Abstract
Articular cartilage injury poses a major challenge for both the patient and orthopaedician. Articular cartilage defects once formed do not regenerate spontaneously, rather replaced by fibrocartilage which is weaker in mechanical competence than the normal hyaline cartilage. Mesenchymal stem cells (MSCs) along with different growth factors and scaffolds are currently incorporated in tissue engineering to overcome the deficiencies associated with currently available surgical methods and to facilitate cartilage healing. MSCs, being readily available with a potential to differentiate into chondrocytes which are enhanced by the application of different growth factors, are considered for effective repair of articular cartilage after injury. However, therapeutic application of MSCs and growth factors for cartilage repair remains in its infancy, with no comparative clinical study to that of the other surgical techniques. The present review covers the role of MSCs, growth factors and scaffolds for the repair of articular cartilage injury.
Keywords: Cartilage injury, growth factors, mesenchymal stem cells, scaffolds
Introduction
The articular cartilage is a unique connective tissue that furnishes the diarthrodial joints with an exceptional resiliency and almost frictionless movement owing to its distinctive structural, biochemical and metabolic characteristics1. Cartilage is a highly differentiated tissue with no direct blood, lymph or nerve supply and a scarce number of less proliferative chondrocytes and has limited regeneration potential2,3,4. Articular cartilage is made up of various unique layers, each with unique properties allowing it to be a suitable cushion for weight dispersement5. The tissue comprises approximately 75 per cent water, 15 per cent type II collagen, 10 per cent proteoglycans and <2 per cent chondrocytes6. Proteoglycans provide resistance against the compression, while tensile strength comes from collagen fibers7. Chondrocytes that reside in the lacunae interact with extracellular matrix (ECM) by means of cell surface receptors called integrins. These receptors act as mechanical links between the cells and ECMs and aid in cell homoeostasis (Fig. 1). Many cytokines and growth factors that may be present in diarthrodial joints stimulate chondrocytes and synovial cells to synthesize proteinases such as aspartic, cysteine, serine and metalloproteinases. The proteinases are normally involved in maintaining the homoeostasis; however, sometimes, these may lead to pathological destruction of articular cartilage involving multiple pathways. Matrix metalloproteinases (MMPs) that can degrade all elements of ECM, have been regarded to be involved in arthritic degeneration of the joint8.
Fig. 1.
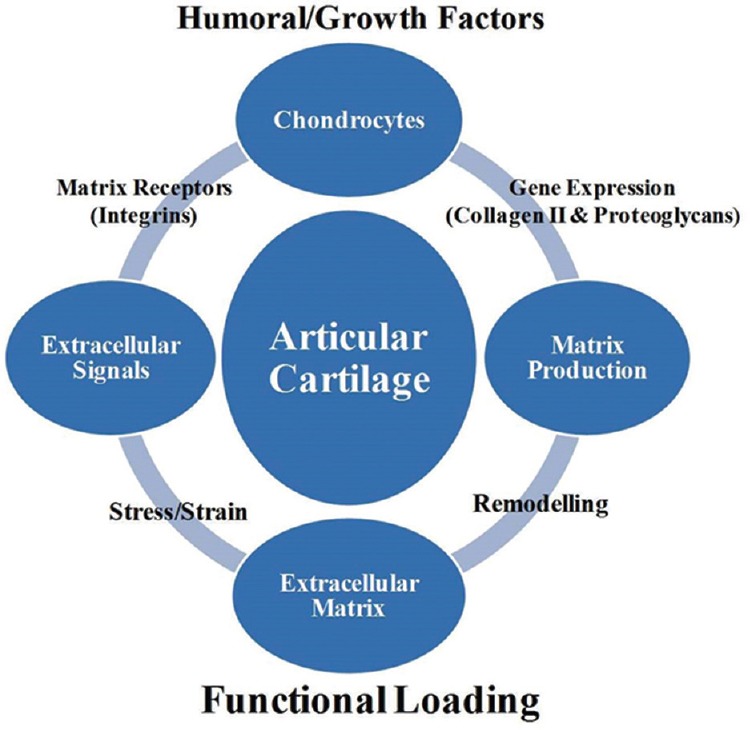
Mechanical signals and humoral factors interaction with chondrocytes for the maintenance of homoeostasis.
Articular cartilage defects are generally classified into partial or full thickness, with former confined to the cartilage tissue itself and the latter penetrating the subchondral bone (Fig. 2). In partial thickness defects, the site of lesion remains devoid of fibrin clot and thus of the reparative cells from bone marrow. These lesions do not heal spontaneously and appear similar even after several months and are analogous to the clefts or fissures seen in the early stages of the osteoarthritis (OA)9. Full thickness defects, an access to a limited number of reparative cells from bone marrow, result in the formation of fibrocartilage which is weaker in structure and mechanical competence9,10,11. Pain and consequent loss of function resulting from the articular cartilage insult emphasize the need for the development of advanced techniques for improved management of cartilage injury5,11,12.
Fig. 2.
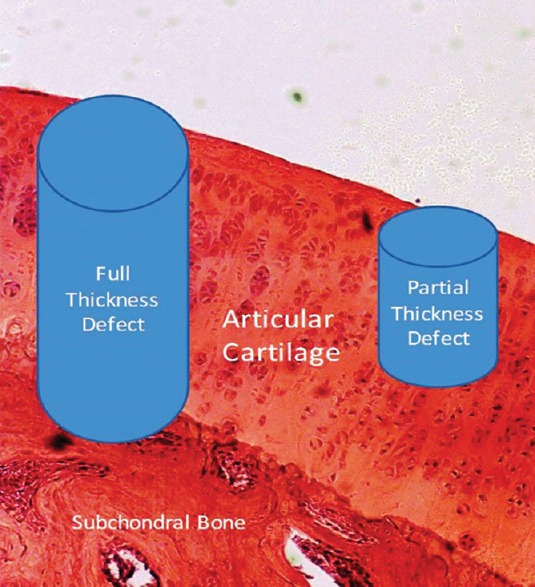
Articular cartilage defects: full thickness penetrating the subchondral bone and partial thickness within the cartilage tissue.
