Abstract
Background & objectives:
Allostatic load (AL) is a cumulative measure of physiological deregulation and is influenced by multiple factors including nutrition. The objectives of the study were to assess AL among adolescent boys (15-19 yr) and delineate its association with psychological stress and micronutrient status.
Methods:
A cross-sectional, school-based study was conducted among 370 adolescent boys of five higher secondary schools from Hyderabad, India. Perceived stress, adolescent life event stress (ALES), psychological morbidity and coping were measured. Biomarkers of AL included dehydroepiandrosterone sulphate, 12-h urinary cortisol, interleukin-6, C-reactive protein, lipid profile, body mass index and blood pressure. Micronutrient status with respect to iron (haemoglobin, ferritin, hepcidin, soluble transferrin receptor), folate, vitamins B12, C and A were analyzed in a sub-sample of 146 boys. AL score ≥3 was calculated from eight biomarkers.
Results:
Fourteen per cent participants had no AL but 34.3 per cent had AL score of ≥ 3. Unadjusted means of ALES scores were significantly different (P = 0.045) among participants with low [mean, 95% confidence interval (CI): 580, 531-629] and high (663, 605-721) AL. After controlling for confounders, the means were significantly different for controllable life event sub-scale of ALES (P = 0.048). Adjusted hepcidin concentrations were significantly higher among participants with high AL (means, 95% CI, 27.2, 24.0-30.8 for high AL; 22.1, 20.2-24.2 μg/l for low AL, P = 0.014).
Interpretation & conclusions:
Build-up of AL was found in adolescent boys and was positively associated with life event stress. Iron nutrition and stress exhibited a positive association through hepcidin. The study provides a link between iron nutrition, physiological deregulation and stress.
Keywords: Adolescents, allostatic load, hepcidin, iron, psychological stress
Allostatic load (AL) is defined as the cumulative wear and tear on physiologic processes due to recurrent or chronic stress and uses multiple biomarkers to quantify this physiological deregulation. AL has proved to be beneficial in quantifying the overall stress accumulation, well before the actual clinical manifestations set in1. Conceptually, psychological stress is an important dimension of AL. There are a few studies looking for a relationship between stress and AL2. However, the measurements are largely done among elderly population3. Exploring AL among adolescent population is an emerging area since puberty is marked by an increase in the susceptibility to various psychological disorders, such as anxiety and depression4,5,6. The developmental plasticity associated with certain areas of the brain during adolescence provides more chances of resilience7 and is therefore, of focus. In India, the biopsychological model of stress and AL has not been tested and is of interest due to the increasing reports on stress among adolescents8,9.
Lifestyle factors including nutrition could influence AL. Healthy eating index has shown a negative association with AL among US adolescents4. Multiple micronutrient deficiencies are a significant problem in India and have not been addressed so far in relation to AL10. The effects could be bi-directional, with specific deficiency of micronutrients leading to more AL accumulation or AL leading to exacerbation of deficiency. Therefore, an understanding on whether the accumulation of physiological wear and tear starts early and has a relationship with stress is warranted. If an association with micronutrient status exists, an effective strategy can be delineated to combat AL and/or micronutrient deficiency.
The present study was aimed to understand whether there was build-up of AL among adolescent boys (15-19 yr), and if so, was it associated with psychological stress and/or micronutrient status.
Material & Methods
The study was carried out in Greater Hyderabad Municipal Corporation, Telangana erstwhile Andhra Pradesh, India, wherein all (n = 5) government boys’ schools9 were covered. Since the study intended to detect early changes in adolescents, boys were selected for the study due to reports of men being more susceptible to AL11. The study protocol was approved by the Institutional Ethics Committee in 2007, and was carried out in the academic year 2009-2010 with approval from the Directorate of Intermediate Education, Hyderabad. The detailed study design and methodology can be found elsewhere9,12.
Study design, sample size and recruitment: For stress variables, a sample size of 353 was fixed assuming a 40 per cent prevalence of stress from pilot studies9,12. To test the mean difference in stress across categorical scores of AL, a difference of 5 units for Perceived Stress Scale-14 (PSS-14) with standard deviation of 5 and 100 units for adolescent life event stress (ALES) with SD of 1689 was assumed and the sample size required per group was 44. The above sample size was adequate to detect a delta in the range of 0.5-0.75 SD unit for all micronutrients on the categorical scores of AL.
