Abstract
Background & objectives:
Amitraz is a member of formamidine family of pesticides. Poisoning from amitraz is underrecognized even in areas where it is widely available. It is frequently misdiagnosed as organophosphate poisoning. This systematic review provides information on the epidemiology, toxicokinetics, mechanisms of toxicity, clinical features, diagnosis and management of amitraz poisoning.
Methods:
Medline and Embase databases were searched systematically (since inception to January 2014) for case reports, case series and original articles using the following search terms: ‘amitraz’, ‘poisoning’, ‘toxicity’, ‘intoxication’ and ‘overdose’. Articles published in a language other than English, abstracts and those not providing sufficient clinical information were excluded.
Results:
The original search yielded 239 articles, of which 52 articles described human cases. After following the inclusion and exclusion criteria, 32 studies describing 310 cases (151 females, 175 children) of human poisoning with amitraz were included in this systematic review. The most commonly reported clinical features of amitraz poisoning were altered sensorium, miosis, hyperglycaemia, bradycardia, vomiting, respiratory failure, hypotension and hypothermia. Amitraz poisoning carried a good prognosis with only six reported deaths (case fatality rate, 1.9%). Nearly 20 and 11.9 per cent of the patients required mechanical ventilation and inotropic support, respectively. The role of decontamination methods, namely, gastric lavage and activated charcoal was unclear.
Interpretation & conclusions:
Our review shows that amitraz is an important agent for accidental or suicidal poisoning in both adults and children. It has a good prognosis with supportive management.
Keywords: Amitraz, intoxication, morbidity, overdose, pesticide, poisoning, toxicity
Amitraz, chemically 1,5 di-(2,4-dimethylphenyl)-3-methyl-1,3,5-triazapenta-1,4-diene is a member of the formamidine family of pesticides1. It is used as a veterinary ectoparasiticide (acaricide) for dogs and livestock, and as an agricultural insecticide for fruit crops2. It has been available for use since 19743, and is marketed under various trade names. It is supplied as a 12.5-50 per cent aqueous solution to be diluted in water in 1:100-1:1000 ratio before use. Xylene is used as a solvent in most of the preparations4. Other solvents include tetrachloroethylene and mixtures of petroleum products containing predominantly aromatic hydrocarbons5. The United States Environmental Protection Agency (US EPA) classifies oral amitraz exposure as ‘Class III- slightly toxic’ and a group C ‘possible’ human carcinogen6. The first human case of poisoning was reported in 19837 though amitraz is widely available in many regions worldwide. This incongruity is probably attributable to under-reporting of amitraz intoxication in remote rural areas, as awareness about amitraz, its toxicity and its management remains poor among physicians5. In addition, it is often misdiagnosed as organophosphate/carbamate (OPC) poisoning5,8. A sizeable number of cases of human intoxication with amitraz have been reported over the past three decades. There have been several reports from India in the past five years9,10,11. Yilmaz and Yildizdas had reviewed 137 cases of amitraz poisoning in 200312. Proudfoot published a narrative review on amitraz poisoning in 200313. Veale et al5 presented the worldwide demographic data of amitraz poisoning in 2011, but they did not elaborate on the details of clinical features and management. This systematic review on amitraz intoxication describes the demographics, toxicokinetics, mechanisms of toxicity, clinical features and treatment modalities used in amitraz poisoning.
Material & Methods
Search strategy: Medline and EmBase databases (since inception to January 23, 2014) were searched using the following search terms: ‘amitraz’, ‘poisoning’, ‘toxicity’, ‘intoxication’, and ‘overdose’. Citations describing human cases of amitraz poisoning including case reports, case series and original articles were included. The cross references of these articles were searched for more relevant articles. Articles published in a language other than English, those presented only as an abstract and those not providing sufficient clinical information, were excluded.
Initial review of studies: The initial database generated from the electronic searches was compiled in the reference manager package Endnote (version X7; Thomson Reuters, New York, USA), and all duplicate citations were eliminated. The citations were first screened by both the authors and disagreement was resolved by discussion. This database was then screened again to include only relevant articles. The full text of each selected citation was obtained and reviewed in detail.
Data abstraction: Data were recorded on a standard data extraction form. The following items were extracted: (i) publication details (title, authors and year of publication); (ii) country where the study was conducted; (iii) number of females and children (≤13 yr of age) reported in the study; (iv) route (oral, dermal or other) and manner (accidental, suicidal, and therapeutic misadventure) of poisoning; (v) amount of poison consumed; (vi) time of onset of symptoms; (vii) clinical symptoms and signs reported; (viii) life support (mechanical ventilation and inotropic support) and specific treatment (including gastric lavage, activated charcoal, atropine and others) offered to patients; (ix) time to recovery of sensorium, extubation/weaning and discharge from intensive care unit (ICU) or hospital; and (x) death, if any.
Results
The initial search retrieved a total of 239 articles after excluding citations common to the two databases. Fifty two articles describing human cases were found. Twenty articles were excluded due to the following reasons: (i) four were abstracts14,15,16,17; (ii) four had insufficient clinical details of cases18,19,20,21; (iii) one report described cases, the details of whom were included in a later paper with a larger sample size22,23; and (iv) 11 articles (33 patients) were published in a language other than English. The search thus yielded 32 studies reporting 310 cases of amitraz poisoning (Table I)1,3,4,5,7,8,9,10,11,12,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44. Three cases from the series described by Bonsall and Turnbull7 were excluded due to lack of clinical data.
Table I.
