Abstract
Background & objectives:
Reduction of viraemia in patients with chronic hepatitis B virus (HBV) infection using nucleoside/nucleotide analogues reduces fatal liver disease-related events, but development of resistance in virus presents serious clinical challenge. Therefore, comparative evaluation of prolonged antiviral monotherapy and combination therapies was prospectively studied to assess their influence on viral suppression, rapidity of response, development of drug resistance and surfacing mutants in chronic liver disease (CLD) patients.
Methods:
A total of 158 (62eAg-ve) chronic hepatitis B patients were prospectively studied for 24 months. Final analysis was performed on patients treated with lamivudine (LAM, n = 28), adefovirdipivoxil (ADV, n = 24), tenofovir disoproxil fumarate (TDF, n = 26), entecavir (ETV, n = 25), LAM + ADV (n = 28) and LAM + TDF (n = 27). Quantitative hepatitis B virus DNA was detected using real-time polymerase chain reaction. Multiple comparisons among drugs and genotypic mutations were analyzed.
Results:
Progressive biochemical and virological response were noted with all the regimens at 24 months except LAM and ADV which were associated with viral breakthrough (VBT) in 46.4 and 25 per cent, respectively. Mutations: rtM204V (39.3%), M204V+L180M (10.7%) while rtA181V (8.1%) and rtN236T (8.3%) were observed with LAM and ADV regimen, respectively. LAM + ADV combination therapy revealed VBT in seven per cent of the cases without mutations whereas TDF, ETV and LAM + TDF therapies neither showed VBT nor mutations.
Interpretation & conclusions:
LAM was the least potent drug among all therapeutic options followed by ADV. TDF and ETV were genetically stable antivirals with a strong efficacy. Among newer combination therapies, LAM + TDF revealed more efficacy in virological remission and acted as a profound genetic barrier on long term. Hence, newer generation molecules (TDF, ETV) and effective combination therapy should be a certain choice.
Keywords: Adefovir, combination therapies, entecavir, lamivudine, monotherapy, mutations, resistance, tenofovir, VBT
Chronic hepatitis B virus (HBV) infection affects an estimated 400 million people worldwide with a million deaths annually1. About one third of chronically infected patients have liver-related morbidity, namely, cirrhosis and hepatocellular carcinoma (HCC), and the remaining two-third though asymptomatic are at an increased risk of developing liver damage2. Eradication of HBV infection, as corroborated by hepatitis B surface antigen (HBsAg) seroconversion, is usually not possible with the currently available therapies. However, reduction of viraemia or DNA negativity is likely to prevent the emergence of fatal liver disease-related events, such as cirrhosis, decompensation and HCC3.
Treatment of HBV has greatly improved with the availability of nucleoside/nucleotide analogues (NAs) such as lamivudine (LAM), adefovir dipivoxil (ADV), tenofovir disoproxil fumarate (TDF), entecavir (ETV) and telbivudine. However, the clinical benefit of prolonged therapy with these drugs has often been eroded by the emergence of resistant mutant strains generated by the spontaneous error rate of the viral polymerase4, leading to the development of drug resistance. Emergence of drug resistance in naïve cases has been reported to be high with LAM (67% at four years) and ADV (29% at five years) and extremely low with ETV (1% at five years) and TDF (none at three years)5. LAM+ADV combination therapy has revealed lower (2%) incidence of mutation than in those receiving LAM alone (20%)6. Patients on combination therapy usually had viral clearance by 12 weeks than patients receiving adefovirmonotherapy7. LAM+TDF may reduce viraemia better than LAM+ADV as TDF has higher genetic barrier and potency than ADV. Sequential therapy with two drugs has resulted in the sequential selection of mutations conferring resistance to the primary therapy and the consequent rescue therapy8.
This prospective pilot study was undertaken to do a comparative evaluation of prolonged antiviral therapy as monotherapies and combination therapies to assess their influence on viral suppression, rapidity of response along with the development of drug resistance and surfacing of mutants in chronic liver disease (CLD) patients.