To minimize the influence of acute stress associated with school examinations, the study was carried out during a period of no terminal examinations. Written consent was obtained from the participants and their parents. Apparently healthy students were included in the study. Exclusion criteria were diagnosed hormonal abnormalities, congenital anomalies, chronic illnesses, current medication or intake of multivitamin or mineral supplements for the past one year. Enrollment was carried out using random selection from each school12.
Study variables
Standard of living index (SLI): SLI, which is a proxy for economic status, was calculated using 17 household assets, and a weighted scoring pattern was used13.
Anthropometric measurements: Anthropometry was done as per the standard methodology described previously12,14.
Blood pressure: The blood pressure was measured using a mercury sphygmomanometer (Omron, Bengaluru, India). The participants were seated for 15 min before measuring BP. The procedure was repeated three times at an interval of five minutes while resting in a seated position15.
Stress variables: The variables studied were ALES, perceived stress, psychological distress and coping using pre-tested, culturally appropriate scales12. The ALES scale16 has 40 items and measures the stress due to life events experienced by the participants in the past year scored on a dichotomous scale of ‘yes’ or ‘no’. The reported internal consistencies for controllable and uncontrollable events were more than 0.816. PSS-14 is a 14-item scale which measures the degree to which situations in one's life are appraised as stressful during the past month17. The participants were required to choose from a scale of five alternatives on a 0-4 scale. The seven positive items were reverse scored and added up to the seven negative items to get the total score. Coefficient alpha reliability for PSS-14 was 0.84 among college student population. Greater scores indicated higher perceived stress. General Health Questionnaire-12 (GHQ-12) has 12 items and is a measure of psychological morbidity experienced during the past week. For scoring, a 0-3 Likert scale followed by bimodal scoring (0-0-1-1) was used. A score of 3 was used as the cut-off for psychological morbidity18.
Methods of coping were assessed using coping strategies scale (50 items). The test consisted of five sub-scales combined into two major constructs of approach and avoidance coping. The split-half reliability reported in the scale was 0.78 for approach and 0.69 for avoidance and the test-retest reliability was 0.9219.
Collection of blood and urine: Blood sample collection, transportation and processing were carried out as have been described previously and were done within one day of administration of behavioural stress measures12. Twelve hour urine samples were collected using 2 l leak-free wide mouth bottles, pre-coated with 100 mg/l thiomersal (Sisco research laboratories Pvt. Ltd., Hyderabad). The samples were transported, measured, filtered using glass wool, aliquoted and stored at −20°C until analysis.
Allostatic load measures
Primary mediators: Dehydroepiandrosterone sulphate (DHEAS) was determined in plasma and cortisol in urine using RIA kit (Immunotech, Beckman Coulter, Marseille Cedex 9, France). The analytical sensitivity of DHEAS was 6 µg/100 ml. Twelve hour urinary cortisol was expressed as µg/g creatinine. Interleukin-6 (IL-6) assay was done using sandwich enzyme immunoassay (R&D Systems Inc., MN, USA), with a minimum detectable dose of 0.70 pg/ml and assay range of 3.12-300 pg/ml. The OD for samples below the lowest standard concentrations was extrapolated using 4-PL logistic curve fit (Sigma plot version 11.0) till the minimum detectable dose of 0.7 pg/ml. C-reactive protein (CRP) was assayed using human CRP assay kit (Alpha Diagnostic International, USA). Assay range was 5-100 ng/ml. The minimum detectable dose was 0.35 ng/ml. For response below the assay sensitivity, a value of 0.3 ng/ml was assigned.
Secondary mediators: Total cholesterol and high-density lipoprotein (HDL) cholesterol were measured in the plasma using a commercial kit as per the manufacturer's instructions (Biosystems, Barcelona, Spain).
Biomarkers of micronutrient status: Haemoglobin was analyzed in the blood samples by cyanmethemoglobin method (Hemocor-D, Coral Systems, Goa) and plasma ascorbic acid by α,α-bipyridyl micro method20 on the day of blood collection. A sandwich ELISA was used to measure serum ferritin concentrations21. Folic acid and vitamin B12 (Siemens Medical Solutions Diagnostics, USA), soluble transferrin receptor (R&D Systems Inc., USA) and hepcidin (DRG International Inc., GmbH, Germany) were estimated using commercial kits as per the manufacturers’ instructions. Plasma retinol was estimated22 by high-performance liquid chromatography (Thermo Finnigan, Herts, UK). The laboratory carrying out these analyses is being continuously validated for ferritin and retinol (VITAL-EQA program, Centers for Disease Control, Atlanta, USA).