Details of studies included in this systematic review of amitraz poisoning
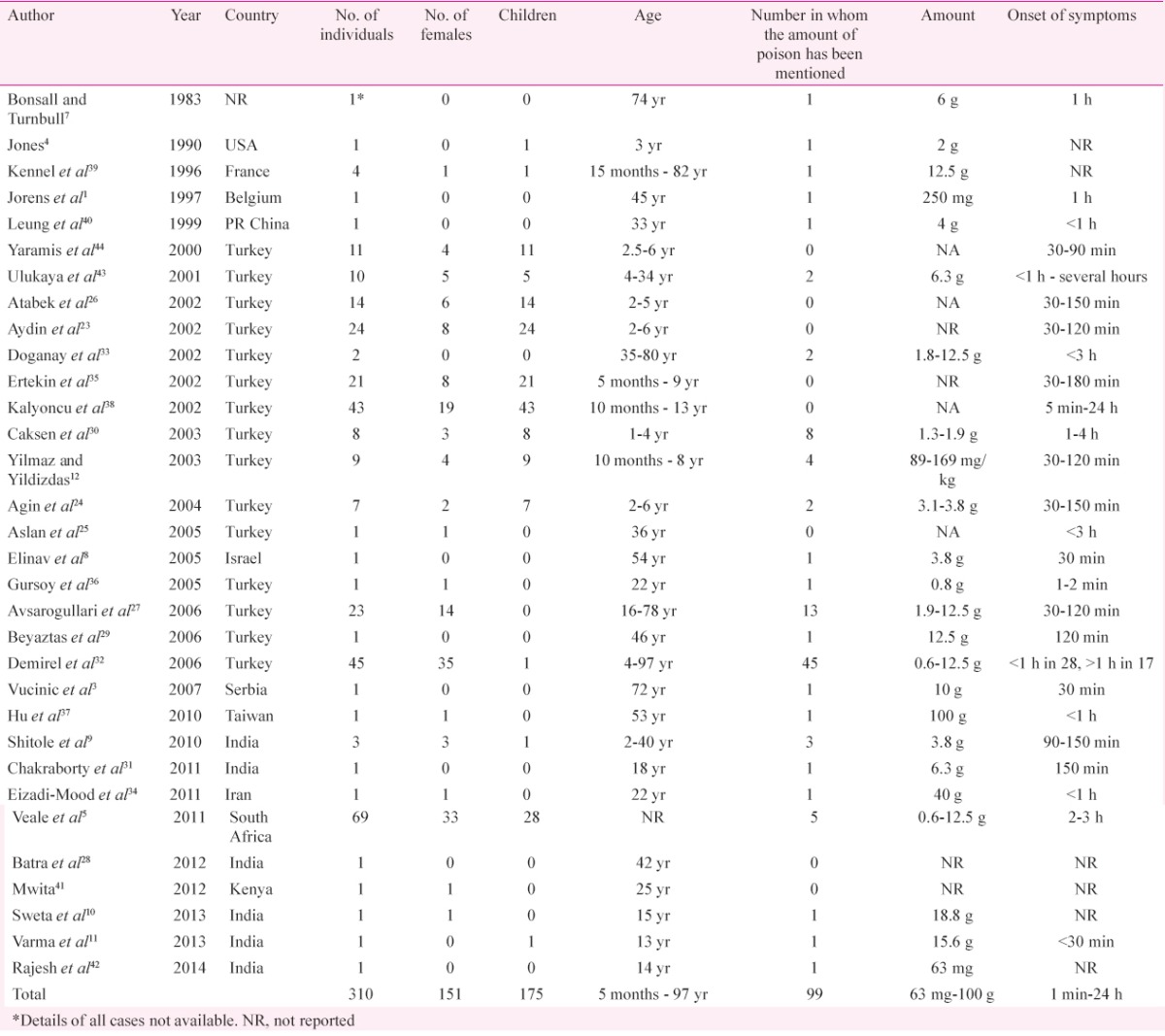
Epidemiology: There was no gender predilection (51.3% males and 48.7% females) among the cases. A majority (56.5%) of the patients were children. The predominant route of exposure to the poison was by ingestion (91.9%) followed by the percutaneous route (7.4%). One patient had exposure by inhalation, while another patient presented with intravenous injection of the toxin27,36. The manner of poisoning was accidental (56.5%) in the majority while 30 per cent of the patients presented with suicidal ingestion. Sixteen patients had intentional percutaneous exposure as in some regions in Turkey, amitraz had been used to treat scabies and pediculosis in humans38. The manner of intoxication was unknown or not reported in 8.4 per cent of the patients. One homicidal poisoning was also reported35. No peculiarities of this poisoning were found in the subgroups of females and children.
Toxicokinetics: Majority of studies included (22/32, 68.8%) reported an onset of symptoms within three hours (Table I). The duration of action is short as the elimination half-life in serum is only four hours, the major terminal metabolite being 3-methyl-4-aminobenzoic acid, which is excreted by the kidneys1,7. Appearance of symptoms was earlier, and the recovery was delayed with oral ingestion as compared to percutaneous exposure38.
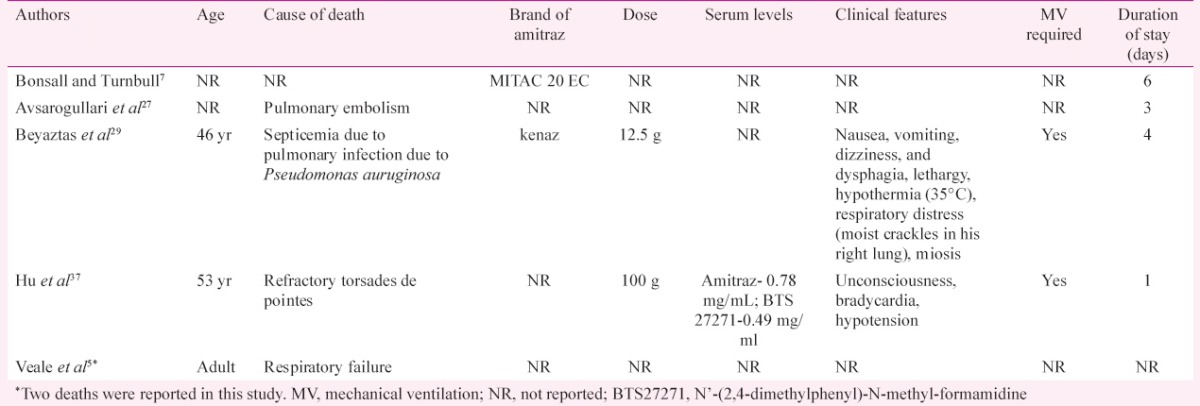
Dose-response relationship: The amount of poison consumed was reported in 99 (31.9%) cases (Table I). It ranged from 63 mg to 100 g. A dose of more than 12 g was reported in ten studies. The proposed lethal dose of the toxin is 200 mg/kg35,45. Accordingly, with an average adult weight of 60 kg, a dose of 12 g is supposedly lethal. Of those ten studies with an intake of more than 12 g amitraz, death was reported in only two patients. The only death for which the information on the exact dose and clinical course was available and the death was attributable to the poisoning itself was the patient reported by Hu et al37. This patient was a 53 yr old female who had consumed 100 g of the poison and developed refractory torsades de pointes. The amitraz level in the serum of this patient was 0.78 mg/ml, while that of BTS-27271, a metabolite of amitraz was 0.49 mg/ml.
