Abstract
Background:
Diabetes is one of the world's biggest health problems and the disease affects almost all organ systems. The relationship between type 2 diabetes mellitus (T2DM) and bone mineral density (BMD) has been controversial. Early identification of reduction in bone mass in a diabetic patient may be helpful in preventing the bone loss and future fracture risks.
Objective:
The aim is to study the effect of T2DM on BMD among patients in South Karnataka.
Materials and Methods:
A cross-sectional study was conducted on 150 patients between 40 and 70 years of age which included 75 diabetic and 75 nondiabetic subjects. BMD was measured using qualitative ultrasound and the data were compared among age-matched subjects of both the groups. Statistical analysis was performed using unpaired Student's t-test and test of equality of proportions.
Results:
No significant difference was observed in bone density of both the groups. On further analyzing the data, incidence of osteoporosis was higher among diabetic subjects, whereas incidence of osteopenia was higher among nondiabetic subjects.
Conclusion:
Although significant difference in bone mineral density was not observed in both the groups, the incidence of osteoporosis was higher among type 2 diabetics. Hence, all type 2 diabetics should be evaluated for the risk of osteoporosis and should be offered appropriate preventive measures.
Key words: Bone mineral density, osteoporosis, qualitative ultrasound, type 2 diabetes
INTRODUCTION
Both diabetes and osteoporosis are common human diseases. Diabetes has evolved as one of the world's biggest health problems and the disease affects almost all organ systems with substantial morbidity and mortality. Osteoporosis is a silent disease with a harmful impact on bone health. Diabetes is often associated with changes in bone health and the term “Diabetic osteopathy” needs to be defined.
Most studies indicate less bone mineral density (BMD) with insulin dependent diabetes mellitus[1] but with type 2 diabetes some authors report increased,[2] some report decreased[3] and some others report unaltered[4] BMD. Metabolic bone disease is underestimated in our country due to unawareness of the same, both among patients as well as health providers. Early identification of reduction in bone mass in a diabetic patient may be helpful in preventing the bone loss and future fracture risk.
Interpretation of fracture data as a measure for bone health is particularly difficult in patients with long-standing diabetes. Visual and neurologic complications can predispose patients to accidents resulting in an increased fracture risk not necessarily dependent on bone density alone. Other factors that make studies difficult to interpret include the presence of diabetic renal disease, autonomic, and neuropathic changes that could contribute to a loss of BMD and a low level of physical activity related to diabetic complications.[1]
Diabetes could influence bone through several mechanisms, some of which may have contradictory effects. Obesity, widespread in type 2 diabetes mellitus (T2DM), is strongly associated with higher BMD, probably through mechanical loading and hormonal factors, including insulin, estrogen, and leptin.[5,6,7] Hyperinsulinemia may promote bone formation.[8] However, low levels of insulin and the progression of T2DM may cause reductions in BMD.
Higher glucose levels in the blood interact with several proteins to generate a higher concentration of advanced glycation end-products (AGEs). Yamagishi et al. hypothesized that AGEs in collagen may interact with bone to reduce bone strength, resulting in osteoporosis in patients with diabetes.[8,9] Accumulated AGEs in the body may stimulate apoptosis of osteoblasts, thereby contributing to the defective bone formation.[10]
Another indirect effect of hyperglycemia is glycosuria, which causes hypercalciuria, leading to decreased levels of calcium in the body and poor bone quality, thus hastening bone loss.[11,12] Some studies have shown low levels of Vitamin D with altered Vitamin D metabolism in patients with diabetic osteopenia.[13,14] In addition, microvascular complications of diabetes lead to reduced blood flow to bone and may contribute to bone loss and fragility.[15,16]
Diabetic osteopathy may need attention as one of the common disease complications. Recently, BMD has been identified as a key determinant of future fracture risks. Each standard deviation of a decrease in BMD yields three-fold increases in fracture risk.[17] Quantitative ultrasound (QUS) is a noninvasive, patient-friendly method used for estimation of the bone mass. It would also provide information regarding the bone elasticity and architecture.