Computation of AL: For each of the biomarkers of AL, the high risk threshold was determined empirically on the basis of the distribution of that biomarker in the sample23. Top quartile was considered as risk, except for HDL cholesterol and DHEAS for which below 25th percentile corresponded to risk. Each participant was assigned one point for a biomarker reading beyond the threshold value. AL was measured by summing the number of parameters, for which the participant showed biomarkers in the risk quartile. A maximum score of eight was possible for AL.
Cortisol was not included in the algorithm since only 107 participants provided urine sample. However, the AL model was tested with and without cortisol and was found to have an intra-class correlation coefficient of 0.884, P < 0.001 signifying agreement between the two models.
Statistical analysis: The analysis was carried out using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). Normal distribution of the variables was tested using Kolmogorov–Smirnov test. Log transformation was done for variables which were not normally distributed. The final sample included 370 boys for behavioural measures and 146 for analyzing the biomarkers of AL and micronutrient status. One participant with CRP >10 mg/l was excluded from the sub-sample, and therefore, data for 145 were analyzed for AL and micronutrient status. The ALES and PSS-14 scores were treated as continuous variable for analysis, bivariate correlations were tested and median split was used for descriptive statistics. The inadequacy of micronutrients was defined as described previously12.
The relationship between stress variables and AL was tested using independent student t test, followed by ANCOVA, if the results were significant. The covariates considered for the relationship between ALES and AL were participant's age, coping variables, SLI, GHQ-12 and PSS-14.
Results
The mean age of the participants was 16.7 yr [95% confidence interval (CI), 16.5-16.9]. Forty two per cent of families had SLI below median. About three-fourth reported GHQ above the cut-off and one-fourth of participants showed low coping behaviour; around 25 per cent of participants fell in the topmost quartile in individual biomarkers of AL, with an overall AL median of 2.02 (interquartile range, IQR 1.34, Table I). Fourteen per cent of participants had no AL and 34.3 per cent had an AL score of 3 and above (Figure). With the inclusion of cortisol, the proportion showing AL was 41 per cent.
Table I.
Psychological stress variables and biomarkers of allostatic load (AL); descriptive and cut-off values
Figure.
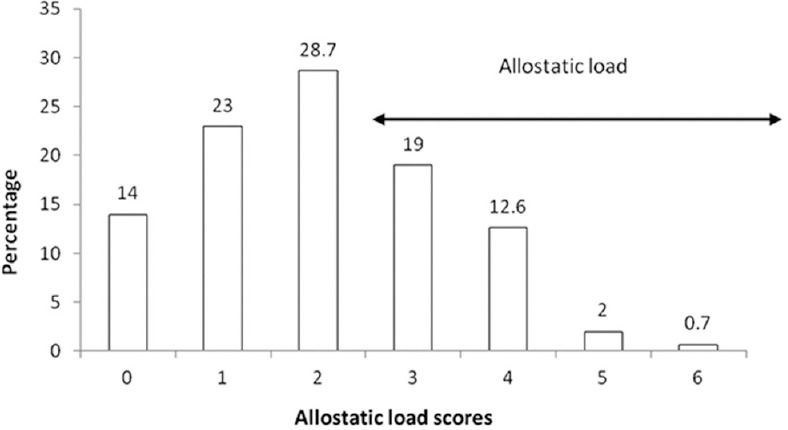
Distribution of allostatic load scores. The cumulative scores were calculated using quartiles of each parameter; counting the number of markers for which the individual has fallen in the risk quartile and summing up. Equal weighting was provided for all markers. A score equal to or above 3.0 was considered as allostatic load (n=143).
SLI showed a negative correlation with ALES (r = -0.318, P < 0.001) and a positive correlation with avoidance coping (r = 0.238, P = 0.004). There was a negative trend between AL and SLI (r = -0.140, P = 0.096).