Clinical features: The clinical characteristics of the cases reported in the included studies are shown in Table II. The toxic effects of this compound on various organ systems of the human body are summarized below.
Table II.
Frequencies of various clinical features described in amitraz poisoning
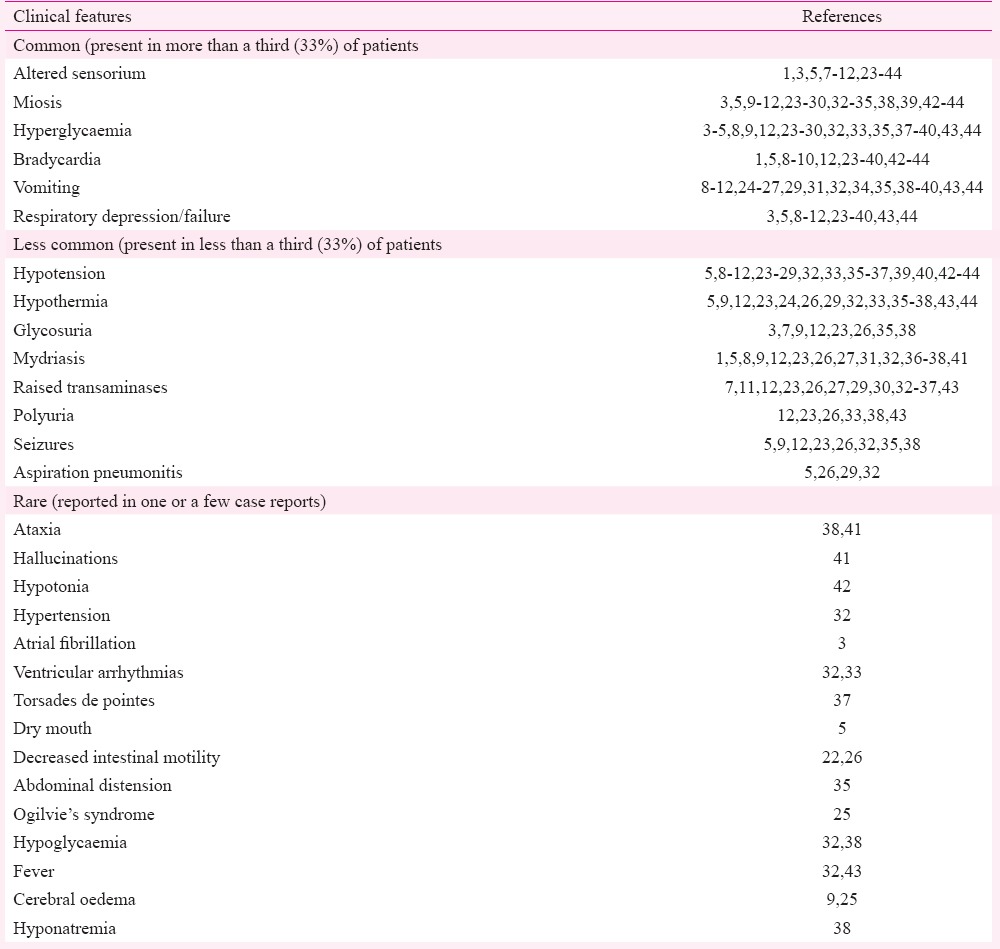
Effects on the nervous system: Central nervous system (CNS) depression was the most common neurological abnormality in this poisoning. It occurs in the form of sleepiness, drowsiness, or complete loss of consciousness depending on the dose of the toxin consumed43. There was a positive correlation observed between the amount of amitraz taken and duration of CNS depression32. It is noteworthy that nearly all patients regain consciousness by 48 h (Table III). This is possibly due to the short elimination half-life of the toxin. If altered sensorium persists beyond this duration, alternative causes should be looked for. Cerebral oedema was documented by brain imaging in two studies9,25. Seizures occurred in <33 per cent of the patients (Table II). Amitraz exposure causes constriction of pupils at lower doses (in about 50% of the patients), but may cause dilation at higher doses due to different mechanisms of action (Table IV)4,12,13,21,26,27,32,35,36,38,43,46,47,48,49,50,51,52,53. Both miosis and mydriasis can occur in the same patient at different times. Rarely reported neurological defects include ataxia, hallucinations, and hypotonia38,41,42.
Table III.
