Abstract
Context:
Oxidative stress is associated with the pathogenesis of many systemic diseases including chronic periodontitis. Periodontal pathogen activated neutrophils liberate the reactive oxygen species (ROS), which causes the destruction of periodontal tissues. Antioxidants modulate the ROS production and inhibit the tissue destruction.
Aim:
We aimed to evaluate the oxidative stress marker malondialdehyde (MDA) in chronic periodontitis patients following scaling and root planing (SRP) after systemic lycopene supplementation.
Materials and Methods:
This was an interventional single arm study. Twenty systemically healthy patients with chronic periodontitis were recruited. Clinical parameters modified gingival index, probing depth, clinical attachment loss were recorded, and serum MDA levels were assessed by thiobarbituric acid reactive substances assay. Patients were supplemented with 8 mg lycopene daily for 2 months following SRP treatment. All the parameters were assessed at pretreatment and 2 months and 6 months posttreatment.
Results:
From pretreatment to posttreatment at 2 months, the mean values of all parameters were reduced. While from 2 to 6 months when lycopene was not administered, an increase in the mean values of all the parameters was observed; however, these values were still below baseline values.
Conclusion:
There was a reduction in oxidative stress and improvement in clinical parameters following systemic antioxidant therapy along with SRP, which was maintained up to 4 months after discontinuation of lycopene treatment.
Key words: Antioxidant therapy, chronic periodontitis, malondialdehyde, oxidative stress
INTRODUCTION
Oxidative stress is defined as a persistent imbalance between the production of highly reactive molecular species (e.g., reactive oxygen species [ROS], reactive nitrogen species) and anti-oxidant defenses.[1] The produced ROS, such as superoxide anion, hydroxyl radical, and peroxyl radical, can damage many biological molecules including DNA, lipids, and proteins. Prolonged existence of these ROS promotes severe tissue damage and cell death.[2] Oxidative stress is thought to play a causative role in the pathogenesis of many diseases such as cardiovascular disease,[3] rheumatoid arthritis,[4] diabetes,[5] and chronic periodontitis.[6]
Much of the damage done to periodontal tissues and supporting bone structure is due to the emission of ROS or free radicals. The body's antioxidant defense system plays a crucial role in fighting inflammatory chronic diseases such as periodontal disease. Antioxidant deficiencies (both local and systemic) have been directly linked to periodontal disease.[7] Antioxidants, enzymes, and the oxidation products of protein, lipids, and DNA were widely used to indicate the oxidative status and are useful as biomarkers of oxidative stress. There are only few studies, which have shown the biomarker levels of oxidative stress in the peripheral blood of periodontitis patients.[8,9]
Malondialdehyde (MDA) is the principal product of polyunsaturated fatty acid peroxidation that can indicate the increase of oxidative stress.[10] Thiotbarbituric acid reactive substance levels are increased in the peripheral blood of chronic periodontitis patients.[7] The aim of the present study was to evaluate the oxidative stress marker (MDA) before and after scaling and root planing (SRP) along with an antioxidant (lycopene) supplementation in chronic periodontitis patients.
MATERIALS AND METHODS
This study was submitted to www.ctri.nic.in with reference number REF/2016/01/010490 and approved by Dr. NTR University of Health Sciences with number 10/98/12. This was an interventional single arm study. This study was undertaken in a Tertiary Referral Care Centre in Hyderabad. Institutional Ethical Committee approved the study. Forty-two patients were screened, ten were excluded based on the inclusion criteria. Thirty-two systemically healthy subjects aged between 35 and 50 years with moderate periodontitis were explained about the protocol. Twenty subjects (10 males and 10 females) who had given consent were recruited in the study.
Inclusion and exclusion criteria
Patients who had not undergone any nonsurgical and surgical periodontal treatment and patients who had not used any antibiotics, anti-inflammatory drugs, and any other over the counter antioxidants such as Vitamin C and Vitamin E with in past 6 months were included in the study. Chronic periodontitis patients were selected according to the American academy of periodontology criteria, i.e., minimum of 15 teeth being present, at least probing depth (PD) ≥4 mm and clinical attachment loss (CAL) ≥2 mm.[11]
Patients with any systemic disease, pregnant and lactating women and smokers (former smokers and current smokers) were excluded from the study.
The protocol was explained to the patients and written consent was taken from those who were interested to participate in the study.
Assessment of clinical parameters
Modified gingival index (MGI)[12] was recorded to assess the severity of gingival inflammation for all the teeth excepting third molars. PD was recorded from base of the pocket to the gingival margin. CAL was recorded from the cemento enamel junction to the tip of the periodontal probe. All the measurements were recorded with William's periodontal probe (Hufreidy, Chicago).