Based on the data, the AL scores were categorized as normal = 0-2 and risk = 3-6 and tested for their association with stress variables. ALES showed a significant association with AL, P = 0.045 (Table II) after controlling for age, perceived stress, psychological morbidity and approach coping strategies, F(1,135) = 4.15, P = 0.043. The relationship did not persist [adjusted mean, 95% CI, 586, 542-631 for low AL and 650, 590-712 for high AL, F(1,134) = 2.748, P = 0.100] after controlling for economic status. Among the sub-scales of ALES, controllable life events were significantly higher in participants with AL and persisted after controlling for all confounders including SLI [adjusted mean, 95% CI, 174, 160-189 for low AL and 202, 182-223 for high AL, F(1,134) = 4.645, P = 0.048].
Table II.
Association between allostatic load (AL) and stress variables
After controlling for confounders, there was a significant association between AL scores and hepcidin concentrations [adjusted means, 95% CI, 22.1, 20.2-24.2 µg/l for low AL, 27.2, 24.0-30.8 µg/l for high AL, F(1,138) = 6.491, P = 0.014, Table III]. In addition, there existed a positive correlation between primary mediators and hepcidin (r = 0.197, P = 0.018) but not with secondary mediators (r = -0.049, P = 0.55).
Table III.
Relationship between allostatic load (AL) and biomarkers of micronutrient status
Discussion
The study explored the build-up of AL among adolescent boys using AL index representing hypothalamus-pituitary-adrenal (HPA) axis, cardio-metabolic and inflammatory markers. About 34 per cent of participants exhibited AL which showed a significant association with ALES. Among biomarkers of micronutrient status, hepcidin showed a significant association with AL, signifying a link between physiological deregulation and iron metabolism.
Forty two per cent of the families had asset-based SLI below median cut-off and the participants belonged to low to middle socio-economic status (SES). The substantial proportion of stunting, undernutrition and multiple micronutrient deficiencies suggest a background of general undernutrition. The proportion of anaemia reported in this group was comparable to the national average of 24 per cent24. Ascorbic acid and retinol deficiencies were in concordance with the earlier report among adults (75 and 21%, respectively)25.
AL has been considered as a cumulative effect of several stressors including SES, stress and early adversities associated with it. The algorithm which has been used for AL included DHEAS as the marker of HPA axis function, along with two inflammatory markers and five secondary mediators, representing cardio-metabolic function. The defined cut-offs of each biomarker of AL were lower than the clinical cut-offs but comparable to the US adolescent data in terms of blood pressure, lower for cholesterol, body mass index and CRP. HDL risk cut-off was significantly lower in our participants compared to the US23. The AL scores computed with and without cortisol showed a high correlation emphasizing on the cumulative nature of AL and non-dependency on a single marker. In adolescence, physical health is generally at its peak and subtle alterations in allostatic mediators are primarily observed. If not addressed, the continued exposure to stress may lead to exponential increases in later life leading to adverse health outcomes23.
A cumulative index cut-off of 3 corresponding to the 75th percentile was used for AL. This cut-off was similar to the cut-off used in the first study among elderly with ten biomarkers11, which was used to provide more specificity among adolescent boys with respect to AL. The median value of AL observed in the study with eight biomarkers was comparable to what has been reported in two nationally represented studies in American adolescents5,23.
The study demonstrated a build-up of AL among one-third (34%) of the participants, which was in concordance with the 35 per cent reported among the US adolescents aged 12-20 yr with a lower cut-off of two4. At this age, one-third of boys exhibiting three or more biomarkers in the top quartile need caution and the reasons of this early physiological deregulation need to be evaluated. Two prospective cohort studies have reported that AL is associated with health risk later; that among adolescents with high AL, there is an increased risk of asthma and schizotypal personality in adulthood5,26.
The relationship between psychological stress and AL among adolescents has shown a positive association among adults2. One prospective cohort study showed an association between early life stressors and AL three years later but was based on HPA functioning alone27. In the present study, the scores of AL were significantly high for those who had higher ALES. This provides evidence for psychological stress of adolescents leading to build-up of AL. SES, a known modulator of AL28, appeared to play a role in the relationship between AL and ALES in this population.
There was an association between controllable life events sub-scale of ALES scale and AL. Controllable life events are considered modifiable by the individual and include items such as change of major subject, social activities, sleeping habits, trouble with parents/bullies, serious argument with a close friend, and issues such as breakup with girlfriend. Most of these events appear to be affected more by emotional factors than socio-economic issues. Therefore, this observation provides indirect evidence on modifiable factors such as promoting adaptive coping in the population.