Various treatment modalities and outcomes reported for amitraz poisoning
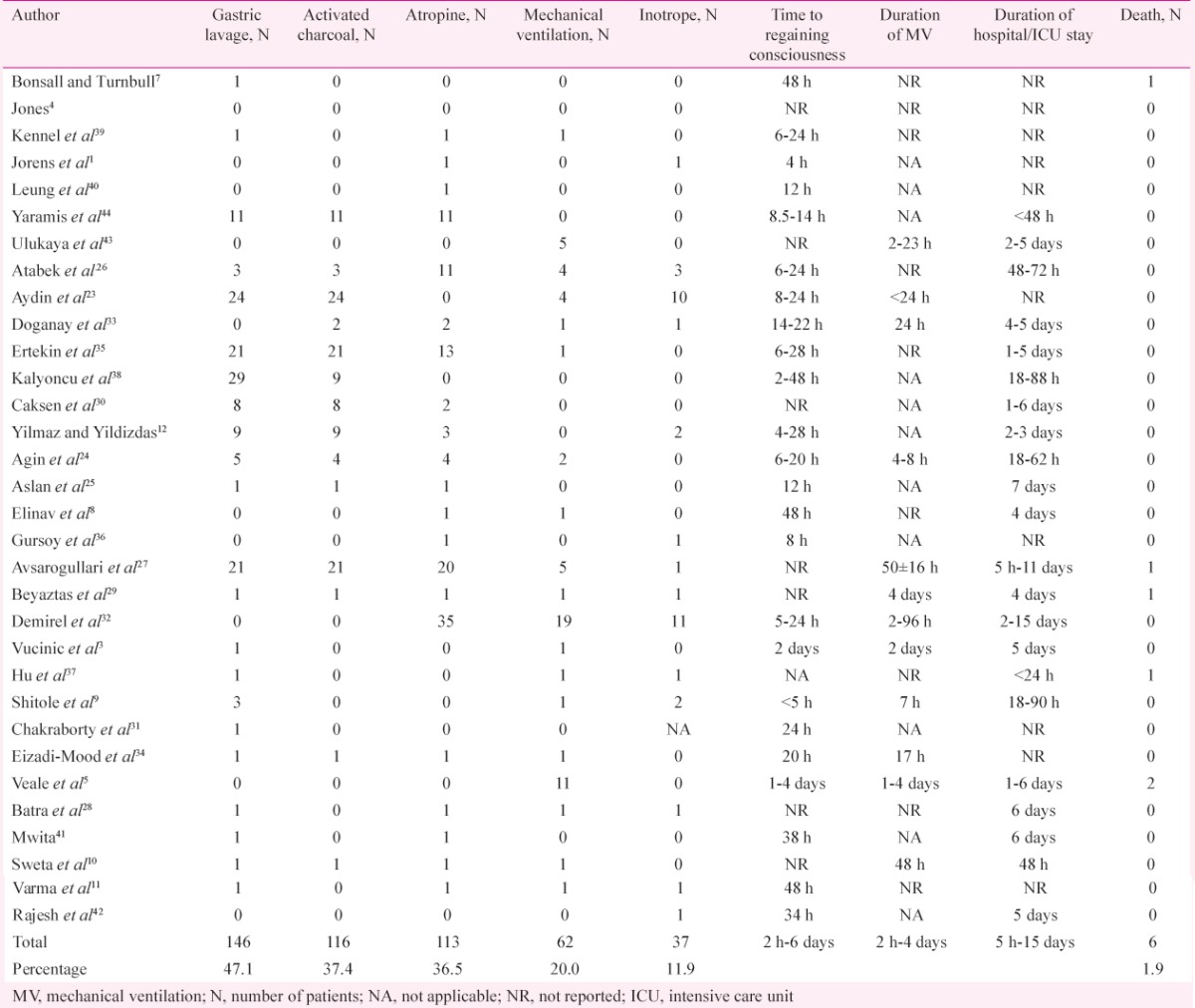
Table IV.
Systemic effects of amitraz and their underlying mechanisms
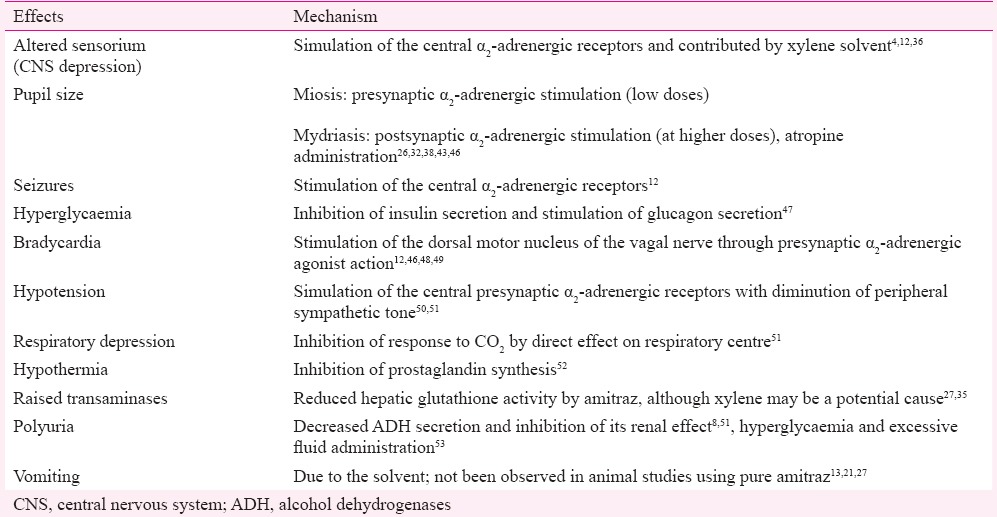
Effects on the cardiovascular system: Bradycardia was the most common cardiovascular manifestation observed in >33 per cent of the patients. Hypotension occurred in a smaller proportion (<33%) of cases (Table II). Cardiovascular manifestations occur mainly due to the stimulation of presynaptic α2-adrenergic receptors (Table IV)12,46,48,49. Hypertension was reported in one study32. Arrhythmias including atrial fibrillation and ventricular arrhythmias occurred, which responded to standard treatment3,33. Fatal torsades de pointes was reported in one case37.
Effects on the respiratory system: Respiratory depression occurred in >33 per cent of the cases (Table II). It occurred in the form of bradypnoea, respiratory acidosis, or respiratory arrest due to direct effect of the poison. Aspiration pneumonia was reported in a small proportion of patients.
