Abstract
Background:
Hemodialysis improves insulin sensitivity. Currently, there is no recommendation for the adjustment of insulin dose on dialysis day and nondialysis day in diabetic patients. This study was undertaken to determine the variations in insulin requirement based on blood glucose levels on dialysis days and nondialysis days in type 2 diabetic patients with chronic kidney disease on maintenance hemodialysis.
Methodology:
Twenty-eight diabetic patients on hemodialysis were recruited into the study after obtaining written informed consent and approval from Azeezia Ethics Committee. Capillary blood glucose levels, just before dialysis and 2 h after dialysis, were checked and compared with fasting and postprandial glucose levels on–off dialysis days.
Results:
Mean age of the patients was 59.5 (±2.3) years. The average duration of dialysis was 20.2 months. There was significant (35.8%) decrease in blood glucose levels 2 h after dialysis in comparison to predialysis levels (from mean level of 258–165 mg/dl). The decrease in the blood glucose levels from predialysis level to 2 h postdialysis level was statistically significant (P < 0.001). Both sets of data showed “strong” positive correlation with r = 0.657 and 0.849. The blood glucose levels on the day of dialysis were significantly lower than the off-day values.
Conclusions:
Diabetic patients with end-stage renal disease on hemodialysis have lower capillary blood glucose levels postdialysis. This has to be addressed clinically to prevent hypoglycemic episodes by reducing exogenous insulin administration on the day of dialysis.
Key words: Capillary blood glucose level, end-stage renal disease, hemodialysis, insulin requirement, on-day and off-day
INTRODUCTION
Glycemic control is a tedious task in patients undergoing hemodialysis. Hemodialysis improves insulin sensitivity and insulin clearance.[1] This compounds the insulin requirement estimation in patients with end-stage renal disease undergoing maintenance hemodialysis. Insulin resistance is a representative feature of type 2 diabetes.[2] Insulin resistance and reduced clearance of insulin are factors that lead to changes in glycemic levels.[3] There is evidence that higher levels of hemoglobin A1C lead to higher death rates in patients with diabetes and chronic kidney disease.[4] There are reports stating repeated episodes of hypoglycemia can lead to impairment of homeostatic mechanisms with the potential to develop hypoglycemia unawareness.[5] If administered insulin is not altered, these patients are at greater risk of acute metabolic complications.[6,7,8] Diabetic patients on hemodialysis may need lesser doses of insulin after dialysis treatments than they do on days when they do not have dialysis.[9] If they continue to take the same dose of insulin after dialysis, they could have a greater chance of hypoglycemia. Currently, there is no recommendation for the adjustment of insulin dose on dialysis day and nondialysis day in diabetic patients. Exogenous insulin requirements may vary in pre- and post-dialysis days.[10] Effects of dialysis on insulin requirement are not certain.
To evaluate day-to-day variations of insulin requirement in type 2 diabetic patients, undergoing hemodialysis is a complex task. Blood glucose levels alter with patient calorie intake and expenditure. To complicate this, there will be heightened sensitivity to circulating insulin, lesser insulin clearance, and alteration in glucose level due to the use of dialysate with or without glucose. A normal plasma glucose level but high hemoglobin A1C levels at predialysis in diabetic patients is often noted.[11] Abe et al. have analyzed fasting plasma glucose and immunoreactive insulin levels at arterial and venous sites at 3 time points (predialysis, 2 and 4 h after starting dialysis) in 16 hemodialysis patients. They noted decreased plasma glucose and immunoreactive levels in venous site in comparison to arterial site. This suggests that hemodialysis leads to lower plasma glucose and immunoreactive insulin levels. Comparison between hemodialysis and nonhemodialysis days revealed that the plasma glucose levels decreased significantly during hemodialysis.[11] The study evaluated plasma glucose and immunoreactive insulin 3 times on every hemodialysis day. This adds significantly to the cost of the study. Same researchers advised caution in selecting dialysate for hemodialysis in diabetic patients.[12] Kazempour-Ardebili et al. have done continuous glucose monitoring in 17 patients undergoing hemodialysis. They found significantly higher glucose profiles on the day off dialysis than the day on dialysis.[13] To complicate the scenario, the validity of the hemoglobin A1C measurement in patients undergoing hemodialysis depends on the methodology used for estimation.[14,15] Factors influencing its levels may be altered life span of red blood cells and other mechanical factors such as carbamylated hemoglobin, and acetylated hemoglobin may play a role in varied levels of hemoglobin A1C.[16,17,18]
The objective of this study was to determine the variations in insulin requirement based on blood glucose levels on-dialysis days and nondialysis days in type 2 diabetic patients with chronic kidney disease on maintenance hemodialysis. Based on blood glucose levels, the alterations in dose of exogenously administered insulin can be recommended.