Therefore, we decided to assess BMD of type 2 diabetic patients with more than 5 years of diabetes using QUS. The results from the diabetic patients were compared with nondiabetic age-matched control subjects, recruited from the same geographical region.
MATERIALS AND METHODS
After obtaining a time bound research committee approval and informed consent from the subjects, a cross-sectional study was conducted at the tertiary care center from December 2010 to July 2012. Adults between 40 and 70 years of age were selected, which included 75 diabetic subjects with at least 5 years of diabetes and 75 nondiabetic subjects. All patients and controls were subjected to detailed clinical evaluation and necessary blood investigations were done. Patients with chronic kidney disease (glomerular filtration rate <60 ml/min),[18] known cases of malabsorption syndromes, malignant diseases, chronic pancreatitis or pancreatotomy, primary hyperparathyroidism, thyroid function abnormalities, paget's disease, and inflammatory disorders such as rheumatoid arthritis, systemic lupus erythematosus, postmenopausal women or those with history of hysterectomy and patients treated with steroids, gonadotropin-releasing hormone agonists, gonadal hormones, immunosuppressants, anticonvulsants and calcium and Vitamin D supplements, thiazolidinediones and bed-ridden patients were excluded from the study.
Bone mineral density measurements
BMD was measured in the distal end of radius using QUS and the data were analyzed on the basis of t-score and Z-score using the WHO criteria.[19]
Osteopenia and osteoporosis were taken as abnormal BMD.
Data analysis
BMD data of type 2 diabetic subjects were compared with those without diabetes matched for age using unpaired Student's t-test. In patients with abnormal BMD, test of equality of proportions was used to compare osteopenia and osteoporosis.
RESULTS
Characteristics of the study populations with and without diabetes are given in Table 1. The mean age for diabetics was 56 years and nondiabetics was 54 years. Basic characteristics when compared, both groups had a similar body mass index (BMI), and no significant difference was found with regard to their smoking habits, alcohol intake, and history of fractures. Prevalence of hypertension was higher in diabetic subjects as compared to nondiabetics. Serum creatinine values also were higher in diabetic subjects. Other metabolic parameters such as serum calcium, phosphorous, and alkaline phosphate levels were similar in both the groups. BMD values were similar and no significant difference was found in both the groups.
Table 1.
Clinical characteristics of study population
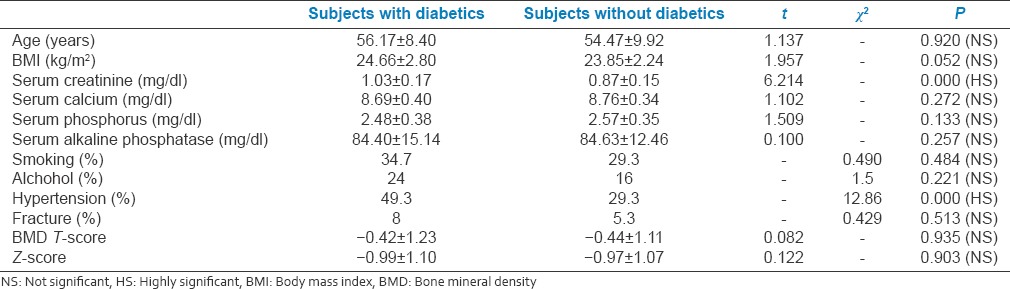
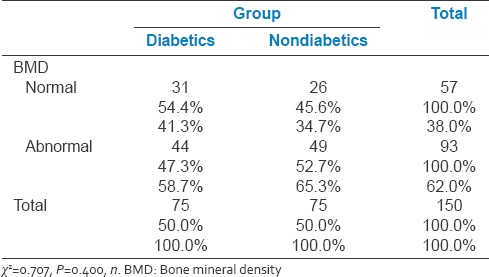
Table 2 depicts the BMD distribution among the study population. The majority of the participants (62%) in the study had abnormal BMD. Among them, 47% were diabetics and 52% were nondiabetics. The rest 38% with normal BMD, 54% were diabetics and 45% were nondiabetics. No significant difference was found in the groups with regard to BMD.