Effects on the gastrointestinal system and liver: Vomiting was reported in >33 per cent of the patients. Asymptomatic rise in liver transaminases may occur usually with a normal bilirubin. Rarely reported features include dry mouth, decreased intestinal motility, and abdominal distension5,22,26,35. A case of Ogilvie's syndrome (acute colonic pseudo-obstruction) was also reported25 (Table II).
Effects on metabolism and homeostasis: Hyperglycaemia is a distinctive feature of this poisoning observed in almost half of the patients. Glycosuria was documented in a small proportion of the patients (Table II). Hypoglycaemia has been rarely reported32,38. Hypothermia was relatively common, while fever was rarely seen32,43. Polyuria may occur due to multiple mechanisms (Table IV).
About 33 per cent of the patients of amitraz poisoning developed respiratory depression (Table II) and 20 per cent of the patients required mechanical ventilation, although most of them were weaned off by 48 h (Table III). The case fatality rate was low (1.9%) with only six deaths reported among 310 cases (Table III). Only one of the six deaths could be directly attributed to the effects of the poison (Table V)37. One patient died 30 days after discharge possibly due to unrelated causes7. Two other deaths occurred due to pulmonary thromboembolism and ventilator-associated pneumonia on the third and fourth days of hospital admission, respectively; and thus, they were not attributable directly to the effects of the poison27,42. The causes of two of the deaths were not available5.
Table V.
Characteristics of patients who expired after amitraz poisoning
Discussion
The results of this systematic review suggest that amitraz poisoning is a widely reported intoxication. It has multitudinous clinical manifestations and generally carries a good prognosis.
A large proportion of cases of amitraz poisoning has been reported from Turkey (15 articles, 220 cases). In the last five years, six reports on amitraz poisoning have been published from India9,10,11,28,31,42. A large number of cases (69 patients) have been reported from South Africa5. A large survey of all acute poisonings in South Africa reported that around one per cent of the 4771 consultations sought from a poison information centre were concerning amitraz18.
Toxic exposure in humans may occur by ingestion, inhalation or skin contact. Absorption from the gut occurs at a high rate, as shown in animal studies54,55. Amitraz concentrations are measurable in the plasma within two hours of ingestion1,21. Amitraz is metabolized rapidly in vivo to produce two primary metabolites: 2,4-dimethyl formanilide (BTS-27919) and an active metabolite N’-(2,4-dimethylphenyl)-N-methyl-formamidine (BTS27271), resulting in a rapid onset of symptoms6,7,37. BTS-27271 acts through the octopamine receptors in invertebrates, which are responsible for its acaricidal and insecticidal activities. In vertebrates, it acts mainly on the α2-adrenergic receptor which is structurally similar to the octopamine receptor4. It acts as an agonist on both pre- and post-synaptic α2-adrenergic receptors43,56. Presynaptic receptor stimulation inhibits norepinephrine discharge, while stimulation of postsynaptic receptors leads to effects similar to α1-stimulation26,43. It also acts as a monoamine synthesis inhibitor and an inhibitor of prostaglandin E2; however, the role of these actions in poisoning is unclear. Table IV shows the proposed mechanisms underlying the systemic effects of this toxin.
Amitraz has been classified as a ‘possible’ human carcinogen by the US EPA based on the studies on mice which have shown an increase in the risk of lymphoreticular malignancies and liver adenoma/carcinoma6. However, there are no reports of increased cancer risk in humans. Amitraz has been found to be teratogenic in frogs57, but there is a lack of human data. Among human cases, a pregnant woman who consumed amitraz at 18 wk of pregnancy subsequently made a complete recovery with treatment and gave birth to a healthy neonate at term58.
In a report from a poison information centre, the clinicians were unfamiliar with amitraz in 89 per cent of the instances18. Physicians should always insist on recovering the poison container and discerning the active compound in the preparation. Altered sensorium, miosis and bradycardia are three most common features of this poisoning. These features often mislead physicians into diagnosing the patient with OPC poisoning. Certain features point towards amitraz poisoning as opposed to OPC toxicity. These include presence of hyperglycaemia, hypothermia, and reduced gastrointestinal motility. Further, the absence of fasciculations and a hypersecretory state (salivation, lacrimation, perspiration, and diarrhoea) points against OPC poisoning. Smell of a solvent or a ‘mothball-like’ odour may be noticed on presentation in amitraz poisoning, while a garlic-like smell is encountered with OPC poisoning3,38,59. Besides, serum cholinesterase levels remain normal in amitraz poisoning, while these are low in OPC poisoning5,28,42. Gas chromatography-mass spectrometry and gas liquid chromatography have been used to detect the presence and measure the levels of amitraz and its metabolites in the serum and urine1,3,37.
There is no specific antidote for amitraz poisoning. Management is supportive. Monitoring of respiratory, cardiovascular and CNS functions is essential12. In case of skin exposure, the contaminated clothing should be removed and the skin should be washed with soap and water. Endotracheal intubation should be done early in unconscious patients to avoid the risk of aspiration. Intravenous fluids and vasopressors/inotropes must be administered in hypotensive patients. There are no randomized controlled trials performed to date to clarify the true clinical benefit of gastric lavage and activated charcoal. In the studies included in this review, gastric lavage was performed in only 47.1 per cent of the patients (146/310) and activated charcoal was administered to only 37.4 per cent (116/310) of them (Table III). There are concerns that organic solvents present in the formulation may increase the risk of aspiration if gastric lavage is attempted13. Therefore, it should be performed only after endotracheal intubation, in cases of massive ingestion12,21.
Activated charcoal is relatively safer; however the clinical benefit is again uncertain. Role of atropine is controversial. It has been shown to abrogate bradycardia in many of the patients, while in others dopamine was used for the treatment of bradycardia1,12,23,26. In this review, bradycardia was reported in 47.1 per cent of the patients, while only 36.5 per cent are reported to have received atropine (Table III). Yilmaz and Yildizdas12 suggest that atropine is effective only in patients with symptomatic bradycardia in amitraz poisoning and is not required for those with only asymptomatic bradycardia and/or miosis.