METHODOLOGY
After obtaining the clearance from Azeezia Ethics Committee, thirty type 2 diabetic patients meeting the inclusion criteria were recruited into the study.
Inclusion criteria
Patients who were diagnosed of type 2 diabetes mellitus, with established kidney failure defined as glomerular filtration rate <15 mL/min/1.73 m2 and on permanent renal replacement therapy, i.e. hemodialysis (4–5 h 3 times weekly) at least for 3 months from the date of beginning of the study were included in the study.
Exclusion criteria
Patients with type 1 diabetes; those who have current acute illness; those who are <90 days from the initial dialysis; those taking drugs that can modify glucose metabolism, excluding insulin and other oral antidiabetes drugs; and those who are pregnant, those who are of age <19 years, hospital admission in the previous 2 months, and a diagnosis of diabetes for <1 year were excluded from the study.
Detailed personal data of patients were collected which includes age, sex, body mass index, age at which diabetes was diagnosed, duration of diabetes, duration of hemodialysis (months), list of currently used drugs, type, and dose of insulin administered.
Blood glucose level was estimated by capillary blood samples. Blood samples were always collected by the same person in dialysis unit. This procedure minimized user dependent preanalytical bias. The area of blood sampling was disinfected before pricking, and the first drop of blood was removed with a sterile swab. All the measurements were carried out with one single subsequent drop of blood from the same area. The drop of blood was applied to the strips of device DiabeCHECK® that uses the glucose dehydrogenase method and was used as the reference method in this study.
Blood glucose estimation
Capillary blood glucose estimations were done for 4 weeks comprising three dialysis days and three nondialysis days every week. On dialysis days, estimations were done just before dialysis and 2 h after dialysis. On nondialysis days, estimations were done at fasting (before breakfast), postprandial (120 min after breakfast). A total of 24 readings were noted from each patient at the end of 4 weeks of study.
Statistical analysis
Demographic, biochemical data were expressed as mean ± standard deviation and as percentages. Chi-square and independent t-tests were used to compare the blood glucose of dialysis days with nondialysis days. Pearson's correlation coefficient was calculated to assess the strength of the relationship between these variables. Statistical Package for the Social Sciences for Windows Version 19.0 (SPSS Inc., Chicago, IL, USA) was used and P < 0.05 was considered statistically significant.
RESULTS
Thirty patients (twenty males; ten females) were recruited in the study, and two patients were subsequently excluded (one because of noncooperation of the patient in recording off-day glucose levels and other because of transfer from our hemodialysis center to another). The age of the patient, age at which diabetes got diagnosed, duration of diabetes, duration of hemodialysis (months), list of currently used drugs, type, and dose of insulin administered was tabulated in Table 1.
Table 1.
Tabulation of patient details enrolled in the study
On-day and off-day capillary blood glucose levels shown in paired linear graphs, [Figures 1 and 2]. Tabulations are depicted in Table 2 and Figure 3. There was significant 35.8% decrease in blood glucose levels 2 h after dialysis in comparison to predialysis levels (from the mean level of 258–165 mg/dl). There was a strong positive correlation between predialysis and 2 h after dialysis values with Pearson's r = 0.65. Similarly, fasting and postprandial blood sugar levels too had a strong positive correlation with r = 0.84 [Table 2 and Figure 4]. The decrease in the blood sugar levels from predialysis level to 2 h postdialysis level was statistically highly significant. The increase in postprandial levels in comparison to fasting was also statistically significant [Table 2].
Figure 1.
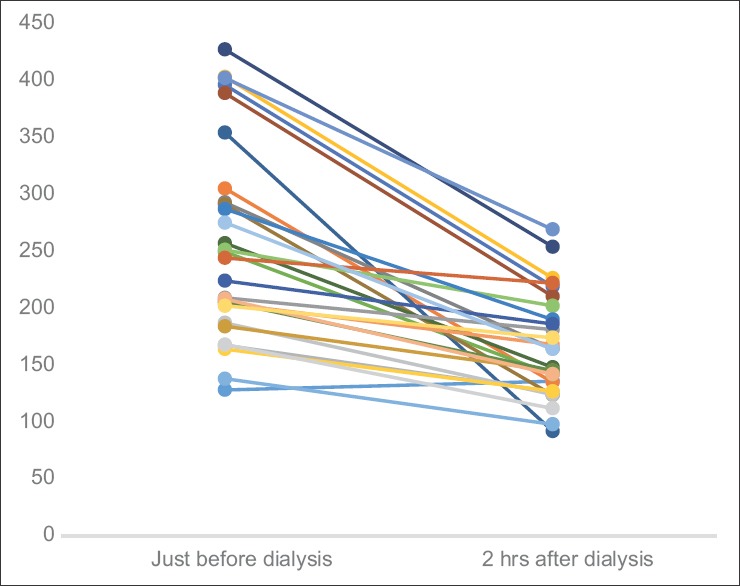
Graph showing paired tabulation of capillary glucose levels in hemodialysis patients, just before and 2 h after dialysis (all values in mg/dl) (n = 28)
Figure 2.