Table 2.
Distribution of bone mineral density among diabetics and nondiabetics
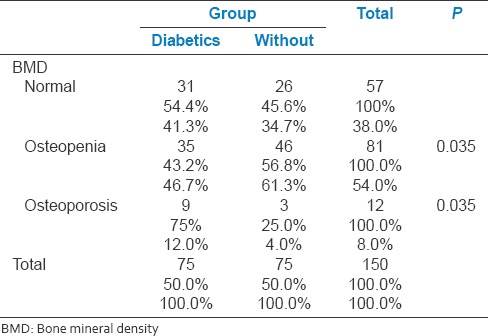
Sub-analysis of abnormal BMD was performedas osteopenia and osteoporosis, and on further studying the data, osteoporosis was found to be higher in diabetics and osteopenia was found higher in nondiabetics. Of those who had osteoporosis, 75% of them were diabetics and of those who had osteopenia 56% of them were non-diabetics. Table 3 illustrates the distribution of osteopenia and osteoporosis among the study population.
Table 3.
Distribution of osteopenia and osteoporosis among diabetics and nondiabetics
The diabetic subjects who had normal BMD, the mean duration of diabetes was 6 years as compared to 9 years in those with abnormal BMD. The mean hemoglobin A1c (HbA1c) value of diabetics with abnormal BMD was 8.1% as compared to 6.8% in those normal BMD.
DISCUSSION
Osteoporosis is a prevalent metabolic bone disease, and its occurrence in diabetic patients further increases their burden of disease. BMD is used as an indicator for assessing susceptibility to osteoporosis.[19,20,21] The study presents the status of BMD among a cohort of diabetic patients attending tertiary care center in South Karnataka. The total number of patients studied was 150 which included 75 diabetic and 75 nondiabetic subjects. The mean duration of diabetes among cases was 8 years.
In this study, no significant difference was observed in BMD among both the groups. These findings were supported by studies carried out by Sosa et al.[4] and Wakasugi et al.[5] When further analysis of abnormal BMD was performed as osteopenia and osteoporosis, the incidence of osteoporosis was found higher among diabetics, whereas incidence of osteopenia was found higher among nondiabetics.
These findings were against the observations seen in few studies. Ishida et al. in their study assessed the degree of diabetic osteopenia and serum Vitamin D metabolic level in type 2 diabetics and found decreased bone mass in diabetic subjects.[11] Gregorio et al. observed reduced bone mineral content in poorly controlled diabetic subjects.[3] Interestingly, some other studies had shown diabetes as a promoter for bone health. Barrett-Connor and Holbrook found that women with T2DM had a significantly higher BMD level than women with normal glucose tolerance.[2] Meema and Meema postulated in 1967 that diabetes was an anti-osteoporotic condition.[22]
In this study, the prevalence of hypertension was higher among diabetic subjects and similar was their serum creatinine value, although the mean serum creatinine values of both the groups were within normal range. The mean serum creatinine among diabetics was 1.0 mg/dl and among nondiabetics was 0.8 mg/dl. Diabetic subjects with abnormal BMD had a mean creatinine of 1.11 mg/dl and with normal BMD had a mean creatinine of 0.9 mg/dl. Thus, a negative correlation was found between serum creatinine values and BMD. These findings stress on the role of kidneys in the maintenance of bone health.