Standardization of clinical measurements
This was possible by using an acrylic stent, which was fabricated from the plaster cast poured after taking an alginate impression.
Outcome measures
The primary outcome measure assessed was serum MDA levels and secondary outcome measures assessed were the clinical parameters such as MGI, PD, and CAL.
Examiner calibration
Investigator (Koduganti Rekha Rani) examined the patients at base line for the clinical parameters and again after 24 h to check the reproducibility of the measurements. The calibrations were considered reproducible when the baseline and 24-h measurements tallied within 1 mm, 95% of the time.
Treatment protocol
Investigator (Manasa Ambati) performed SRP at the second visit (after 24 h) after which patients were given lycopene soft gels to be taken 8 mg daily (2 mg of Lycored soft gels, 2 soft gels twice daily) for 2 months.
Composition of lycored (Jagsonpal pharma) soft gels
Lycopene – 2 mg, zinc sulfate monohydrate – 7.5 μg, monohydrated selenium oxide – 35 μg. Patient compliance toward the intake of lycopene was evaluated at the end of 1–2 months by checking the bottles physically.
Blood sample collection and serum separation
Blood samples were collected by venipuncture of antecubital vein. One milliliter of blood was collected in a test tube. Ten minutes after collection, blood was subjected to centrifugation at 3000 rpm for 10 min; the supernatant straw colored fluid (serum) was separated and collected in storage vials for serum MDA estimation.
Assessment of biochemical parameter: Serum malondialdehyde
Oxidative stress in the cellular environment results in the formation of highly reactive and unstable lipid hydroperoxides. Decomposition of the unstable peroxides derived from polyunsaturated fatty acids results in the formation of MDA,[13] which was quantified calorimetrically following its controlled reaction with thiobarbituric acid (TBA). MDA assessment was done by spectrophotometric estimation of serum TBA reactive substances (TBARS),[14] i.e., TBARS assay. The TBARS assay measures MDA, a reactive compound formed from lipid peroxides that are generated under conditions of oxidative stress. MDA forms an adduct with TBA. Results are calculated from a standard curve constructed with authentic MDA.
Principle of the test
Lipid peroxidation is estimated based on the formation of MDA. MDA is a stable product of lipid peroxidation which reacts with TBA reagent under acidic conditions to form a colorless to faint pink product. TBA adducts of MDA is a stable cromophore with maximal absorbance at 532 nm. This absorbance was recorded and was converted to nano moles/ml comparing them with standard solutions of known concentration.
Statistical analysis
The observations recorded were subjected to statistical analysis by one-way analysis of variance with post hoc Bonferroni test.
RESULTS
All the patients recruited for the study reported for the follow-up both at 2 months and 6 months. No patients complained of adverse effects due to lycopene. Secondary outcome measures MGI, PD, CAL have shown statistically significant improvements after treatment at 2 months follow-up but at 6 months there was a slight increase in these parameters which were, however, still less than that of baseline values.
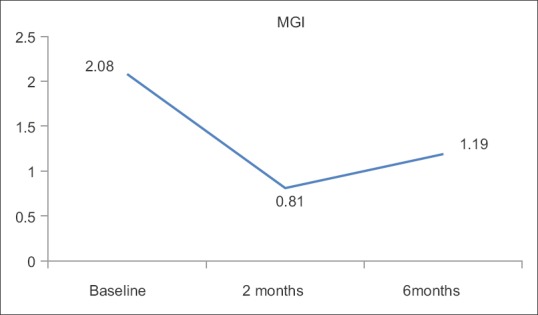
MGI is the most common measurement to assess gingival inflammation. At base line, the mean MGI was 2.08 ± 0.39, which indicates moderate inflammation and after treatment reduced to 0.81 ± 0.29 (clinically the gingiva was free of inflammation). At 6 months, mild inflammation reappeared with score 1.19 ± 0.29.
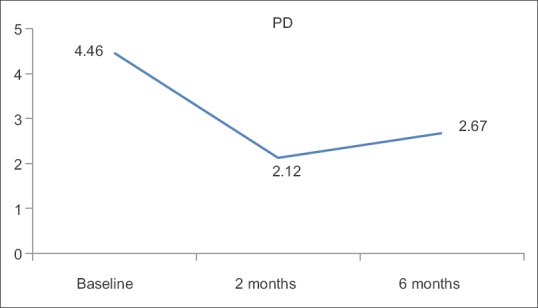
PD is the key measurement for periodontal disease but it does not take into consideration the amount of periodontal tissue destruction. In this regard, measurement of CAL is helpful. The mean reduction of PD and CAL at 2 months (2.12 ± 0.45 and 2.99 ± 0.67, respectively) shows decrease in periodontal tissue destruction but this state was not stable and increased at 6 months (2.67 ± 0.39 and 3.57 ± 0.58) indicating the need for maintenance therapy.