Although there is no antidote, animal studies have demonstrated that α2-adrenergic antagonists such as yohimbine and atipamezole can reverse most of the clinical and laboratory signs of amitraz poisoning60,61. These drugs, however, have not been used in human poisoning. Occasionally, patients receive pralidoxime for the mistaken diagnosis of OPC poisoning, which is however not recommended27. Naloxone used successfully for clonidine poisoning (α2-adrenergic agonist) has proved to be ineffective in animal studies of amitraz poisoning62,63.
Amitraz poisoning carries a good prognosis with a low case fatality rate. The most important factors affecting the clinical course and prognosis seem to be the dose and route of exposure to the poison. The possible reason for the low case fatality rate was that the compound was most commonly available in a 12.5 per cent solution. At this dilution, a large quantity needs to be consumed for a lethal effect.
There were a few limitations of this review. We did not have access to patient level data for all studies, therefore, certain factors such as the relationship of dose of poison consumed and the severity of the effects could not be analyzed for the entire patient population. As there are no randomized trials, no conclusions can be drawn on the ideal management strategy for this poisoning.
In conclusion, the present analysis shows that amitraz poisoning occurs in either accidental or suicidal manner and is more common in children than adults. Though majority of the cases have been reported from Turkey, there has been a recent rise in the number of cases reported from South Africa and India. There is no antidote for this toxin. It has an excellent prognosis with supportive management.
Footnotes
Conflicts of Interest: None.
References
- 1.Jorens PG, Zandijk E, Belmans L, Schepens PJ, Bossaert LL. An unusual poisoning with the unusual pesticide amitraz. Hum Exp Toxicol. 1997;16:600–1. doi: 10.1177/096032719701601008. [DOI] [PubMed] [Google Scholar]
- 2.Gosselin RE, Smith RP, Hodge HC. Clinical toxicology of commercial products. 5th ed. Baltimore: Williams and Wilkins; 1984. pp. 397–404. [Google Scholar]
- 3.Vucinic S, Jovanovic D, Vucinic Z, Joksovic D, Segrt Z, Zlatkovic M, et al. A near-fatal case of acute poisoning by amitraz/xylene showing atrial fibrillation. Forensic Toxicol. 2007;25:41–4. [Google Scholar]
- 4.Jones RD. Xylene/amitraz: a pharmacologic review and profile. Vet Hum Toxicol. 1990;32:446–8. [PubMed] [Google Scholar]
- 5.Veale DJ, Wium CA, Muller GJ. Amitraz poisoning in South Africa: a two year survey (2008-2009) Clin Toxicol (Phila) 2011;49:40–4. doi: 10.3109/15563650.2010.542159. [DOI] [PubMed] [Google Scholar]
- 6.Reregistration Eligibility Decision. Amitraz: Washington, USA; [accessed on April 30, 2014]. United States Environmental Protection Agency. Available from: www.epa.gov/oppsrrd1/reregistration/REDs/0234red.pdf . [Google Scholar]
- 7.Bonsall JL, Turnbull GJ. Extrapolation from safety data to management of poisoning with reference to amitraz (a formamidine pesticide) and xylene. Hum Toxicol. 1983;2:587–92. doi: 10.1177/096032718300200403. [DOI] [PubMed] [Google Scholar]
- 8.Elinav E, Shapira Y, Ofran Y, Hassin T, Ben-Dov IZ. Near-fatal amitraz intoxication: the overlooked pesticide. Basic Clin Pharmacol Toxicol. 2005;97:185–7. doi: 10.1111/j.1742-7843.2005.pto_97399.x. [DOI] [PubMed] [Google Scholar]
- 9.Shitole DG, Kulkarni RS, Sathe SS, Rahate PR. Amitraz poisoning – an unusual pesticide poisoning. J Assoc Physicians India. 2010;58:317–9. [PubMed] [Google Scholar]
- 10.Sweta, Srivastava U, Agarwal A. Amitraz: an unfamiliar poisoning with familiar pesticide. J Anaesthesiol Clin Pharmacol. 2013;29:420–1. doi: 10.4103/0970-9185.117092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varma PV, Bhatt S, Bhat RY. Amitraz poisoning. Indian J Pediatr. 2013;80:349–50. doi: 10.1007/s12098-012-0772-2. [DOI] [PubMed] [Google Scholar]
- 12.Yilmaz HL, Yildizdas DR. Amitraz poisoning, an emerging problem: epidemiology, clinical features, management, and preventive strategies. Arch Dis Child. 2003;88:130–4. doi: 10.1136/adc.88.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Proudfoot AT. Poisoning with amitraz. Toxicol Rev. 2003;22:71–4. doi: 10.2165/00139709-200322020-00001. [DOI] [PubMed] [Google Scholar]
- 14.Veale DJH, Muller GJ, Wium CA. Amitraz poisoning in South Africa: an analysis of cases over the past 5 years. Clin Toxicol. 2009;47:507–8. [Google Scholar]
- 15.Tavanaei M, Safari KS. Amitraz poisoning. Clin Toxicol. 2010;48:303. [Google Scholar]
- 16.Jacob J, Schaeffer TH. Self-medication with amitraz for delusions of parasitosis: dermal exposure and a delayed presentation. Clin Toxicol. 2011;49:563–4. [Google Scholar]