In this study, no significant difference was observed in the BMI of two groups and the study did not signify BMI as a predictor for BMD. In diabetic subjects who had normal BMD, mean BMI was 25.3 kg/m2 and in those with abnormal BMD, mean BMI was 24.1 kg/m2. This was against a meta-analysis that had demonstrated BMI as an important predictor of BMD and that low BMI is associated with decreased BMD, increased risk of osteoporosis and increased risk of fracture.[23]
In this study, a negative association was observed between the duration of diabetes and BMD. These results were consistent with findings of Wakasugi et al.[5] and Kao et al.[15] They demonstrated duration of diabetes as a risk factor for decreased BMD in T2DM subjects. This study also demonstrated a negative association between glycemic control and BMD. Those with abnormal BMD had a mean HbAIc of 8.1% and normal BMD had a mean HbAIc of 6.8% which was statistically significant. The mean fasting blood sugar among diabetics with normal BMD was 122 mg/dl and abnormal BMD was 174 mg/dl, the differences were statistically significant. These findings were supported by studies carried out by Okazaki et al. who found that metabolic improvements in poorly controlled T2DM decreased bone loss within a short period.[24] However, these findings were against the observation of Weinstock et al. who found no significant relationship between BMD, duration of diabetes or HbAIc.[25]
Although, in this study diabetic subjects had no significant difference in BMD when compared to nondiabetic counterparts, the incidence of osteoporosis was higher among them. The study revealed a negative association between glycemic control, duration of diabetes and BMD. The study also observed an increasing creatinine values among those with abnormal BMD. Henceforth, all diabetics should be evaluated for the risk of osteoporosis and appropriate preventive measures may be offered.
CONCLUSION
Although diabetic subjects had no significant difference in BMD as compared to the nondiabetic counterparts, prevalence of osteoporosis was higher among them
BMI had no effect on BMD scores
BMD scores had negative correlation with duration of diabetes and glycemic control.
However, additional studies are required to determine whether osteoporosis is aggravated by T2DM and whether it should be considered as one of the long-term complications of diabetes. Further studies with Vitamin D levels and assessment of BMD using DEXA scan may be required. Thus, identifying and evaluating populations at increased risk of developing osteoporosis is critical in disease prevention and management.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Strotmeyer ES, Cauley JA, Orchard TJ, Steenkiste AR, Dorman JS. Middle-aged premenopausal women with type 1 diabetes have lower bone mineral density and calcaneal quantitative ultrasound than nondiabetic women. Diabetes Care. 2006;29:306–11. doi: 10.2337/diacare.29.02.06.dc05-1353. [DOI] [PubMed] [Google Scholar]
- 2.Barrett-Connor E, Holbrook TL. Sex differences in osteoporosis in older adults with non-insulin-dependent diabetes mellitus. JAMA. 1992;268:3333–7. [PubMed] [Google Scholar]
- 3.Gregorio F, Cristallini S, Santeusanio F, Filipponi P, Fumelli P. Osteopenia associated with non-insulin-dependent diabetes mellitus: What are the causes? Diabetes Res Clin Pract. 1994;23:43–54. doi: 10.1016/0168-8227(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 4.Sosa M, Dominguez M, Navarro MC, Segarra MC, Hernández D, de Pablos P, et al. Bone mineral metabolism is normal in non-insulin-dependent diabetes mellitus. J Diabetes Complications. 1996;10:201–5. doi: 10.1016/1056-8727(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 5.Wakasugi M, Wakao R, Tawata M, Gan N, Koizumi K, Onaya T. Bone mineral density measured by dual energy x-ray absorptiometry in patients with non-insulin-dependent diabetes mellitus. Bone. 1993;14:29–33. doi: 10.1016/8756-3282(93)90252-6. [DOI] [PubMed] [Google Scholar]
- 6.Reid IR, Evans MC, Cooper GJ, Ames RW, Stapleton J. Circulating insulin levels are related to bone density in normal postmenopausal women. Am J Physiol. 1993;265(4 Pt 1):E655–9. doi: 10.1152/ajpendo.1993.265.4.E655. [DOI] [PubMed] [Google Scholar]