Many cartilage repair methods have been developed so far, however, without a satisfactory long-term solution. The main problem that arises is the formation of biomechanically weaker regeneration tissue that lacks integration with the native osteochondral tissue. At the site of injury, death of zone of cells hampers the production of matrix that may integrate laterally with the native cartilage tissue. Surgical techniques such as microfracture13, subchondral bone drilling14, lavage and debridement and perichondral arthroplasty15, periosteal arthroplasty11,16, autologous osteochondral transplantation17, autologous chondrocyte implantation (ACI)12,18,19,20 and autogenetic cancellous bone grafts21,22 have been attempted to form a new chondral surface. However, these techniques are limited to a small focal or medium-sized osteochondral defect and lack the potential to regenerate true hyaline cartilage23. ACI though has shown some good results, but due to the limited availability of chondrocytes, their proneness to dedifferentiate into fibroblasts and degeneration in pre-damaged cartilage, has limited its usefulness24,25.
Cartilage rehabilitation should be aimed at elimination of pain and prevention of onset of OA15,17,26, which can be achieved through the formation of actual hyaline cartilage. Currently, tissue engineering is being considered for better cartilage rehabilitation. For successful tissue engineering, three main components that are required include scaffold, cells and growth factors or cytokines. A scaffold provides a three-dimensional (3D) structure into which cells can grow making them less prone to deleterious environment. Growth factors or cytokines stimulate the cellular pathways for the proper functioning of cells. The present review discusses the possible roles of mesenchymal stem cells (MSCs), growth factors and scaffolds in the process of articular cartilage repair.
Mesenchymal stem cells (MSCs)
Existence of MSCs was first established by Friedenstein et al27, who demonstrated that certain cells present in the bone marrow can differentiate into the bone and cartilage. Later on, several other workers confirmed the finding and reported that MSCs isolated from the bone marrow have potential to proliferate extensively, to self-renew and to differentiate into cells of several lineages including chondrocytes28,29,30,31,32,33. To harvest their potential in cellular therapy, certain criteria were put forth by the International Society for Cell Therapy to confirm the cells as MSCs. The cells that are plastic adherent and express CD105, CD73, CD29 and CD90 surface molecules, but lack the expression of CD45, CD34, CD14 or CD11b, CD79a or CD19 and human leukocyte antigen-antigen D related (HLA-DR) surface molecules and can differentiate towards osteogenic, chondrogenic and adipogenic lineages, are regarded as MSCs34. Since MSCs have multiple sources, possess extensive proliferation potential and can differentiate into multiple lineages, these are currently perceived as attractive cell source for experimental and clinical studies in the area of regenerative medicine including cartilage repair29,35,36.
Johnstone et al28 first evaluated MSCs for chondrogenesis under in vitro conditions in 1998 using a specific medium. It was later found that addition of growth factors such as transforming growth factor-beta (TGF-β), bone morphogenetic proteins (BMPs), insulin-like growth factor (IGF) and parathyroid hormone-related peptide (PTHrP) may enhance chondrogenic potential of MSCs under in vitro conditions28,37,38,39,40. Currently, in vitro micromass culture method is in vogue to evaluate chondrogenic potential of MSCs. However, it may not produce the cartilage tissue comparable to the native one as the process does not mimic the sequences of cartilage formation that normally occur during foetal development. However, under in vivo conditions, MSCs do have a potential to differentiate into chondrocytes, stimulated by the signals arising from the microenvironment of the cartilage41,42,43,44. MSCs when implanted into osteochondral defects differentiate into chondrocytes41,45; however, when cartilage pellets differentiated from MSCs in vitro are transplanted subcutaneously, these either disappear46 or are calcified with vascular invasion24. In vivo MSC differentiation can be affected by the existing microenvironment, plausibly by the molecular signals generated by other resident cells of the tissue44,47. The induction can thus occur by cell surface receptor stimulation, through growth factors, ECM or the direct interaction with the surface proteins of other resident cells (chondrocytes)48,49. With the progression in understanding of embryonic development and biological features of stem cells, the tissue engineering approaches also improved. The repaired cartilage tissue approaching to native articular cartilage both in physiologic stratification and biomechanical features has been developed from stem cells under in vitro conditions. This has been possible after recapitulating different developmental processes of mesenchymal condensation induced by the growth factors, especially TGF-β50,51. During the condensation process, MSCs condense into cellular bodies [condensed mesenchymal cell bodies (CMBs)], undergo chondrogenic differentiation and ultimately form cartilaginous tissue. CMBs after loading onto the osseous tissue were found to generate cartilage on the superficial surface that interfaced with the underlying bone in in vitro studies. CMBs also develop mechanically strong cartilage to cartilage interface, leading to the production of seamless interface and thus complete integration52.
MSCs are generally considered to have a limited potential to undergo chondrogenesis both in vivo43 and in vitro53,54 conditions. This may be due to their limited potential to divide or decrease in number upon apoptosis54. This necessitates the implantation of higher cell density for the effective healing of the cartilage. One report has shown better healing upon transplantation of higher cell density compared to lower cell density43. Tiwary et al55 implanted 2.96 ± 0.18 × 106 mononuclear cells (2.96 ± 0.18 × 103 to 104 MSCs) in the cartilage defect of knee joint and reported better healing compared to control group owing to the presence of the humoral factors.
To treat cartilage defects using MSC transplantation, a vehicle is required to hold them, allow their growth and make them less prone to deleterious environmental effects. Generally, the common problem that arises while transplanting cells into the cartilage defects is their leakage. These cells do not stick at the site of defect, and thus, a scaffold is required for their in situ transplantation. Scaffold selection is made on the basis its biocompatibility, ability to be retained at the implantation site and to integrate with the adjacent tissue, sufficient porosity to allow ingrowth of host tissue yet maintain adequate mechanical strength and properties to deliver cells without any toxic effect upon them56,57,58. In osteochondral defects, scaffolds that are being replaced by neocartilage should survive until two types of tissues, bone and cartilage, are formed58. Growth of the superficially placed cartilage depends on the availability of subchondral bone, and if latter is not formed within a requisite period, cartilage regeneration at the superficial surface may be hampered59. During healing of articular cartilage, integration of regenerated tissue with that of the adjacent native tissue is another problem. Cartilage islands formed after regeneration fail to survive unless not integrated with the surrounding normal cartilage9. Thus, the scaffolds that encourage the growth and survival of implanted cells and also promote the colonization of native cells should be transplanted60. Scaffold design for cartilage repair should be aimed at normalizing the biochemical (affecting cellular behaviour and activity) and physical (scaffold architecture, mechanical function and degradability) properties61. A number of materials including both natural (fibrin62,63,64,65, agarose and alginate66, collagen67,68,69,70, hyaluronan71,72,73), or synthetic scaffolds (polylactic acid74,75,76, polyglycolic acid77 and polylactic and polyglycolic acid78,79) have been used as scaffolds for cartilage regeneration. Natural scaffolds though bear good biocompatibility (leading to better cell attachment and thus differentiation) but lack in ease of fabrication, suitable mesh properties and controllable biodegradability. Natural scaffolds are also associated with the risk of immunological reactions, disease transmission and are limited in availability. The synthetic scaffolds, chemically modified for desired fabrication, better versatility, suitable mesh properties and controllable biodegradability, lack optimal cyto-compatibility and may also elicit host response upon release of toxic by-products56,57. To overcome such limitations, it was desirable to design composite scaffolds that could combine the respective properties of both synthetic as well as natural scaffolds. This has led to the development of the hybrid scaffolds that utilizes the solid polymer backbone (providing mechanical strength) and hydrogel (supporting the cell delivery) resembling the biphasic nature of cartilage, namely, solid and water phases80. Hydrogel was found to retain cells in the three dimensional stage in a friendly environment along with their homogenous distribution in the solid polymer scaffold pores81. However, such designs demand further in vitro as well as in vivo investigations, especially with respect to the mechanical strength and biocompatibility, to employ clinically.