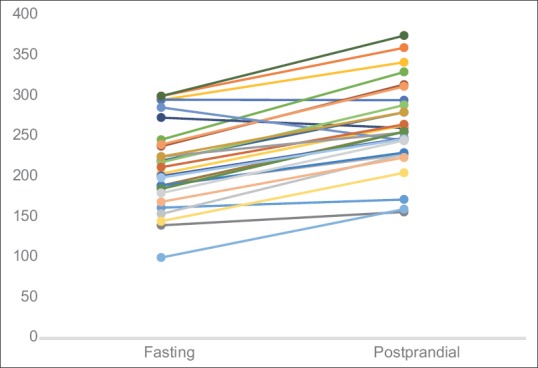
Graph showing paired tabulation of fasting and postprandial capillary glucose levels in hemodialysis patients on–off days (all values in mg/dl) (n = 28)
Table 2.
On- and off-day capillary blood glucose levels of patients enrolled in the study
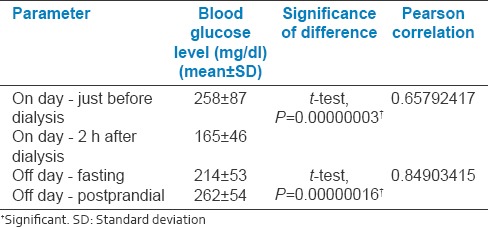
Figure 3.
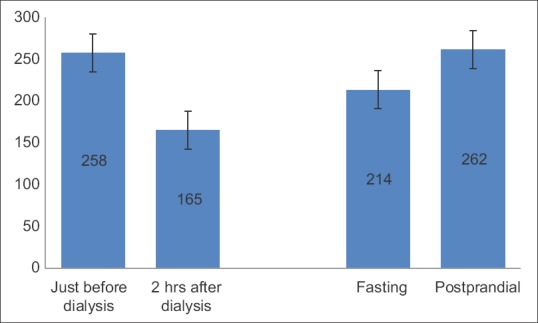
Bar chart representation of blood sugar levels in patients on- and off-days of dialysis (n = 28)
Figure 4.
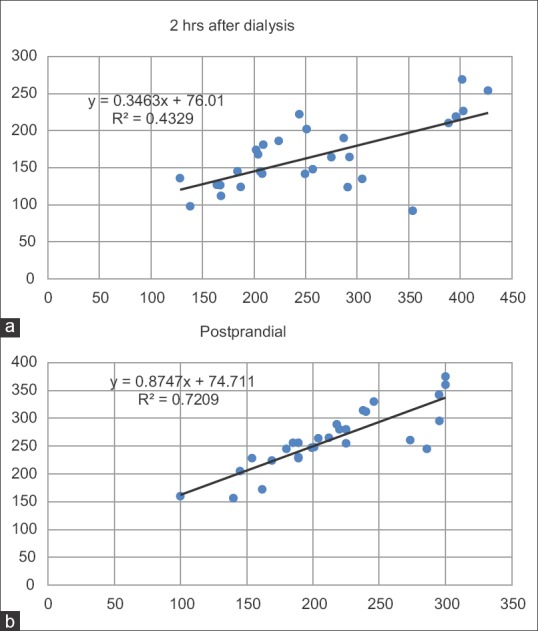
Scattered plot graph showing correlation of predialysis sugar levels to 2 h postdialysis (a) and fasting blood sugar levels to postprandial levels (b). Both sets of data showed “strong” positive correlation with r = 0.657 and 0.849. In both graphs, the linear equation of the trend line and R2 value is displayed
No patient had any change in frequency or dosing medications, including dose of insulin throughout 4 weeks.