Material & Methods
Treatment naïve consecutive patients with HBV-related chronic hepatitis or cirrhosis, with or without decompensation, were selected for this prospective study from gastroenterology outpatient and wards of Sir Sunderlal Hospital, Institute of Medical Sciences (IMS), Banaras Hindu University (BHU), Varanasi, India during May 2008 to March 2011. The study protocol was approved by the Ethical Committee of IMS, BHU. Participants involved in the study were informed and written consent was obtained.
Inclusion criteria included age >16 yr with persistent elevation of alanine amino transaminase (ALT) level greater than two times the upper limit of normal (ULN) and HBV DNA >105 copies/ml in hepatitis B e antigen (HBeAg)-positive and >104 copies/ml in HBeAg-negative cases with or without decompensation. Patients with co-infection with hepatitis C virus (HCV), hepatitis E virus (HEV), hepatitis A virus (HAV) or HIV, presence of sepsis or hepatorenal syndrome, presence of HCC and active alcohol abuse over the past three months were excluded from the study. Patients with CLD fulfilling the above selection criteria underwent detailed clinical examination (endoscopy), routine haematologic and biochemical tests and serology for serum HBsAg, HBeAg and its antibody (anti-HBe), anti-HBc IgM (DIA.PRO, Milano, Italy), anti-HCV, anti-HEV IgM, anti-HAV IgM (Orgenics, Yavne, Israel) and HIV I and II (ELISA kits, Standard Diagnostics, Inc. Korea) were detected through ELISA. Blood samples (5 ml) were collected at inclusion and during follow up and stored at −80°C in aliquots. Patients with evidence of hepatic necroinflammation and/or fibrosis underwent quantitative HBV DNA estimation.
Patients with chronic hepatitis B (CHB, chronic necroinflammatory disease of the liver caused by persistent infection) and cirrhosis (further worsening of liver by destructive damage of liver parenchymal cells) were differentiated at the entry point after reviewing their parameters. For those with decompensation, Child-Turcotte-Pugh (CTP) and Model for End-stage Liver Disease (MELD) scores were calculated1 along with biochemical/serological tests to list the patients among the two subgroups.
Initially, 198 treatment naïve patients were enrolled in this study. The study protocol was focused on six treatment regimens (including conventional and newer molecules): LAM-100mg/day (n = 35), ADV-10mg/day (n = 32), TDF-300mg/day (n = 33), ETV-0.5mg/day (n = 32), LAM + ADV (n = 34) and LAM + TDF (n = 32) for 24 months. Forty patients were lost during the first year of follow up (LAM 7, ADV 8, TDF 7, ETV 7, LAM + ADV 6 and LAM + TDF 5) and were considered as dropouts. Final analysis was done on 158 patients who completed and were compliant for one year of the drug administration [LAM (n = 28), ADV (n = 24), TDF (n = 26), ETV (n = 25), LAM + ADV (n = 28) and LAM + TDF (n = 27)].
The follow up data (at 6, 12 and 24 months) were evaluated through monitoring of patients at the outpatients clinics and in the ward for inpatients. Clinical, biochemical/serological tests and HBV DNA levels were evaluated for every six months (or earlier if any adverse symptoms) till 24 months of initiation of therapy (18 month data not given). Clinical evaluation was performed by monitoring MELD score using the UNOS formula i.e.: 9.6 ×loge (creatinine mg/dl) + 3.8 ×loge (bilirubin mg/dl) + 11.2× loge (INR) + 6.4 (aetiology: biliary or alcoholic 0; others 1 and INR is international normalized ratio) as per Mayo clinic calculator (https://optn.transplant.hrsa.gov/resources/allocation-calculators/meld-calculator/) as well as CTP score. Biochemical response (decrease in serum ALT to within normal range) and virological response (decrease in HBV DNA to achieve undetectable level by <1000 copies/ml during continued treatment) and/or loss of HBeAg were the primary endpoints for the cessation of therapy. Patients were monitored regularly for greater than six months after the end point was reached. Biochemical breakthrough (BBT) was defined as an increase in serum ALT above ULN after achieving normalization and virological breakthrough (VBT) as rise of HBV DNA by >1 log10 above nadir after achieving virological response during continued treatment. HBV polymerase gene was sequenced for the detection of mutations in patients with VBT or with viral load >105 copies/ml.