Other types of tissue engineered scaffolds, namely, biomimetic zonal scaffold and nanofibrous/nanoporous scaffold, have also been developed to overcome the drawbacks associated with conventional scaffolds, namely, compatibility and functional properties. Zonal scaffolds involve different distinct zones/layers with or without the cells resembling the natural cartilage. This zonal system mimics the physical properties of the native articular cartilage and the cells, if implanted, secrete ECM resembling different layers of cartilage82. The biomimetic zonal scaffold technology, although a promising one, is still in its infancy and further investigations are required in its design and fabrication technology. The non-fibrous/nanoporous scaffolds due to their nanosize mimic the biological as well as physico-chemical properties of the native nanosize ECM and thus, play a key role in stem cell and/or chondrocyte growth as well as tissue regeneration83. In pre-clinical phase, the cells are encapsulated in nanofibrous scaffolds fabricated by electrospinning. However, the main problem that arises is of cellular homogeneity as the cells get clumped on such fabrication84. To avoid cell clumping, other fabrication techniques (particulate leaching, chemical etching, 3D printing and phase separation) warrant investigation.
There are numerous intrinsic or extrinsic growth factors that work independently or complement each other for the maintenance of cartilage homoeostasis85. Studies conducted on different growth factors, namely, TGF-β, IGF, BMP, fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF), have shown promising results in cartilage repair55,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102. All these growth factors stimulate chondrocytic matrix synthesis and decrease catabolic effect of MMPs and cytokines such as interleukin-1, except FGF-2 that antagonizes the proteoglycan synthesis and upregulates MMPs86,88,99. The growth factors also stimulate MSC proliferation, increase their matrix production and downregulate their collagen type I gene expression. BMP-7, however, has alone been reported to inhibit MSC proliferation but does allow proliferation in the presence of TGF-β91,93. Growth factors when used in combinations work synergistically such as BMP-7 and IGF-1 lead to enhanced cartilage matrix synthesis103. IGF-1, FGF-2 and TGF-β under in vitro conditions regulate their own and each other's gene expression and protein production104. It was also demonstrated that the combination of IGF-1 and TGF-β has better healing potential compared to individual effect with IGF-1 involved in protection of synovium, showing reduced thickening depicting lack of chronic inflammation105. All these observations suggest that the growth factors have an essential role to play in cartilage tissue engineering. Some drawbacks such as osteophyte formation89,96 and synovial thickening87,106 have been reported upon such transplantation which can be managed by standardizing their dosages107 and using them in right combinations105.
Clinical studies on cartilage tissue injury
Use of MSCs, with or without scaffolds and growth factors, has been reported increasingly for the treatment of cartilage defects. In a case report on a single patient, MSCs were reported to form a hyaline type cartilage tissue with improved arthroscopic score108. In another case report, Improved Knee and Osteoarthritis Outcome Score and International Knee Documentation Committee Score were recorded on transplantation of autologous MSCs109. In an institutional study, implantation of MSCs along with mononuclear cells and platelets resulted in better visual analogue score (VAS) and increased meniscal and femoral cartilage volume on magnetic resonance imaging (MRI) than the control110. MSCs were found as effective as ACI in the management of cartilage defects. A cohort study, comparing MSCs and ACI in 72 patients with almost similar symptoms111, showed no difference between the groups in terms of clinical outcome, except the physical function that improved over time in MSCs group111. Bone marrow-MSCs suspended in a collagen type I gel and transplanted in knee of the osteoarthritic patients showed better arthroscopic and histological scores compared to the control group70. The same authors reported improvement in clinical symptoms in three patients112,113. Another study wherein five patients with full thickness cartilage (3-12 cm2) were treated with MSCs laden on platelet-rich fibrin glue showed better clinical, arthroscopic and MRI results as compared to the control patients114. The efficacy of infrapatellar fat pad-derived MSCs in the treatment of human OA has also been proved in two studies. Intra-articular injection of MSC was found to be safe and provided assistance in reducing pain and improving function in patients with knee OA115,116.
Among animals, equines and dogs are more prone to the articular cartilage injuries. Autologous fat-derived MSCs clinically evaluated for the treatment of chronic OA in dogs showed improved scores of lameness, pain and range of motions117. A multicentre clinical trial was conducted on 39 horses using intra-articular injection of autologous MSCs to treat OA (74% cases of stifle joint), with a follow up of 21 months. Seventy seven per cent were found to resume some work, 38 per cent returning or exceeding the level observed before OA and 38 per cent requiring additional medicinal treatment118. Sato et al119 studied the outcome of intra-articular transplantation of MSCs suspended in hyaluronic acid in spontaneous arthritis of Hartley strain guinea pigs. Partial cartilage repair was noted at five weeks post-operation with higher type II collagen and low levels of MMP-13. Migration, differentiation and proliferation of MSCs in the hyaluronic acid in treated animals were also observed. However, there are reports that do not suggest positive outcome following MSC application in human patients. A study on four elderly OA patients (55-65 yr) treated with MSCs therapy did not show any significant knee outcome score, except that the patients could climb number of stairs and had improved VAS120. In clinical settings, the differences in the extent of articular lesion, the duration of the lesion, age of the patient, the methods of application, the number of cells used, concurrent use of growth factors and scaffolds, etc., may have bearing on the outcome of the treatment. It may, therefore, be imperative to consider all the above-mentioned factors while selecting a patient for MSC therapy and interpreting the results of such therapy.