In the present study, there was a significant association between AL and hepcidin, especially the primary mediators of AL, IL-6, CRP and DHEAS. The hepcidin concentrations reported for participants were comparable to the previously reported hepcidin concentrations in 13-15 yr old boys of the same region29. Earlier, an association between stress and hepcidin through IL-6 has been demonstrated as an outcome of stress upregulating the inflammatory pathway of hepcidin release11. Therefore, the pathway of influence of AL and hepcidin could be through IL-6 and CRP. A recent study has reported an inverse association between AL scores and serum beta-carotene concentrations in middle-aged men, providing evidence for a link between nutritional status and AL30.
In conclusion, this study showed that there was build-up of AL among adolescent boys. AL was associated with stress but not with micronutrient status, except for hepcidin. Attempts need to be made to target AL and bring about resilience. The implications of the association between AL and hepcidin on iron status need to be investigated further.
Acknowledgment
Authors acknowledge Dr Shahnaz Vazir, Former Deputy Director and ICMR Emeritus Scientist, Dr B. Sesikeran MD, Former Director, National Institute of Nutrition for critical inputs. The assistance provided by Shri G. Subbarao in the administration of psychological scales, the field assistance by Shriyut Venkat Narasimha Reddy, K. Narasimha Reddy, Technical Assistants, Behavioural Sciences; haemoglobin and retinol estimation by Shriyut Vikas Rao and JS Acharya, Technical Officers, Micronutrient Research, are acknowledged.
Footnotes
Conflicts of Interest: None.
References
- 1.McEwen BS. Interacting mediators of allostasis and allostatic load: towards an understanding of resilience in aging. Metabolism. 2003;52(10 Suppl 2):10–6. doi: 10.1016/s0026-0495(03)00295-6. [DOI] [PubMed] [Google Scholar]
- 2.Beckie TM. A systematic review of allostatic load, health, and health disparities. Biol Res Nurs. 2012;14:311–46. doi: 10.1177/1099800412455688. [DOI] [PubMed] [Google Scholar]
- 3.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Theall KP, Drury SS, Shirtcliff EA. Cumulative neighborhood risk of psychosocial stress and allostatic load in adolescents. Am J Epidemiol. 2012;176(Suppl 7):S164–74. doi: 10.1093/aje/kws185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahreinian S, Ball GD, Vander Leek TK, Colman I, McNeil BJ, Becker AB, et al. Allostatic load biomarkers and asthma in adolescents. Am J Respir Crit Care Med. 2013;187:144–52. doi: 10.1164/rccm.201201-0025OC. [DOI] [PubMed] [Google Scholar]
- 6.Hastings PD, Shirtcliff EA, Klimes-Dougan B, Allison AL, Derose L, Kendziora KT, et al. Allostasis and the development of internalizing and externalizing problems: changing relations with physiological systems across adolescence. Dev Psychopathol. 2011;23:1149–65. doi: 10.1017/S0954579411000538. [DOI] [PubMed] [Google Scholar]
- 7.Chen E, Miller GE, Lachman ME, Gruenewald TL, Seeman TE. Protective factors for adults from low-childhood socioeconomic circumstances: the benefits of shift-and-persist for allostatic load. Psychosom Med. 2012;74:178–86. doi: 10.1097/PSY.0b013e31824206fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arun P, Chavan BS. Stress and suicidal ideas in adolescent students in Chandigarh. Indian J Med Sci. 2009;63:281–7. [PubMed] [Google Scholar]
- 9.Augustine LF, Vazir S, Rao SF, Rao MV, Laxmaiah A, Nair KM. Perceived stress, life events and coping among higher secondary students of Hyderabad, India: a pilot study. Indian J Med Res. 2011;134:61–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Diet and nutritional status of rural population, prevalence of hypertension and diabetes among adults and infant and young child feeding practices -report of third repeat survey. Technical report No. 26. Hyderabad, India: National Institute of Nutrition; 2012. National Nutrition Monitoring Bureau. [Google Scholar]
- 11.Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci U S A. 2001;98:4770–5. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Augustine LF, Nair KM, Rao SF, Rao MV, Ravinder P, Balakrishna N, et al. Adolescent life-event stress in boys is associated with elevated IL-6 and hepcidin but not hypoferremia. J Am Coll Nutr. 2014;33:354–62. doi: 10.1080/07315724.2013.875417. [DOI] [PubMed] [Google Scholar]