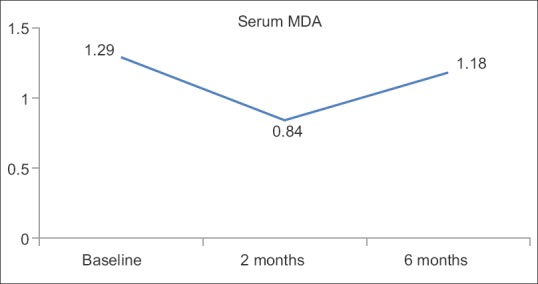
Primary outcome measure serum MDA levels (as oxidative stress marker) was reduced at 2 months (0.84 ± 0.20) and 6 months (1.18 ± 0.26) follow-up from baseline values (1.29 ± 0.25) showing reduction in oxidative stress and effectiveness of SRP and adjunctive antioxidant lycopene.
DISCUSSION
Chronic periodontitis is a bacterially-induced inflammatory disease that destroys the connective tissue and alveolar bone support of the teeth. In a report by Kornman,[15] it was described that although bacterial activation of immune inflammatory mechanisms are the prime cause, the biochemical changes caused by innate immunity play a specific role in the pathogenesis of periodontal disease. Polymorphonuclear (PMN) leukocytes act as the primary mediators of the host response against proliferating periodontal pathogenic microorganisms. Activated PMN's produce a large amount of ROS and result in destruction of periodontal tissues.[6,16] ROS causes peroxidation of proteins, lipids, and DNA. MDA is one of the end products of lipid peroxidation whose levels are increased in periodontitis patients. The present study was undertaken to evaluate the serum MDA levels in chronic periodontitis patients following SRP along with systemic antioxidant (lycopene) supplementation.
It is well known that SRP remains a gold standard treatment for chronic periodontitis.[17,18] Some studies observed that there was an increase in oxidative stress markers such as 4-Hydroxy-2-nonenal,[19] 8-hydroxy-2'-deoxyguanosine[20] (products of DNA peroxidation) in chronic periodontitis patients. Decrease in antioxidant enzymes such as superoxide dismutase,[21] glutathione peroxidase,[22] and total antioxidant capacity in chronic periodontitis patients has been observed and their levels improved following SRP. The present study correlates with the findings of other observational studies[22,23,24,25] in showing that lipid peroxidation is high in chronic periodontitis. In this study, it was observed that there was a decrease in serum MDA levels at 2 months and 6 months after SRP [Table 1 and Figure 4].
Table 1.
Comparison of each parameter at pre- and post-treatment (2 months and 6 months follow-up)
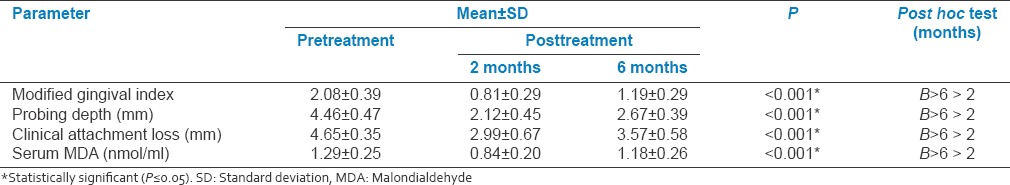
Figure 4.
Serum malondialdehyde in nmol/ml
The present study also focused on the systemic antioxidant treatment by lycopene supplementation for 2 months, following which there was a significant reduction in the clinical parameters, serum MDA, which were well maintained for 6 months [Table 1 and Figures 1–4].
Figure 1.
Modified gingival index
Figure 2.
Probing depth in mm
Figure 3.
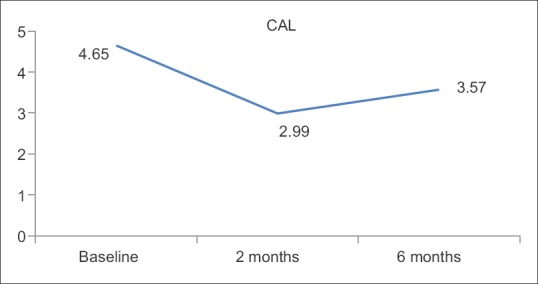
Clinical attachment loss in mm
Studies done by Chandra et al.[26] and Arora et al.[27] have shown that systemic lycopene supplementation was effective in gingivitis and periodontitis patients, for 2 months. However, in the present study, long-term follow-up (i.e.., 6 months) of the patients was done and it was found that serum MDA levels were still less than that of baseline values [Table 1 and Figure 4].