Conclusion and future perspective
MSCs from different sources have shown potential to repair cartilage defects by differentiation into chondrocytes and synthesis of cartilage matrix. Inclusion of suitable growth factors and scaffold may support the regeneration and integration of neocartilage with the surrounding native tissue. The combined and precise use of MSCs, growth factors and scaffolds may offer new modalities that can overcome the limitations associated with currently available surgical techniques. The survival on transplantation and integration of cells with the host tissue remain the major causes of concern. Processes of mesenchymal condensation into cellular bodies under the influence of growth factors may be a promising technology to develop mechanically strong cartilage to cartilage interface leading to the production of seamless interface and complete integration. Further research is needed to investigate the technology under in vivo trials for its actual potential of cartilage repair. Moreover, CMBs should be assessed for their phenotypic identity as the cells may lose their identity under in vitro conditions. Suitable cell source should also be investigated to find out whether only autogenous cells or both autogenic and allogenic/xenogenic cells can be utilized for CMBs production. Although scaffold properties clearly affect the chondrogenesis, the exact mechanisms that facilitate such cartilage formation remain to be elucidated. Besides, comparisons between conventional and non-conventional scaffold technology need to be drawn to check the functional benefits of the later. For better comparison of scaffold design, standard mechanical and biological tests should be developed.
Footnotes
Conflicts of Interest: None.
References
- 1.Mankin HJ. Synovium and cartilage in health and disease. In: Newton CD, Nunamaker DM, editors. Textbook of Small Animal Orthopaedics. Philadelphia: JB. Lippincott Company; 1984. p. 90. [Google Scholar]
- 2.Kinner B, Capito RM, Spector M. Regeneration of articular cartilage. Adv Biochem Eng Biotechnol. 2005;94:91–123. doi: 10.1007/b100001. [DOI] [PubMed] [Google Scholar]
- 3.Kang SW, Bada LP, Kang CS, Lee JS, Kim CH, Park JH, et al. Articular cartilage regeneration with microfracture and hyaluronic acid. Biotechnol Lett. 2008;30:435–9. doi: 10.1007/s10529-007-9576-2. [DOI] [PubMed] [Google Scholar]
- 4.Duarte Campos DF, Drescher W, Rath B, Tingart M, Fischer H. Supporting biomaterials for articular cartilage repair. Cartilage. 2012;3:205–21. doi: 10.1177/1947603512444722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilgili H, Yildiz C, Kurum B, Soysal Y, Bahce M. Repair of osteochondral defects with autologous chondrocyte implantation: clinical study on the stifle joint of 9 dogs. Ankara Univ J Vet Fac. 2006;53:103–9. [Google Scholar]
- 6.Poole AR. Cartilage in health and disease. In: Koopman WJ, editor. Arthritis and Allied Conditions. Philadelphia: Lippincott Williams and Wilkins; 2001. pp. 226–84. [Google Scholar]
- 7.Maroudas A. Physicochemical properties of articular cartilage. In: Freeman M, editor. Adult Articular Cartilage. London: Pitman Medical; 1979. pp. 215–90. [Google Scholar]
- 8.Woessner JF, Nagase H. Matrix Metalloproteinases and TIMPs. Oxford, UK: Oxford University Press; 2003. [Google Scholar]
- 9.Hunziker EB. Biologic repair of articular cartilage. Defect models in experimental animals and matrix requirements. Clin Orthop Relat Res. 1999;367:S135–46. [PubMed] [Google Scholar]
- 10.Arican M, Koylu O, Uyaroglu A, Erol M, Calim KN. The Effect of (Hylan G-F 20) on bone metabolism in dogs with experimental osteochondral defects. J Turk Vet Surg. 2006;12:20–3. [Google Scholar]
- 11.Günes T, Sen C, Erdem M, Köseoglu RD, Filiz NO. Combination of microfracture and periosteal transplantation techniques for the treatment of full-thickness cartilage defects. Acta Orthop Traumatol Turc. 2006;40:315–23. [PubMed] [Google Scholar]
- 12.Breinan HA, Minas T, Hsu HP, Nehrer S, Sledge CB, Spector M. Effect of cultured autologous chondrocytes on repair of chondral defects in a canine model. J Bone Joint Surg Am. 1997;79:1439–51. doi: 10.2106/00004623-199710000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19:477–84. doi: 10.1053/jars.2003.50112. [DOI] [PubMed] [Google Scholar]
- 14.Sgaglione NA, Miniaci A, Gillogly SD, Carter TR. Update on advanced surgical techniques in the treatment of traumatic focal articular cartilage lesions in the knee. Arthroscopy. 2002;18:9–32. doi: 10.1053/jars.2002.31783. [DOI] [PubMed] [Google Scholar]
- 15.O’Driscoll SW. The healing and regeneration of articular cartilage. J Bone Joint Surg Am. 1998;80:1795–812. [PubMed] [Google Scholar]
- 16.Tsai CL, Liu TK, Fu SL, Perng JH, Lin AC. Preliminary study of cartilage repair with autologous periosteum and fibrin adhesive system. J Formos Med Assoc. 1992;91:S239–45. [PubMed] [Google Scholar]
- 17.Outerbridge HK, Outerbridge AR, Outerbridge RE. The use of a lateral patellar autologous graft for the repair of a large osteochondral defect in the knee. J Bone Joint Surg Am. 1995;77:65–72. doi: 10.2106/00004623-199501000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Grande DA, Halberstadt C, Naughton G, Schwartz R, Manji R. Evaluation of matrix scaffolds for tissue engineering of articular cartilage grafts. J Biomed Mater Res. 1997;34:211–20. doi: 10.1002/(sici)1097-4636(199702)34:2<211::aid-jbm10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 19.Brittberg M, Nilsson A, Lindahl A, Ohlsson C, Peterson L. Rabbit articular cartilage defects treated with autologous cultured chondrocytes. Clin Orthop Relat Res. 1996;326:270–83. doi: 10.1097/00003086-199605000-00034. [DOI] [PubMed] [Google Scholar]