- 17.Tavanaei M, Akhondi Nematabad V, Safari Kamalabadi S. Incidence of poisoning in Rafsanjan, Iran. Clin Toxicol. 2012;50:295. [Google Scholar]
- 18.Veale DJ, Wium CA, Müller GJ. Toxicovigilance. I: a survey of acute poisonings in South Africa based on tygerberg poison information centre data. S Afr Med J. 2013;103:293–7. doi: 10.7196/samj.6647. [DOI] [PubMed] [Google Scholar]
- 19.Mutlu M, Cansu A, Karakas T, Kalyoncu M, Erduran E. Pattern of pediatric poisoning in the East Karadeniz region between 2002 and 2006: increased suicide poisoning. Hum Exp Toxicol. 2010;29:131–6. doi: 10.1177/0960327109357141. [DOI] [PubMed] [Google Scholar]
- 20.Sabzghabaee AM, Eizadi-Mood N, Gheshlaghi F, Adib N, Safaeian L. Is there a relationship between admission blood glucose level following acute poisoning and clinical outcome? Arch Med Sci. 2011;7:81–6. doi: 10.5114/aoms.2011.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garnier R, Chataigner D, Djebbar D. Six human cases of amitraz poisoning. Hum Exp Toxicol. 1998;17:294. doi: 10.1177/096032719801700515. [DOI] [PubMed] [Google Scholar]
- 22.Aydin K, Kurtoglu S, Poyrazoglu MH, Uzüm K, Ustünbas HB, Hallaç IK. Amitraz poisoning in children: clinical and laboratory findings of eight cases. Hum Exp Toxicol. 1997;16:680–2. doi: 10.1177/096032719701601109. [DOI] [PubMed] [Google Scholar]
- 23.Aydin K, Per H, Kurtoglu S, Poyrazoglu MH, Narin N, Aslan D. Amitraz poisoning in children. Eur J Pediatr. 2002;161:349–50. doi: 10.1007/s00431-002-0945-5. [DOI] [PubMed] [Google Scholar]
- 24.Agin H, Calkavur S, Uzun H, Bak M. Amitraz poisoning: clinical and laboratory findings. Indian Pediatr. 2004;41:482–6. [PubMed] [Google Scholar]
- 25.Aslan S, Bilge F, Aydinli B, Ocak T, Uzkeser M, Erdem AF, et al. Amitraz: an unusual aetiology of Ogilvie's syndrome. Hum Exp Toxicol. 2005;24:481–3. doi: 10.1191/0960327105ht550cr. [DOI] [PubMed] [Google Scholar]
- 26.Atabek ME, Aydin K, Erkul I. Different clinical features of amitraz poisoning in children. Hum Exp Toxicol. 2002;21:13–6. doi: 10.1191/0960327102ht207oa. [DOI] [PubMed] [Google Scholar]
- 27.Avsarogullari L, Ikizceli I, Sungur M, Sözüer E, Akdur O, Yücei M. Acute amitraz poisoning in adults: clinical features, laboratory findings, and management. Clin Toxicol (Phila) 2006;44:19–23. doi: 10.1080/15563650500357545. [DOI] [PubMed] [Google Scholar]
- 28.Batra B, Verma PK, Pramanik V, Gogia AR. Amitraz poisoning – Familiar presentation, unfamiliar diagnosis. Anaesth Intensive Care. 2012;40:363–4. [PubMed] [Google Scholar]
- 29.Beyaztas FY, Gursoy S, Demirel Y, Kaygusuz K, Mimaroglu C. Aspiration and death from amitraz-xylene poisoning. Middle East J Fam Med. 2006;4:42–4. [Google Scholar]
- 30.Caksen H, Odabas D, Arslan S, Akgün C, Atas B, Akbayram S, et al. Report of eight children with amitraz intoxication. Hum Exp Toxicol. 2003;22:95–7. doi: 10.1191/0960327103ht333sr. [DOI] [PubMed] [Google Scholar]
- 31.Chakraborty J, Nagri SK, Gupta AN, Bansal A. An uncommon but lethal poisoning – Amitraz. Australas Med J. 2011;4:439–41. doi: 10.4066/AMJ.2011.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demirel Y, Yilmaz A, Gursoy S, Kaygusuz K, Mimaroglu C. Acute amitraz intoxication: Retrospective analysis of 45 cases. Hum Exp Toxicol. 2006;25:613–7. doi: 10.1177/096032706072472. [DOI] [PubMed] [Google Scholar]
- 33.Doganay Z, Aygun D, Altintop L, Guven H, Bildik F. Basic toxicological approach has been effective in two poisoned patients with amitraz ingestion: case reports. Hum Exp Toxicol. 2002;21:55–7. doi: 10.1191/0960327102ht204cr. [DOI] [PubMed] [Google Scholar]
- 34.Eizadi-Mood N, Sabzghabaee AM, Gheshlaghi F, Yaraghi A. Amitraz poisoning treatment: still supportive? Iran J Pharm Res. 2011;10:155–8. [PMC free article] [PubMed] [Google Scholar]
- 35.Ertekin V, Alp H, Selimoglu MA, Karacan M. Amitraz poisoning in children: retrospective analysis of 21 cases. J Int Med Res. 2002;30:203–5. doi: 10.1177/147323000203000215. [DOI] [PubMed] [Google Scholar]
- 36.Gursoy S, Kunt N, Kaygusuz K, Kafali H. Intravenous amitraz poisoning. Clin Toxicol (Phila) 2005;43:113–6. [PubMed] [Google Scholar]
- 37.Hu SY, Hsu CL, Tsan YT, Hung DZ, Hu WH, Li HP. Torsades de pointes in amitraz poisoning. Resuscitation. 2010;81:366–7. doi: 10.1016/j.resuscitation.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Kalyoncu M, Dilber E, Okten A. Amitraz intoxication in children in the rural Black Sea region: analysis of forty-three patients. Hum Exp Toxicol. 2002;21:269–72. doi: 10.1191/0960327102ht241oa. [DOI] [PubMed] [Google Scholar]
- 39.Kennel O, Prince C, Garnier R. Four cases of amitraz poisoning in humans. Vet Hum Toxicol. 1996;38:28–30. [PubMed] [Google Scholar]