- 13.National family and health survey (NFHS-2) Mumbai, India: IIPS; 2000. International Institute for Population Sciences (IIPS) and ORC Macro. [Google Scholar]
- 14.Jelliffe DB. Assessment of nutritional status of the community. Geneva, Switzerland: World Health Organization; 1988. [Google Scholar]
- 15.Victor RG. Sysemic hypertension: mechanisms and diagnosis. In: Bonow RO, Mann DL, Zipes DP, Libby P, editors. Braunwald’s heart disease: a textbook of cardiovascular medicine. 9th ed. Philadelphia: Saunders-Elsevier; 2011. pp. 934–52. [Google Scholar]
- 16.Aggarwal S, Prabhu HRA, Anand A, Kotwal A. Stressful life events among adolescents: the development of a new measure. Indian J Psychiatry. 2007;49:96–102. doi: 10.4103/0019-5545.33255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 18.Goldberg DP, Williams P. A user's guide to the general health questionnaire. Berkshire, UK: NFER-Nelson; 1988. [Google Scholar]
- 19.Srivastava AK. Manual of coping strategies scale. Varanasi, India: Rupa Psychological Centre, Banaras Hindu University; 2001. [Google Scholar]
- 20.Zannoni V, Lynch M, Goldstein S, Sato P. A rapid micromethod for the determination of ascorbic acid in plasma and tissues. Biochem Med. 1974;11:41–8. doi: 10.1016/0006-2944(74)90093-3. [DOI] [PubMed] [Google Scholar]
- 21.Pawashe AB, Raman L, Nair M, Sarma J. Validity of using capillary blood for the measurement of plasma ferritin. Clin Chim Acta. 1987;163:119–20. doi: 10.1016/0009-8981(87)90041-6. [DOI] [PubMed] [Google Scholar]
- 22.Bieri JG, Tolliver TJ, Catignani GL. Simultaneous determination of alpha-tocopherol and retinol in plasma or red cells by high pressure liquid chromatography. Am J Clin Nutr. 1979;32:2143–9. doi: 10.1093/ajcn/32.10.2143. [DOI] [PubMed] [Google Scholar]
- 23.Rainisch BKW, Upchurch DM. Sociodemographic correlates of allostatic load among a national sample of adolescents: findings from the National Health and Nutrition Examination Survey, 1999-2008. J Adolesc Health. 2013;53:506–11. doi: 10.1016/j.jadohealth.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National family health survey-3, IIPS and ORC Macro. Mumbai, India: IIPS; 2007. International Institute for Population Sciences. [Google Scholar]
- 25.Chiplonkar SA, Agte VV, Mengale SS, Tarwadi KV. Are lifestyle factors good predictors of retinol and Vitamin C deficiency in apparently healthy adults? Eur J Clin Nutr. 2002;56:96–104. doi: 10.1038/sj.ejcn.1601291. [DOI] [PubMed] [Google Scholar]
- 26.Peskin M, Raine A, Gao Y, Venables PH, Mednick SA. A developmental increase in allostatic load from ages 3 to 11 years is associated with increased schizotypal personality at age 23 years. Dev Psychopathol. 2011;23:1059–68. doi: 10.1017/S0954579411000496. [DOI] [PubMed] [Google Scholar]
- 27.Essex MJ, Shirtcliff EA, Burk LR, Ruttle PL, Klein MH, Slattery MJ, et al. Influence of early life stress on later hypothalamic-pituitary-adrenal axis functioning and its covariation with mental health symptoms: a study of the allostatic process from childhood into adolescence. Dev Psychopathol. 2011;23:1039–58. doi: 10.1017/S0954579411000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dowd JB, Simanek AM, Aiello AE. Socio-economic status, cortisol and allostatic load: a review of the literature. Int J Epidemiol. 2009;38:1297–309. doi: 10.1093/ije/dyp277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nair KM, Brahmam GNV, Radhika MS, Dripta RC, Ravinder P, Balakrishna N, et al. Inclusion of guava enhances non-heme iron bioavailability but not fractional zinc absorption from a rice-based meal in adolescents. J Nutr. 2013;143:852–8. doi: 10.3945/jn.112.171702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenberg N, Park CG, Eldeirawi K. Relationship of serum carotenoid concentrations with allostatic load as a measure of chronic stress among middle-aged adults in the USA. Public Health Nutr. 2015;18:313–21. doi: 10.1017/S1368980014000056. [DOI] [PMC free article] [PubMed] [Google Scholar]