- 20.Tins BJ, McCall IW, Takahashi T, Cassar-Pullicino V, Roberts S, Ashton B, et al. Autologous chondrocyte implantation in knee joint: MR imaging and histologic features at 1-year follow-up. Radiology. 2005;234:501–8. doi: 10.1148/radiol.2342031970. [DOI] [PubMed] [Google Scholar]
- 21.van Dyk GE, Dejardin LM, Flo G, Johnson LL. Cancellous bone grafting of large osteochondral defects: an experimental study in dogs. Arthroscopy. 1998;14:311–20. doi: 10.1016/s0749-8063(98)70148-3. [DOI] [PubMed] [Google Scholar]
- 22.Gunay C, Sagliyan A, Unsaldi E, Yaman M. Repair of experimentally induced osteochondral defects of dog knee joint with cancellous autograft. Firat Univ J Health Sci. 2005;19:107–13. [Google Scholar]
- 23.Reddy S, Pedowitz DI, Parekh SG, Sennett BJ, Okereke E. The morbidity associated with osteochondral harvest from asymptomatic knees for the treatment of osteochondral lesions of the talus. Am J Sports Med. 2007;35:80–5. doi: 10.1177/0363546506290986. [DOI] [PubMed] [Google Scholar]
- 24.Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–66. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 25.Punwar S, Khan WS. Mesenchymal stem cells and articular cartilage repair: clinical studies and future direction. Open Orthop J. 2011;5:296–301. doi: 10.2174/1874325001105010296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reinholz GG, Lu L, Saris DB, Yaszemski MJ, O’Driscoll SW. Animal models for cartilage reconstruction. Biomaterials. 2004;25:1511–21. doi: 10.1016/s0142-9612(03)00498-8. [DOI] [PubMed] [Google Scholar]
- 27.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–74. [PubMed] [Google Scholar]
- 28.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–72. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 29.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 30.Prockop DJ, Gregory CA, Spees JL. One strategy for cell and gene therapy: harnessing the power of adult stem cells to repair tissues. Proc Natl Acad Sci U S A. 2003;100:11917–23. doi: 10.1073/pnas.1834138100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–36. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 32.Gade NE, Pratheesh MD, Nath A, Dubey PK, Amarpal, Sharma B, et al. Molecular and cellular characterization of buffalo bone marrow-derived mesenchymal stem cells. Reprod Domest Anim. 2013;48:358–67. doi: 10.1111/j.1439-0531.2012.02156.x. [DOI] [PubMed] [Google Scholar]
- 33.Udehiya RK, Amarpal, Kinjavdekar P, Aithal HP, Nath N, Pawde AM, et al. Isolation, ex vivo expansion and characterization of rabbit bone marrow derived mesenchymal stem cells (rBM-MSCs) Indian J Vet Surg. 2013;34:41–6. [Google Scholar]
- 34.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 35.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–50. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 36.Csaki C, Matis U, Mobasheri A, Ye H, Shakibaei M. Chondrogenesis, osteogenesis and adipogenesis of canine mesenchymal stem cells: a biochemical, morphological and ultrastructural study. Histochem Cell Biol. 2007;128:507–20. doi: 10.1007/s00418-007-0337-z. [DOI] [PubMed] [Google Scholar]
- 37.Sekiya I, Colter DC, Prockop DJ. BMP-6 enhances chondrogenesis in a subpopulation of human marrow stromal cells. Biochem Biophys Res Commun. 2001;284:411–8. doi: 10.1006/bbrc.2001.4898. [DOI] [PubMed] [Google Scholar]
- 38.Sekiya I, Larson BL, Vuoristo JT, Reger RL, Prockop DJ. Comparison of effect of BMP-2, -4, and -6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell Tissue Res. 2005;320:269–76. doi: 10.1007/s00441-004-1075-3. [DOI] [PubMed] [Google Scholar]
- 39.Kim YJ, Kim HJ, Im GI. PTHrP promotes chondrogenesis and suppresses hypertrophy from both bone marrow-derived and adipose tissue-derived MSCs. Biochem Biophys Res Commun. 2008;373:104–8. doi: 10.1016/j.bbrc.2008.05.183. [DOI] [PubMed] [Google Scholar]
- 40.Pei M, He F, Vunjak-Novakovic G. Synovium-derived stem cell-based chondrogenesis. Differentiation for cartilage repair: monitoring its success by magnetic resonance imaging and histology. Arthritis Res Ther. 2008;5:R60–3. [Google Scholar]
- 41.Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579–92. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Chen J, Wang C, Lü S, Wu J, Guo X, Duan C, et al. In vivo chondrogenesis of adult bone-marrow-derived autologous mesenchymal stem cells. Cell Tissue Res. 2005;319:429–38. doi: 10.1007/s00441-004-1025-0. [DOI] [PubMed] [Google Scholar]
- 43.Koga H, Muneta T, Ju YJ, Nagase T, Nimura A, Mochizuki T, et al. Synovial stem cells are regionally specified according to local microenvironments after implantation for cartilage regeneration. Stem Cells. 2007;25:689–96. doi: 10.1634/stemcells.2006-0281. [DOI] [PubMed] [Google Scholar]
- 44.Steck E, Lorenz H, Gotterbarm T, Jung M, Richter W. Spontaneous chondrogenic MSC-differentiation in a porcine articular cartilage defect. J Bone Joint Surg Br. 2009;91:459–60. [Google Scholar]
- 45.Wang F, Li Z, Tamama K, Sen CK, Guan J. Fabrication and characterization of prosurvival growth factor releasing, anisotropic scaffolds for enhanced mesenchymal stem cell survival/growth and orientation. Biomacromolecules. 2009;10:2609–18. doi: 10.1021/bm900541u. [DOI] [PubMed] [Google Scholar]
- 46.De Bari C, Dell’Accio F, Luyten FP. Failure of in vitro-differentiated mesenchymal stem cells from the synovial membrane to form ectopic stable cartilage in vivo. Arthritis Rheum. 2004;50:142–50. doi: 10.1002/art.11450. [DOI] [PubMed] [Google Scholar]
- 47.Loveridge N, Farquharson C, Hesketh JE, Jakowlew SB, Whitehead CC, Thorp BH. The control of chondrocyte differentiation during endochondral bone growth in vivo: changes in TGF-beta and the proto-oncogene c-myc. J Cell Sci. 1993;105:949–56. doi: 10.1242/jcs.105.4.949. [DOI] [PubMed] [Google Scholar]