- 40.Leung VK, Chan TY, Yeung VT. Amitraz poisoning in humans. J Toxicol Clin Toxicol. 1999;37:513–4. doi: 10.1081/clt-100102523. [DOI] [PubMed] [Google Scholar]
- 41.Mwita CC. Amitraz poisoning in a patient from rural Africa. Case report. JCR. 2012;2:39–41. [Google Scholar]
- 42.Rajesh NT, Pandian NS, Mathai J. Poisoning with Amitraz, a veterinary drug, in an adolescent boy. Sri Lanka J Child Health. 2014;43:55–6. [Google Scholar]
- 43.Ulukaya S, Demirag K, Moral AR. Acute amitraz intoxication in human. Intensive Care Med. 2001;27:930–3. doi: 10.1007/s001340100934. [DOI] [PubMed] [Google Scholar]
- 44.Yaramis A, Soker M, Bilici M. Amitraz poisoning in children. Hum Exp Toxicol. 2000;19:431–3. doi: 10.1191/096032700682694215. [DOI] [PubMed] [Google Scholar]
- 45.Dreisbach RH. Diagnosis and treatment. Handbook of poisoning. California: Lange Medical Publishing; 1977. p. 126. [Google Scholar]
- 46.Hsu WH, Kakuk TJ. Effect of amitraz and chlordimeform on heart rate and pupil diameter in rats: mediated by alpha 2-adrenoreceptors. Toxicol Appl Pharmacol. 1984;73:411–5. doi: 10.1016/0041-008x(84)90093-0. [DOI] [PubMed] [Google Scholar]
- 47.Abu-Basha EA, Yibchok-Anun S, Hopper DL, Hsu WH. Effects of the pesticide amitraz and its metabolite BTS 27271 on insulin and glucagon secretion from the perfused rat pancreas: involvement of alpha2D-adrenergic receptors. Metabolism. 1999;48:1461–9. doi: 10.1016/s0026-0495(99)90160-9. [DOI] [PubMed] [Google Scholar]
- 48.Tsuchiya Y, Hosokawa T, Kasuya Y. Involvement of alpha 2-adrenergic receptors in the vagal reflex-induced tracheal constriction. J Pharmacobiodyn. 1990;13:30–5. doi: 10.1248/bpb1978.13.30. [DOI] [PubMed] [Google Scholar]
- 49.Robertson HA, Leslie RA. Noradrenergic alpha 2 binding sites in vagal dorsal motor nucleus and nucleus tractus solitarius: autoradiographic localization. Can J Physiol Pharmacol. 1985;63:1190–4. doi: 10.1139/y85-195. [DOI] [PubMed] [Google Scholar]
- 50.Reynoldson JA, Cullen LK. Amitraz depresses cardiovascular responses to bilateral carotid occlusion. J Vet Pharmacol Ther. 1996;19:22–6. doi: 10.1111/j.1365-2885.1996.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 51.Maze M, Tranquilli W. Alpha-2 adrenoceptor agonists: defining the role in clinical anesthesia. Anesthesiology. 1991;74:581–605. [PubMed] [Google Scholar]
- 52.Yim GK, Holsapple MP, Pfister WR, Hollingworth RM. Prostaglandin synthesis inhibited by formamidine pesticides. Life Sci. 1978;23:2509–15. doi: 10.1016/0024-3205(78)90176-5. [DOI] [PubMed] [Google Scholar]
- 53.Harvey PW, Cockburn A, Davies WW. Commentary on ‘an unusual poisoning with the unusual pesticide amitraz’ with respect to the pharmacology of amitraz. Hum Exp Toxicol. 1998;17:191–2. doi: 10.1177/096032719801700312. [DOI] [PubMed] [Google Scholar]
- 54.Folz SD, Kakuk TJ, Henke CL, Rector DL, Tesar FB. Clinical evaluation of amitraz as a treatment for canine demodicosis. Vet Parasitol. 1984;16:335–41. doi: 10.1016/0304-4017(84)90051-7. [DOI] [PubMed] [Google Scholar]
- 55.Grossman MR. Amitraz toxicosis associated with ingestion of an acaricide collar in a dog. J Am Vet Med Assoc. 1993;203:55–7. [PubMed] [Google Scholar]
- 56.Queiroz-Neto A, Zamur G, Gonçalves SC, Carregaro AB, Mataqueiro MI, Harkins JD, et al. Characterization of the antinociceptive and sedative effect of amitraz in horses. J Vet Pharmacol Ther. 1998;21:400–5. doi: 10.1046/j.1365-2885.1998.00150.x. [DOI] [PubMed] [Google Scholar]
- 57.Osano O, Oladimeji AA, Kraak MH, Admiraal W. Teratogenic effects of amitraz, 2,4-dimethylaniline, and paraquat on developing frog (Xenopus) embryos. Arch Environ Contam Toxicol. 2002;43:42–9. doi: 10.1007/s00244-002-1132-4. [DOI] [PubMed] [Google Scholar]
- 58.Dadpour B, Forooghian M, Talebi M, Tavassoli S. A case report of deep coma in a pregnant women with amitraz poisoning. IJOGI. 2013;16:9–12. [Google Scholar]
- 59.Parrish A, Lancaster R. Does the nose know? Amitraz poisoning and olfaction. S Afr Med J. 2012;102:223–4. [PubMed] [Google Scholar]
- 60.Hugnet C, Buronrosse F, Pineau X, Cadoré JL, Lorgue G, Berny PJ. Toxicity and kinetics of amitraz in dogs. Am J Vet Res. 1996;57:1506–10. [PubMed] [Google Scholar]
- 61.Smith BE, Hsu WH, Yang PC. Amitraz-induced glucose intolerance in rats: antagonism by yohimbine but not by prazosin. Arch Toxicol. 1990;64:680–3. doi: 10.1007/BF01974698. [DOI] [PubMed] [Google Scholar]
- 62.Nichols MH, King WD, James LP. Clonidine poisoning in Jefferson County, Alabama. Ann Emerg Med. 1997;29:511–7. doi: 10.1016/s0196-0644(97)70225-7. [DOI] [PubMed] [Google Scholar]
- 63.Schaffer DD, Hsu WH, Hopper DL. The effects of yohimbine and four other antagonists on amitraz-induced depression of shuttle avoidance responses in dogs. Toxicol Appl Pharmacol. 1990;104:543–7. doi: 10.1016/0041-008x(90)90176-u. [DOI] [PubMed] [Google Scholar]