This study is also in accordance with the findings of the study done by Aziz et al.[28] where in nonsurgical periodontal therapy significantly reduced the serum MDA levels at 3 months follow-up, whereas the present study included antioxidant treatment along with SRP showing it as an effective adjuvant. In a recent study by Nguyen et al.,[29] they investigated the MDA levels in acute coronary syndrome patients with chronic periodontitis and the results indicated that MDA can serve as a biomarker in periodontitis patients with cardiovascular diseases.
Limitations
Since the sample size is small the results obtained may not be conclusive therefore larger sample would further strengthen the study. MDA was the only biomarker taken in this study as it is the major lipid peroxidation product; however, the measurement of other lipid peroxidation products such as conjugated dienes, volatile hydrocarbons was not done. We also have not controlled for any potential placebo effects.
CONCLUSION
In this study, it was observed that the serum MDA levels as well as the clinical parameters (MGI, PD, CAL) showed a significant improvement posttreatment when chronic periodontitis patients were administered systemic lycopene for a period of 2 months. Hence, such antioxidant supplementation as adjunct therapy for the clinical management of chronic periodontitis should be further explored.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We are thankful to Mr. Shivaprakash, faculty of National Institute of Nutrition for spectrophotometrical assessment of malondialdehyde and Dr. Kalyan Chakravarthy for statistical analysis. Jagsonpal pharma for providing lycopene soft gels.
REFERENCES
- 1.Halliwell B. Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. Am J Med. 1991;91:14S–22S. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- 2.Arnhold J. Free radicals – Friends or foes? Properties, functions, and secretion of human myeloperoxidase. Biochemistry (Mosc) 2004;69:4–9. doi: 10.1023/b:biry.0000016344.59411.ee. [DOI] [PubMed] [Google Scholar]
- 3.Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18:655–73. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 4.Hitchon CA, El-Gabalawy HS. Oxidation in rheumatoid arthritis. Arthritis Res Ther. 2004;6:265–78. doi: 10.1186/ar1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24:816–23. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 6.Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000. 2007;43:160–232. doi: 10.1111/j.1600-0757.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 7.Liu Z, Liu Y, Song Y, Zhang X, Wang S, Wang Z. Systemic oxidative stress biomarkers in chronic periodontitis: A meta-analysis. Dis Markers [Article on the Internet] 2014. [Cited on 2015 Mar 15]. Article ID 931083: [about 10 p.]. Available from: http://www.hindawi.com/jounals/dm/2014/931083/cta/ [DOI] [PMC free article] [PubMed]
- 8.Abou Sulaiman AE, Shehadeh RM. Assessment of total antioxidant capacity and the use of Vitamin C in the treatment of non-smokers with chronic periodontitis. J Periodontol. 2010;81:1547–54. doi: 10.1902/jop.2010.100173. [DOI] [PubMed] [Google Scholar]
- 9.Wei D, Zhang XL, Wang YZ, Yang CX, Chen G. Lipid peroxidation levels, total oxidant status and superoxide dismutase in serum, saliva and gingival crevicular fluid in chronic periodontitis patients before and after periodontal therapy. Aust Dent J. 2010;55:70–8. doi: 10.1111/j.1834-7819.2009.01123.x. [DOI] [PubMed] [Google Scholar]
- 10.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–28. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Cionca N, Giannopoulou C, Ugolotti G, Mombelli A. Amoxicillin and metronidazole as an adjunct to full-mouth scaling and root planing of chronic periodontitis. J Periodontol. 2009;80:364–71. doi: 10.1902/jop.2009.080540. [DOI] [PubMed] [Google Scholar]
- 12.Lobene RR, Weatherford T, Ross NM, Lamm RA, Menaker L. A modified gingival index for use in clinical trials. Clin Prev Dent. 1986;8:3–6. [PubMed] [Google Scholar]