- 48.Csaki C, Schneider PR, Shakibaei M. Mesenchymal stem cells as a potential pool for cartilage tissue engineering. Ann Anat. 2008;190:395–412. doi: 10.1016/j.aanat.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 49.Solchaga LA, Penick KJ, Welter JF. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells: tips and tricks. Methods Mol Biol. 2011;698:253–78. doi: 10.1007/978-1-60761-999-4_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall BK, Miyake T. All for one and one for all: condensations and the initiation of skeletal development. Bioessays. 2000;22:138–47. doi: 10.1002/(SICI)1521-1878(200002)22:2<138::AID-BIES5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 51.DeLise AM, Fischer L, Tuan RS. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage. 2000;8:309–34. doi: 10.1053/joca.1999.0306. [DOI] [PubMed] [Google Scholar]
- 52.Bhumiratana S, Eton RE, Oungoulian SR, Wan LQ, Ateshian GA, Vunjak-Novakovic G. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. Proc Natl Acad Sci U S A. 2014;111:6940–5. doi: 10.1073/pnas.1324050111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–41. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- 54.Ichinose S, Yamagata K, Sekiya I, Muneta T, Tagami M. Detailed examination of cartilage formation and endochondral ossification using human mesenchymal stem cells. Clin Exp Pharmacol Physiol. 2005;32:561–70. doi: 10.1111/j.1440-1681.2005.04231.x. [DOI] [PubMed] [Google Scholar]
- 55.Tiwary R, Amarpal, Aithal HP, Kinjavdekar P, Pawde AM, Singh R. Effect of IGF-1 and uncultured autologous bone-marrow-derived mononuclear cells on repair of osteochondral defect in rabbits. Cartilage. 2014;5:43–54. doi: 10.1177/1947603513499366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu L, Zhu X, Valenzuela RG, Currier BL, Yaszemski MJ. Biodegradable polymer scaffolds for cartilage tissue engineering. Clin Orthop Relat Res. 2001;391:S251–70. doi: 10.1097/00003086-200110001-00024. [DOI] [PubMed] [Google Scholar]
- 57.Risbud MV, Sittinger M. Tissue engineering: advances in in vitro cartilage generation. Trends Biotechnol. 2002;20:351–6. doi: 10.1016/s0167-7799(02)02016-4. [DOI] [PubMed] [Google Scholar]
- 58.Frenkel SR, Di Cesare PE. Scaffolds for articular cartilage repair. Ann Biomed Eng. 2004;32:26–34. doi: 10.1023/b:abme.0000007788.41804.0d. [DOI] [PubMed] [Google Scholar]
- 59.Hutmacher DW. Scaffold design and fabrication technologies for engineering tissues – State of the art and future perspectives. J Biomater Sci Polym Ed. 2001;12:107–24. doi: 10.1163/156856201744489. [DOI] [PubMed] [Google Scholar]
- 60.Coutts RD, Healey RM, Ostrander R, Sah RL, Goomer R, Amiel D. Matrices for cartilage repair. Clin Orthop Relat Res. 2001;391:S271–9. doi: 10.1097/00003086-200110001-00025. [DOI] [PubMed] [Google Scholar]
- 61.Hutmacher D, Woodfield T, Dalton PD, Lewis JA. Scaffold design and fabrication. In: Van Blitterswijk C, Thomsen P, Hubbell J, Cancedda R, de Bruijn J, Lindahl A, et al., editors. Tissue engineering. London, UK: Elsevier Academic Press; 2008. pp. 403–54. [Google Scholar]
- 62.Hendrickson DA, Nixon AJ, Grande DA, Todhunter RJ, Minor RM, Erb H, et al. Chondrocyte-fibrin matrix transplants for resurfacing extensive articular cartilage defects. J Orthop Res. 1994;12:485–97. doi: 10.1002/jor.1100120405. [DOI] [PubMed] [Google Scholar]
- 63.Brittberg M, Sjögren-Jansson E, Lindahl A, Peterson L. Influence of fibrin sealant (Tisseel) on osteochondral defect repair in the rabbit knee. Biomaterials. 1997;18:235–42. doi: 10.1016/s0142-9612(96)00117-2. [DOI] [PubMed] [Google Scholar]
- 64.Fortier LA, Nixon AJ, Lust G. Phenotypic expression of equine articular chondrocytes grown in three-dimensional cultures supplemented with supraphysiologic concentrations of insulin-like growth factor-1. Am J Vet Res. 2002;63:301–5. doi: 10.2460/ajvr.2002.63.301. [DOI] [PubMed] [Google Scholar]
- 65.Fortier LA, Mohammed HO, Lust G, Nixon AJ. Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg Br. 2002;84:276–88. doi: 10.1302/0301-620x.84b2.11167. [DOI] [PubMed] [Google Scholar]
- 66.Marijnissen WJ, van Osch GJ, Aigner J, Verwoerd-Verhoef HL, Verhaar JA. Tissue-engineered cartilage using serially passaged articular chondrocytes. Chondrocytes in alginate, combined in vivo with a synthetic (E210) or biologic biodegradable carrier (DBM) Biomaterials. 2000;21:571–80. doi: 10.1016/s0142-9612(99)00218-5. [DOI] [PubMed] [Google Scholar]
- 67.Grande DA, Pitman MI, Peterson L, Menche D, Klein M. The repair of experimentally produced defects in rabbit articular cartilage by autologous chondrocyte transplantation. J Orthop Res. 1989;7:208–18. doi: 10.1002/jor.1100070208. [DOI] [PubMed] [Google Scholar]
- 68.Lee CR, Grodzinsky AJ, Hsu HP, Spector M. Effects of a cultured autologous chondrocyte-seeded type II collagen scaffold on the healing of a chondral defect in a canine model. J Orthop Res. 2003;21:272–81. doi: 10.1016/S0736-0266(02)00153-5. [DOI] [PubMed] [Google Scholar]
- 69.Nehrer S, Breinan HA, Ramappa A, Shortkroff S, Young G, Minas T, et al. Canine chondrocytes seeded in type I and type II collagen implants investigated in vitro. J Biomed Mater Res. 1997;38:95–104. doi: 10.1002/(sici)1097-4636(199722)38:2<95::aid-jbm3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 70.Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10:199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 71.Knudson W, Casey B, Nishida Y, Eger W, Kuettner KE, Knudson CB. Hyaluronan oligosaccharides perturb cartilage matrix homeostasis and induce chondrocytic chondrolysis. Arthritis Rheum. 2000;43:1165–74. doi: 10.1002/1529-0131(200005)43:5<1165::AID-ANR27>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 72.Gao J, Dennis JE, Solchaga LA, Goldberg VM, Caplan AI. Repair of osteochondral defect with tissue-engineered two-phase composite material of injectable calcium phosphate and hyaluronan sponge. Tissue Eng. 2002;8:827–37. doi: 10.1089/10763270260424187. [DOI] [PubMed] [Google Scholar]