- 7.Paul RG, Bailey AJ. Glycation of collagen: The basis of its central role in the late complications of ageing and diabetes. Int J Biochem Cell Biol. 1996;28:1297–310. doi: 10.1016/s1357-2725(96)00079-9. [DOI] [PubMed] [Google Scholar]
- 8.Yamagishi S, Nakamura K, Inoue H. Possible participation of advanced glycation end products in the pathogenesis of osteoporosis in diabetic patients. Med Hypotheses. 2005;65:1013–5. doi: 10.1016/j.mehy.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Alikhani M, Alikhani Z, Boyd C, MacLellan CM, Raptis M, Liu R, et al. Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways. Bone. 2007;40:345–53. doi: 10.1016/j.bone.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raskin P, Stevenson MR, Barilla DE, Pak CY. The hypercalciuria of diabetes mellitus: Its amelioration with insulin. Clin Endocrinol (Oxf) 1978;9:329–35. doi: 10.1111/j.1365-2265.1978.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 11.Ishida H, Seino Y, Matsukura S, Ikeda M, Yawata M, Yamashita G, et al. Diabetic osteopenia and circulating levels of Vitamin D metabolites in type 2 (noninsulin-dependent) diabetes. Metabolism. 1985;34:797–801. doi: 10.1016/0026-0495(85)90101-5. [DOI] [PubMed] [Google Scholar]
- 12.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of Vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–29. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wientroub S, Eisenberg D, Tardiman R, Weissman SL, Salama R. Is diabetic osteoporosis due to microangiopathy? Lancet. 1980;2:983. doi: 10.1016/s0140-6736(80)92145-5. [DOI] [PubMed] [Google Scholar]
- 14.Vogt MT, Cauley JA, Kuller LH, Nevitt MC. Bone mineral density and blood flow to the lower extremities: The study of osteoporotic fractures. J Bone Miner Res. 1997;12:283–9. doi: 10.1359/jbmr.1997.12.2.283. [DOI] [PubMed] [Google Scholar]
- 15.Kao WH, Kammerer CM, Schneider JL, Bauer RL, Mitchell BD. Type 2 diabetes is associated with increased bone mineral density in Mexican-American women. Arch Med Res. 2003;34:399–406. doi: 10.1016/j.arcmed.2002.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Bonjour JP, Chevalley T, Rizzoli R, Ferrari S. Gene-environment interactions in the skeletal response to nutrition and exercise during growth. Med Sport Sci. 2007;51:64–80. doi: 10.1159/000103005. [DOI] [PubMed] [Google Scholar]
- 17.Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20:1185–94. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 18.Bacchetta J, Boutroy S, Juillard L, Vilayphiou N, Guebre-Egziabher F, Pelletier S, et al. Bone imaging and chronic kidney disease: Will high-resolution peripheral tomography improve bone evaluation and therapeutic management? J Ren Nutr. 2009;19:44–9. doi: 10.1053/j.jrn.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 19.Genant HK, Cooper C, Poor G, Reid I, Ehrlich G, Kanis J, et al. Interim report and recommendations of the World Health Organization Task-Force for Osteoporosis. Osteoporos Int. 1999;10:259–64. doi: 10.1007/s001980050224. [DOI] [PubMed] [Google Scholar]
- 20.Weaver CM. The role of nutrition on optimizing peak bone mass. Asia Pac J Clin Nutr. 2008;17(Suppl 1):135–7. [PubMed] [Google Scholar]
- 21.Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: A summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–39. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 22.Meema HE, Meema S. The relationship of diabetes mellitus and body weight to osteoporosis in elderly females. Can Med Assoc J. 1967;96:132–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes – A meta-analysis. Osteoporos Int. 2007;18:427–44. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 24.Okazaki R, Totsuka Y, Hamano K, Ajima M, Miura M, Hirota Y, et al. Metabolic improvement of poorly controlled noninsulin-dependent diabetes mellitus decreases bone turnover. J Clin Endocrinol Metab. 1997;82:2915–20. doi: 10.1210/jcem.82.9.4258. [DOI] [PubMed] [Google Scholar]
- 25.Weinstock RS, Goland RS, Shane E, Clemens TL, Lindsay R, Bilezikian JP. Bone mineral density in women with type II diabetes mellitus. J Bone Miner Res. 1989;4:97–101. doi: 10.1002/jbmr.5650040114. [DOI] [PubMed] [Google Scholar]