DISCUSSION
There is a greater need for strict glycemic control in diabetic patients undergoing dialysis to prevent hypoglycemic episodes.[19] Such control can be effectively established if the patient undergoes continuous glucose monitoring, which is not possible in most of the patients in most dialysis centers.[13] There will be higher prevalence of microvascular complications in diabetic patients undergoing dialysis.
We have measured blood glucose levels on- and off-dialysis days. On dialysis days, the measurement of blood glucose levels just before beginning of dialysis and 2 h after dialysis, irrespective of the session time of dialysis in all patients has negated the influence of differential calorie intake and output. This method checked the effect of dialysis on blood glucose levels. We have noted a consistent statistically significant decrease in the blood glucose levels in all patients except two patients on seven occasions. We have used glucose-free dialysate during dialysis. A similar decrease in the plasma blood glucose levels coupled with decrease in immunoreactive insulin was noted in the previous studies.[11,20] Sobngwi et al., using a 24-h euglycemic clamp, have shown a reduction in glucose levels postdialysis translating to a 25% lower insulin requirement immediately after dialysis.[20] We have noted a much higher reduction in glucose levels, in our study. That can be explained by nonstrict calorie intake restriction and 2 h delay in the postdialysis recording.
This study showed that there is a significant decrease in blood glucose levels postdialysis. However, there were no serious hypoglycemic events requiring medical intervention. Patients were not given insulin dosage on the day of dialysis, irrespective of time of their dialysis session. Thereby, it has accounted for higher predialysis glucose levels in patients (especially those having evening sessions). A similar decrease in glucose profiles and the mean glucose values were reported in earlier studies.[3,20] Therefore, it can be predicted that risk of hypoglycemia is the highest after dialysis and enhanced glycemic monitoring is recommended. In this study, since the insulin dose was withheld on dialysis days, severe hypoglycemia was noted.
However, of late, the very importance of strict blood glucose control on possible effects on microvascular and macrovascular complications of diabetes is unclear.[21,22,23,24] Action to Control Cardiovascular Risk in Diabetes has emphasized a strict blood glucose control.[25] However, no additional benefit was noted with strict glycemic control before dialysis in a study by Oomichi et al.[23,26] Therefore, strict glycemic control may not be a must for all patients undergoing dialysis. However, good glycemic control will reduce insulin dose requirement and lower incidence of hypoglycemic episodes.
CONCLUSION
Diabetic patients with end-stage renal disease on hemodialysis have lower capillary blood glucose levels postdialysis. This has to be addressed clinically to prevent hypoglycemic episodes by reducing exogenous insulin administration on the day of hemodialysis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Authors gratefully acknowledge the cooperation extended by the Department of Nephrology during this study.
REFERENCES
- 1.De Boer IH, Mehrotra R. Insulin resistance in chronic kidney disease: A step closer to effective evaluation and treatment. Kidney Int. 2014;86:243–5. doi: 10.1038/ki.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eringa EC, Serne EH, Meijer RI, Schalkwijk CG, Houben AJ, Stehouwer CD, et al. Endothelial dysfunction in (pre) diabetes: Characteristics, causative mechanisms and pathogenic role in type 2 diabetes. Rev Endocr Metab Disord. 2013;14:39–48. doi: 10.1007/s11154-013-9239-7. [DOI] [PubMed] [Google Scholar]
- 3.Unwin N, Whiting D, Guariguata L, Ghyoot G, Gan D, editors. In Diabetes Atlas. 5th ed. Belgium: International Diabetes Federation; 2011. [Google Scholar]
- 4.Samuel VT, Shulman GI. Mechanisms for insulin resistance: Common threads and missing links. Cell. 2012;148:852–71. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalra S, Mukherjee JJ, Venkataraman S, Bantwal G, Shaikh S, Saboo B, et al. Hypoglycemia: The neglected complication. Indian J Endocrinol Metab. 2013;17:819–34. doi: 10.4103/2230-8210.117219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycaemia in type 2 diabetes: A patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2012;55:1577–96. doi: 10.1007/s00125-012-2534-0. [DOI] [PubMed] [Google Scholar]