- 73.Solchaga LA, Gao J, Dennis JE, Awadallah A, Lundberg M, Caplan AI, et al. Treatment of osteochondral defects with autologous bone marrow in a hyaluronan-based delivery vehicle. Tissue Eng. 2002;8:333–47. doi: 10.1089/107632702753725085. [DOI] [PubMed] [Google Scholar]
- 74.Chu CR, Dounchis JS, Yoshioka M, Sah RL, Coutts RD, Amiel D. Osteochondral repair using perichondrial cells. A 1-year study in rabbits. Clin Orthop Relat Res. 1997;340:220–9. doi: 10.1097/00003086-199707000-00029. [DOI] [PubMed] [Google Scholar]
- 75.Dounchis JS, Bae WC, Chen AC, Sah RL, Coutts RD, Amiel D. Cartilage repair with autogenic perichondrium cell and polylactic acid grafts. Clin Orthop Relat Res. 2000;377:248–64. doi: 10.1097/00003086-200008000-00033. [DOI] [PubMed] [Google Scholar]
- 76.Frenkel SR, Chang J, Maurer S, Baitner A, Wright K. Bone protein in a grafton flex carrier for articular cartilage repair. Trans Am Acad Orthop Surg. 2001;26:356. [Google Scholar]
- 77.Liu Y, Chen F, Liu W, Cui L, Shang Q, Xia W, et al. Repairing large porcine full-thickness defects of articular cartilage using autologous chondrocyte-engineered cartilage. Tissue Eng. 2002;8:709–21. doi: 10.1089/107632702760240616. [DOI] [PubMed] [Google Scholar]
- 78.Niederauer GG, Slivka MA, Leatherbury NC, Korvick DL, Harroff HH, Ehler WC, et al. Evaluation of multiphase implants for repair of focal osteochondral defects in goats. Biomaterials. 2000;21:2561–74. doi: 10.1016/s0142-9612(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 79.Cohen SB, Meirisch CM, Wilson HA, Diduch DR. The use of absorbable co-polymer pads with alginate and cells for articular cartilage repair in rabbits. Biomaterials. 2003;24:2653–60. doi: 10.1016/s0142-9612(03)00058-9. [DOI] [PubMed] [Google Scholar]
- 80.Moutos FT, Guilak F. Composite scaffolds for cartilage tissue engineering. Biorheology. 2008;45:501–12. [PMC free article] [PubMed] [Google Scholar]
- 81.Caterson EJ, Nesti LJ, Li WJ, Danielson KG, Albert TJ, Vaccaro AR, et al. Three-dimensional cartilage formation by bone marrow-derived cells seeded in polylactide/alginate amalgam. J Biomed Mater Res. 2001;57:394–403. doi: 10.1002/1097-4636(20011205)57:3<394::aid-jbm1182>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 82.Klein TJ, Schumacher BL, Schmidt TA, Li KW, Voegtline MS, Masuda K, et al. Tissue engineering of stratified articular cartilage from chondrocyte subpopulations. Osteoarthritis Cartilage. 2003;11:595–602. doi: 10.1016/s1063-4584(03)00090-6. [DOI] [PubMed] [Google Scholar]
- 83.Zhang L, Webster TJ. Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nano Today. 2009;4:66–80. [Google Scholar]
- 84.Li WJ, Jiang YJ, Tuan RS. Cell-nanofiber-based cartilage tissue engineering using improved cell seeding, growth factor, and bioreactor technologies. Tissue Eng Part A. 2008;14:639–48. doi: 10.1089/tea.2007.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97:33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 86.Middleton J, Manthey A, Tyler J. Insulin-like growth factor (IGF) receptor, IGF-I, interleukin-1 beta (IL-1 beta), and IL-6 mRNA expression in osteoarthritic and normal human cartilage. J Histochem Cytochem. 1996;44:133–41. doi: 10.1177/44.2.8609369. [DOI] [PubMed] [Google Scholar]
- 87.Bakker AC, van de Loo FA, van Beuningen HM, Sime P, van Lent PL, van der Kraan PM, et al. Overexpression of active TGF-beta-1 in the murine knee joint: evidence for synovial-layer-dependent chondro-osteophyte formation. Osteoarthritis Cartilage. 2001;9:128–36. doi: 10.1053/joca.2000.0368. [DOI] [PubMed] [Google Scholar]
- 88.Gouttenoire J, Valcourt U, Ronzière MC, Aubert-Foucher E, Mallein-Gerin F, Herbage D. Modulation of collagen synthesis in normal and osteoarthritic cartilage. Biorheology. 2004;41:535–42. [PubMed] [Google Scholar]
- 89.Wilke MM, Nydam DV, Nixon AJ. Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. J Orthop Res. 2007;25:913–25. doi: 10.1002/jor.20382. [DOI] [PubMed] [Google Scholar]
- 90.Blaney Davidson EN, van der Kraan PM, van den Berg WB. TGF-beta and osteoarthritis. Osteoarthritis Cartilage. 2007;15:597–604. doi: 10.1016/j.joca.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 91.Goodrich LR, Hidaka C, Robbins PD, Evans CH, Nixon AJ. Genetic modification of chondrocytes with insulin-like growth factor-1 enhances cartilage healing in an equine model. J Bone Joint Surg Br. 2007;89:672–85. doi: 10.1302/0301-620X.89B5.18343. [DOI] [PubMed] [Google Scholar]
- 92.Badlani N, Inoue A, Healey R, Coutts R, Amiel D. The protective effect of OP-1 on articular cartilage in the development of osteoarthritis. Osteoarthritis Cartilage. 2008;16:600–6. doi: 10.1016/j.joca.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 93.Elshaier AM, Hakimiyan AA, Rappoport L, Rueger DC, Chubinskaya S. Effect of interleukin-1beta on osteogenic protein 1-induced signaling in adult human articular chondrocytes. Arthritis Rheum. 2009;60:143–54. doi: 10.1002/art.24151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schmidt MB, Chen EH, Lynch SE. A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthritis Cartilage. 2006;14:403–12. doi: 10.1016/j.joca.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 95.Fan H, Tao H, Wu Y, Hu Y, Yan Y, Luo Z. TGF-ß3 immobilized PLGA-gelatin/chondroitin sulfate/hyaluronic acid hybrid scaffold for cartilage regeneration. J Biomed Mater Res A. 2010;95:982–92. doi: 10.1002/jbm.a.32899. [DOI] [PubMed] [Google Scholar]
- 96.Miyakoshi N, Kobayashi M, Nozaka K, Okada K, Shimada Y, Itoi E. Effects of intraarticular administration of basic fibroblast growth factor with hyaluronic acid on osteochondral defects of the knee in rabbits. Arch Orthop Trauma Surg. 2005;125:683–92. doi: 10.1007/s00402-005-0052-y. [DOI] [PubMed] [Google Scholar]