- 13.Pryor WA. The antioxidant nutrients and disease prevention – What do we know and what do we need to find out? Am J Clin Nutr. 1991;53(1 Suppl):391S–3S. doi: 10.1093/ajcn/53.1.391S. [DOI] [PubMed] [Google Scholar]
- 14.Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9:515–40. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 15.Kornman KS. Mapping the pathogenesis of periodontitis: A new look. J Periodontol. 2008;79(8 Suppl):1560–8. doi: 10.1902/jop.2008.080213. [DOI] [PubMed] [Google Scholar]
- 16.Canakçi CF, Ciçek Y, Canakçi V. Reactive oxygen species and human inflammatory periodontal diseases. Biochemistry (Mosc) 2005;70:619–28. doi: 10.1007/s10541-005-0161-9. [DOI] [PubMed] [Google Scholar]
- 17.Drisko CH. Nonsurgical periodontal therapy. Periodontol 2000. 2001;25:77–88. doi: 10.1034/j.1600-0757.2001.22250106.x. [DOI] [PubMed] [Google Scholar]
- 18.Cobb CM. Clinical significance of non-surgical periodontal therapy: An evidence-based perspective of scaling and root planing. J Clin Periodontol. 2002;29(Suppl 2):6–16. [PubMed] [Google Scholar]
- 19.Pradeep AR, Agarwal E, Bajaj P, Rao NS. 4-Hydroxy-2-nonenal, an oxidative stress marker in crevicular fluid and serum in type 2 diabetes with chronic periodontitis. Contemp Clin Dent. 2013;4:281–5. doi: 10.4103/0976-237X.118342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konopka T, Król K, Kopec W, Gerber H. Total antioxidant status and 8-hydroxy-2'-deoxyguanosine levels in gingival and peripheral blood of periodontitis patients. Arch Immunol Ther Exp (Warsz) 2007;55:417–22. doi: 10.1007/s00005-007-0047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh N, Chander Narula S, Kumar Sharma R, Tewari S, Kumar Sehgal P. Vitamin E supplementation, superoxide dismutase status, and outcome of scaling and root planing in patients with chronic periodontitis: A randomized clinical trial. J Periodontol. 2014;85:242–9. doi: 10.1902/jop.2013.120727. [DOI] [PubMed] [Google Scholar]
- 22.Patel SP, Rao NS, Pradeep AR. Effect of nonsurgical periodontal therapy on crevicular fluid and serum glutathione peroxidase levels. Dis Markers. 2012;32:1–7. doi: 10.3233/DMA-2012-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akalin FA, Baltacioglu E, Alver A, Karabulut E. Lipid peroxidation levels and total oxidant status in serum, saliva and gingival crevicular fluid in patients with chronic periodontitis. J Clin Periodontol. 2007;34:558–65. doi: 10.1111/j.1600-051X.2007.01091.x. [DOI] [PubMed] [Google Scholar]
- 24.Trivedi S, Lal N, Mahdi AA, Mittal M, Singh B, Pandey S. Evaluation of antioxidant enzymes activity and malondialdehyde levels in patients with chronic periodontitis and diabetes mellitus. J Periodontol. 2014;85:713–20. doi: 10.1902/jop.2013.130066. [DOI] [PubMed] [Google Scholar]
- 25.Baltacioglu E, Yuva P, Aydin G, Alver A, Kahraman C, Karabulut E, et al. Lipid peroxidation levels and total oxidant/antioxidant status in serum and saliva from patients with chronic and aggressive periodontitis. Oxidative stress index: A new biomarker for periodontal disease? J Periodontol. 2014;85:1432–41. doi: 10.1902/jop.2014.130654. [DOI] [PubMed] [Google Scholar]
- 26.Chandra RV, Prabhuji ML, Roopa DA, Ravirajan S, Kishore HC. Efficacy of lycopene in the treatment of gingivitis: A randomised, placebo-controlled clinical trial. Oral Health Prev Dent. 2007;5:327–36. [PubMed] [Google Scholar]
- 27.Arora N, Avula H, Avula JK. The adjunctive use of systemic antioxidant therapy (lycopene) in nonsurgical treatment of chronic periodontitis: A short-term evaluation. Quintessence Int. 2013;44:395–405. doi: 10.3290/j.qi.a29188. [DOI] [PubMed] [Google Scholar]
- 28.Aziz AS, Kalekar MG, Benjamin T, Suryakar AN, Prakashan MM, Bijle MN. Effect of nonsurgical periodontal therapy on some oxidative stress markers in patients with chronic periodontitis: A biochemical study. World J Dent. 2013;4:17–23. [Google Scholar]
- 29.Nguyen TT, Ngo LQ, Promsudthi A, Surarit R. Salivary lipid peroxidation in patients with generalized chronic periodontitis and acute coronary syndrome. J Periodontol. 2016;87:134–41. doi: 10.1902/jop.2015.150353. [DOI] [PubMed] [Google Scholar]