- 7.Umpierrez GE, Smiley D, Jacobs S, Peng L, Temponi A, Mulligan P, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery) Diabetes Care. 2011;34:256–61. doi: 10.2337/dc10-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGill JB, Sloan L, Newman J, Patel S, Sauce C, von Eynatten M, et al. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment: A 1-year, randomized, double-blind, placebo-controlled study. Diabetes Care. 2013;36:237–44. doi: 10.2337/dc12-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charpentier G, Riveline JP, Varroud-Vial M. Management of drugs Affecting Blood Glucose in Diabetic Patients with Renal Failure. Management. 2011. [Last cited on 2015 Sep 26]. Available from: http://www.em.consulte.com/en/module/displayarticle/article/79946/impression/vue3 . [PubMed]
- 10.Rhee CM, Leung AM, Kovesdy CP, Lynch KE, Brent GA, Kalantar-Zadeh K. Updates on the management of diabetes in dialysis patients. Semin Dial. 2014;27:135–45. doi: 10.1111/sdi.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abe M, Kaizu K, Matsumoto K. Evaluation of the hemodialysis-induced changes in plasma glucose and insulin concentrations in diabetic patients: Comparison between the hemodialysis and non-hemodialysis days. Ther Apher Dial. 2007;11:288–95. doi: 10.1111/j.1744-9987.2007.00492.x. [DOI] [PubMed] [Google Scholar]
- 12.Abe M, Kaizu K, Matsumoto K. Plasma insulin is removed by hemodialysis: Evaluation of the relation between plasma insulin and glucose by using a dialysate with or without glucose. Ther Apher Dial. 2007;11:280–7. doi: 10.1111/j.1744-9987.2007.00491.x. [DOI] [PubMed] [Google Scholar]
- 13.Kazempour-Ardebili S, Lecamwasam VL, Dassanyake T, Frankel AH, Tam FW, Dornhorst A, et al. Assessing glycemic control in maintenance hemodialysis patients with type 2 diabetes. Diabetes Care. 2009;32:1137–42. doi: 10.2337/dc08-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inaba M, Okuno S, Kumeda Y, Yamada S, Imanishi Y, Tabata T, et al. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: Effect of anemia and erythropoietin injection. J Am Soc Nephrol. 2007;18:896–903. doi: 10.1681/ASN.2006070772. [DOI] [PubMed] [Google Scholar]
- 15.Kalantar-Zadeh K, Kopple JD, Regidor DL, Jing J, Shinaberger CS, Aronovitz J, et al. A1C and survival in maintenance hemodialysis patients. Diabetes Care. 2007;30:1049–55. doi: 10.2337/dc06-2127. [DOI] [PubMed] [Google Scholar]
- 16.Bry L, Chen PC, Sacks DB. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin Chem. 2001;47:153–63. [PubMed] [Google Scholar]
- 17.Rondeau P, Bourdon E. The glycation of albumin: Structural and functional impacts. Biochimie. 2011;93:645–58. doi: 10.1016/j.biochi.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Little RR, Rohlfing CL, Sacks DB. National Glycohemoglobin Standardization Program (NGSP) Steering Committee. Status of hemoglobin A1c measurement and goals for improvement: From chaos to order for improving diabetes care. Clin Chem. 2011;57:205–14. doi: 10.1373/clinchem.2010.148841. [DOI] [PubMed] [Google Scholar]
- 19.Kishimoto M, Noda M. Glucose management in diabetic patients undergoing hemodialysis. Diabetol Int. 2014;5:84–91. [Google Scholar]
- 20.Sobngwi E, Enoru S, Ashuntantang G, Azabji-Kenfack M, Dehayem M, Onana A, et al. Day-to-day variation of insulin requirements of patients with type 2 diabetes and end-stage renal disease undergoing maintenance hemodialysis. Diabetes Care. 2010;33:1409–12. doi: 10.2337/dc09-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang CC. Treatment targets for diabetic patients on peritoneal dialysis: Any evidence? Perit Dial Int. 2007;27(Suppl 2):S176–9. [PubMed] [Google Scholar]
- 22.Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004;79:992–1000. doi: 10.4065/79.8.992. [DOI] [PubMed] [Google Scholar]
- 23.Oomichi T, Emoto M, Tabata T, Morioka T, Tsujimoto Y, Tahara H, et al. Impact of glycemic control on survival of diabetic patients on chronic regular hemodialysis: A 7-year observational study. Diabetes Care. 2006;29:1496–500. doi: 10.2337/dc05-1887. [DOI] [PubMed] [Google Scholar]
- 24.Zoungas S, Kerr PG, Lui M, Teede HJ. The impact of glycaemic control on outcomes in patients with end-stage renal disease and type 2 diabetes. Nephrology (Carlton) 2008;13:124–7. doi: 10.1111/j.1440-1797.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- 25.ACCORD Study Group. Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, et al. Action to control cardiovascular risk in diabetes (ACCORD) trial: Design and methods. Am J Cardiol. 2007;99:21i–33i. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 26.ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]