- 97.Ekenstedt KJ, Sonntag WE, Loeser RF, Lindgren BR, Carlson CS. Effects of chronic growth hormone and insulin-like growth factor 1 deficiency on osteoarthritis severity in rat knee joints. Arthritis Rheum. 2006;54:3850–8. doi: 10.1002/art.22254. [DOI] [PubMed] [Google Scholar]
- 98.Singh NK, Singh GR, Amarpal, Kinjavdekar P, Sharma AK, Mohanty TR, et al. Articular cartilage repair with autografting under the influence of insulin-like growth factor-1 in rabbits. J Vet Med A Physiol Pathol Clin Med. 2007;54:210–8. doi: 10.1111/j.1439-0442.2007.00941.x. [DOI] [PubMed] [Google Scholar]
- 99.Hayashi M, Muneta T, Ju YJ, Mochizuki T, Sekiya I. Weekly intra-articular injections of bone morphogenetic protein-7 inhibits osteoarthritis progression. Arthritis Res Ther. 2008;10:R118. doi: 10.1186/ar2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smyth SS, McEver RP, Weyrich AS, Morrell CN, Hoffman MR, Arepally GM, et al. Platelet functions beyond hemostasis. J Thromb Haemost. 2009;7:1759–66. doi: 10.1111/j.1538-7836.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- 101.Maehara H, Sotome S, Yoshii T, Torigoe I, Kawasaki Y, Sugata Y, et al. Repair of large osteochondral defects in rabbits using porous hydroxyapatite/collagen (HAp/Col) and fibroblast growth factor-2 (FGF-2) J Orthop Res. 2010;28:677–86. doi: 10.1002/jor.21032. [DOI] [PubMed] [Google Scholar]
- 102.Singh NK, Shiwani S, Singh GR, Jeong DK, Kinjavdekar P, Amarpal, et al. TGF-β1 improves articular cartilage damage in rabbit knee. Pak Vet J. 2012;32:412–7. [Google Scholar]
- 103.Loeser RF, Pacione CA, Chubinskaya S. The combination of insulin-like growth factor 1 and osteogenic protein 1 promotes increased survival of and matrix synthesis by normal and osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003;48:2188–96. doi: 10.1002/art.11209. [DOI] [PubMed] [Google Scholar]
- 104.Shi S, Mercer S, Eckert GJ, Trippel SB. Growth factor regulation of growth factors in articular chondrocytes. J Biol Chem. 2009;284:6697–704. doi: 10.1074/jbc.M807859200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davies LC, Blain EJ, Gilbert SJ, Caterson B, Duance VC. The potential of IGF-1 and TGFbeta1 for promoting “adult” articular cartilage repair: an in vitro study. Tissue Eng Part A. 2008;14:1251–61. doi: 10.1089/ten.tea.2007.0211. [DOI] [PubMed] [Google Scholar]
- 106.Boehm AK, Seth M, Mayr KG, Fortier LA. Hsp90 mediates insulin-like growth factor 1 and interleukin-1beta signaling in an age-dependent manner in equine articular chondrocytes. Arthritis Rheum. 2007;56:2335–43. doi: 10.1002/art.22664. [DOI] [PubMed] [Google Scholar]
- 107.Mierisch CM, Cohen SB, Jordan LC, Robertson PG, Balian G, Diduch DR. Transforming growth factor-beta in calcium alginate beads for the treatment of articular cartilage defects in the rabbit. Arthroscopy. 2002;18:892–900. doi: 10.1053/jars.2002.36117. [DOI] [PubMed] [Google Scholar]
- 108.Kuroda R, Ishida K, Matsumoto T, Akisue T, Fujioka H, Mizuno K, et al. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007;15:226–31. doi: 10.1016/j.joca.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 109.Kasemkijwattana C, Hongeng S, Kesprayura S, Rungsinaporn V, Chaipinyo K, Chansiri K. Autologous bone marrow mesenchymal stem cells implantation for cartilage defects: two cases report. J Med Assoc Thai. 2011;94:395–400. [PubMed] [Google Scholar]
- 110.Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11:343–53. [PubMed] [Google Scholar]
- 111.Nejadnik H, Hui JH, Feng Choong EP, Tai BC, Lee EH. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38:1110–6. doi: 10.1177/0363546509359067. [DOI] [PubMed] [Google Scholar]
- 112.Wakitani S, Aoki H, Harada Y, Sonobe M, Morita Y, Mu Y, et al. Embryonic stem cells form articular cartilage, not teratomas, in osteochondral defects of rat joints. Cell Transplant. 2004;13:331–6. doi: 10.3727/000000004783983891. [DOI] [PubMed] [Google Scholar]
- 113.Wakitani S, Nawata M, Tensho K, Okabe T, Machida H, Ohgushi H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 2007;1:74–9. doi: 10.1002/term.8. [DOI] [PubMed] [Google Scholar]
- 114.Haleem AM, Singergy AA, Sabry D, Atta HM, Rashed LA, Chu CR, et al. The clinical use of human culture-expanded autologous bone marrow mesenchymal stem cells transplanted on platelet-rich fibrin glue in the treatment of articular cartilage defects: a pilot study and preliminary results. Cartilage. 2010;1:253–261. doi: 10.1177/1947603510366027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Koh YG, Jo SB, Kwon OR, Suh DS, Lee SW, Park SH, et al. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29:748–55. doi: 10.1016/j.arthro.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 116.Koh YG, Choi YJ. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19:902–7. doi: 10.1016/j.knee.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 117.Black LL, Gaynor J, Gahring D, Adams C, Aron D, Harman S, et al. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trial. Vet Ther. 2007;8:272–84. [PubMed] [Google Scholar]
- 118.Ferris DJ, Frisbie DD, Kisiday JD, McIIwraith CW, Hague BA, Major MD, et al. Clinical follow-up of horses treated with bone marrow derived mesenchymal stem cells for musculoskeletal lesions. Proc Am Ass Equine Practnrs. 2009;55:59–60. [Google Scholar]
- 119.Sato M, Uchida K, Nakajima H, Miyazaki T, Guerrero AR, Watanabe S, et al. Direct transplantation of mesenchymal stem cells into the knee joints of Hartley strain guinea pigs with spontaneous osteoarthritis. Arthritis Res Ther. 2012;14:R31. doi: 10.1186/ar3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Davatchi F, Abdollahi BS, Mohyeddin M, Shahram F, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 2011;14:211–5. doi: 10.1111/j.1756-185X.2011.01599.x. [DOI] [PubMed] [Google Scholar]


