
Previous studies have documented benefits of early intensive insulin therapy in newly diagnosed type 2 DM with increased chances of remission.[16] Remission rates after 1 year are significantly higher in the insulin groups (51.1% in continuous subcutaneous insulin infusion group and 44.9% in multiple dose insulin group) than in the oral hypoglycemic agents group (26.7%).[17] Whether initial intensive insulin therapy followed by low-calorie diet would have resulted in higher rates of reversal of diabetes in our type 2, DM cohort remains questionable.
ADA recommends initiation of insulin in symptomatic type 2 DM with HbA1C >9.0% at diagnosis.[18] However, in our study, all newly diagnosed type 2 DM were initiated on low-calorie diet, exercise and metformin with which almost all achieved glycemic control by 1–3 months. Initial use of insulin or oral combination therapy in our patients might have resulted in a faster achievement of euglycemia. The attempts for reducing the dose of or stopping oral hypoglycemic agents at HbA1c <6.5% left the patients often at prediabetic range which would still put them at some risk for diabetic complications. Keeping a stringent cut-off of HbA1C (<5.7%) to reduce the dose or stop ADD would be better to minimize the diabetic complications on long-term basis.
Most of the young type 2 DM subjects in our cohort had HbA1C ≥9.0% at presentation. This is likely due to referral bias with only those with very high glycemic parameters getting referred to our institute.
CONCLUSION
Seventy-five percent of the young adults with newly diagnosed type 2 DM achieve remission of diabetes at 1 year and 68.75% at 2 years with low-calorie diet and regular physical exercise. Hence, we suggest that all young, newly diagnosed patients with type 2 DM should receive ILT to achieve remission of diabetes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.International Diabetes Federation. IDF Diabetes. 7th ed. Brussels, Belgium: International Diabetes Federation; 2015. [Last accessed on 2015 Apr 10]. http://www.diabetesatlas.org . [Google Scholar]
- 2.Ramachandran A, Snehalatha C, Shetty AS, Nanditha A. Trends in prevalence of diabetes in Asian countries. World J Diabetes. 2012;3:110–7. doi: 10.4239/wjd.v3.i6.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raj M, Kumar RK. Obesity in children & adolescents. Indian J Med Res. 2010;132:598–607. [PMC free article] [PubMed] [Google Scholar]
- 4.Vetter ML, Ritter S, Wadden TA, Sarwer DB. Comparison of bariatric surgical procedures for diabetes remission: Efficacy and mechanisms. Diabetes Spectr. 2012;25:200–10. doi: 10.2337/diaspect.25.4.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah SS, Todkar JS, Shah PS, Cummings DE. Diabetes remission and reduced cardiovascular risk after gastric bypass in Asian Indians with body mass index <35 kg/m(2) Surg Obes Relat Dis. 2010;6:332–8. doi: 10.1016/j.soard.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: Normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. 2011;54:2506–14. doi: 10.1007/s00125-011-2204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steven S, Lim EL, Taylor R. Population response to information on reversibility of type 2 diabetes. Diabet Med. 2013;30:e135–8. doi: 10.1111/dme.12116. [DOI] [PubMed] [Google Scholar]
- 8.Taylor R. Banting Memorial lecture 2012: Reversing the twin cycles of type 2 diabetes. Diabet Med. 2013;30:267–75. doi: 10.1111/dme.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl 1):S64–71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steven S, Taylor R. Restoring normoglycaemia by use of a very low calorie diet in long- and short-duration type 2 diabetes. Diabet Med. 2015;32:1149–55. doi: 10.1111/dme.12722. [DOI] [PubMed] [Google Scholar]
- 11.Karter AJ, Nundy S, Parker MM, Moffet HH, Huang ES. Incidence of remission in adults with type 2 diabetes: The diabetes & aging study. Diabetes Care. 2014;37:3188–95. doi: 10.2337/dc14-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reinehr T, Schober E, Roth CL, Wiegand S, Holl R. DPV-Wiss Study Group. Type 2 diabetes in children and adolescents in a 2-year follow-up: Insufficient adherence to diabetes centers. Horm Res. 2008;69:107–13. doi: 10.1159/000111814. [DOI] [PubMed] [Google Scholar]
- 13.Silverstein JH, Rosenbloom AL. Treatment of type 2 diabetes mellitus in children and adolescents. J Pediatr Endocrinol Metab. 2000;13(Suppl 6):1403–9. doi: 10.1515/jpem-2000-s614. [DOI] [PubMed] [Google Scholar]
- 14.Newfield RS, Dewan AK, Jain S. Dyslipidemia in children with type 2 diabetes vs. obesity. Pediatr Diabetes. 2008;9:115–21. doi: 10.1111/j.1399-5448.2007.00345.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen A, Huang Z, Wan X, Deng W, Wu J, Li L, et al. Attitudes toward diabetes affect maintenance of drug-free remission in patients with newly diagnosed type 2 diabetes after short-term continuous subcutaneous insulin infusion treatment. Diabetes Care. 2012;35:474–81. doi: 10.2337/dc11-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raz I, Mosenzon O. Early insulinization to prevent diabetes progression. Diabetes Care. 2013;36(Suppl 2):S190–7. doi: 10.2337/dcS13-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weng J, Li Y, Xu W, Shi L, Zhang Q, Zhu D, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: A multicentre randomised parallel-group trial. Lancet. 2008;371:1753–60. doi: 10.1016/S0140-6736(08)60762-X. [DOI] [PubMed] [Google Scholar]
- 18.American Diabetes Association. Approaches to glycemic treatment. Diabetes Care. 2015;38(Suppl):S41–8. doi: 10.2337/dc15-S010. [DOI] [PubMed] [Google Scholar]