Quantitative polymerase chain reaction (qPCR) for HBV DNA detection was done as follows: HBV DNA was extracted from 200 µl serum using High-pure Viral Nucleic Acid Kit as instructed by manufacturers (Roche Diagnostics, Mannheim, Germany). Extracted DNA was stored at −20°C till the assay was done.
The WHO International standards for HBV DNA used in the assay were procured from National Institute of Biological Standards and Control (NIBSC, Code 97/750, UK). The lowest and highest detection limits of this assay were 67 and 108 copies/ml, respectively. Serum HBV DNA levels were quantified using qPCR assay (Miniopticon™, Biorad, USA). This diagnostic PCR was carried out on 10µl of extracted template DNA using HBV Geno-Sense kit (Genome Diagnostics, New Delhi, India) by TaqMan probe analysis. The reaction conditions were as follows: initial denaturation: 95°C for 10 min, followed by 45 cycles of denaturation: 95°C for 15 sec, annealing: 55°C for 20 sec, and primer extension: 72°C for 15 sec. The PCR amplicon length was 50 bp.
The extracted DNA was subjected to polymerase gene amplification by nested PCR using the following pair of primers: forward primer pol1 (5’-CTT CCT GCT GGT GGC TCC AGT TC-3’ nt 53-75) and reverse primer pol2 (5’-CGT CAG CAA ACA CTT GGC-3’ nt 1175-1192). A total of 25µl reaction mixture was prepared using 2.5 mmol dNTPs, 10xDream Taq™ Buffer, 5U/µl Dream Taq™ DNA polymerase (Fermentas, USA) and 10 pmol forward and reverse primers. The PCR amplification protocol for the first round PCR was at 93°C for two minutes followed by 35 cycles of 93°C for 50 sec, 55°C for 50 sec and 72°C for one minute ending with the final extension of 72°C for five minutes (PCR amplicon length: 1100bp); 1µl of the first round amplified DNA was subjected to nested PCR (PCR amplicon length: 766 bp) using the same cycling profile in 25µl of reaction mixture containing 1 pmol of forward primer pol-3 (5’-CTC GTG GTG GAC TTC TCT C-3’ nt 253-272) and reverse primer pol-4 (5’-GCA AAG CCC AAA AGA CCC AC-3’nt 1000-1019) was prepared. All primers were self-designed and procured from Integrated DNA Technology, USA. Mutation in YMDD motif (M204) was analyzed with the help of restriction fragment length polymorphism of polymerase gene by restriction digestion with FokI (New England Biolabs, USA). The restriction enzyme FokI could digest wild type fragment but not the mutantone9.
Further mutations were confirmed by sequencing as follows: the nested PCR product was eluted from gel purified using PCR purification kit (Real Biotech, Taiwan). Cycle sequencing PCR was carried out using primer pol3 and pol4 individually (i.e., both forward and reverse). The protocol for cycle sequencing was as follows: initial denaturation at 96°C for one minute followed by 25 cycles of 96°C for 10 sec, 50°C for five sec and 60°C for four minute. Further, the cycle sequencing product was purified using 100 per cent ethanol and 3M sodium acetate. The dried pellet was dissolved in Hi-Di Formamide (Applied Biosystems, USA). Subsequently, the product was run on automated DNA sequencer as per manufactures’ instructions (3130 Genetic Analyzer, Applied Biosystems, USA)for further analysis.
Statistical analysis: Statistical testing was performed using SPSS version 16.0 for windows (SPSS Inc., Chicago, IL, USA). Two-sided Fisher's exact test and Chi-square test were performed for categorical data (for different drug regimens). Wilcoxon's signed rank test was used to evaluate non-parametric data (especially the change in ALT and HBV DNA load at different time intervals). Box plot graph was plotted for changes in the serum ALT level and HBV DNA using their median [interquartile range (IQR)] values. Parametric tests were also performed between CHB and patients with decompensation as well as between the two serostatus (HBeAg +ve and -ve). One-way ANOVA was used for multiple drug comparison using post hoc (Bonferroni) analysis for the comparative analysis of reduction in viral load between different drug regimens and to find the significant association as well as the potency of drug. Data were log transformed (for plotting box plot graph) wherever found necessary, and no deviation in normal distribution was found.
Results
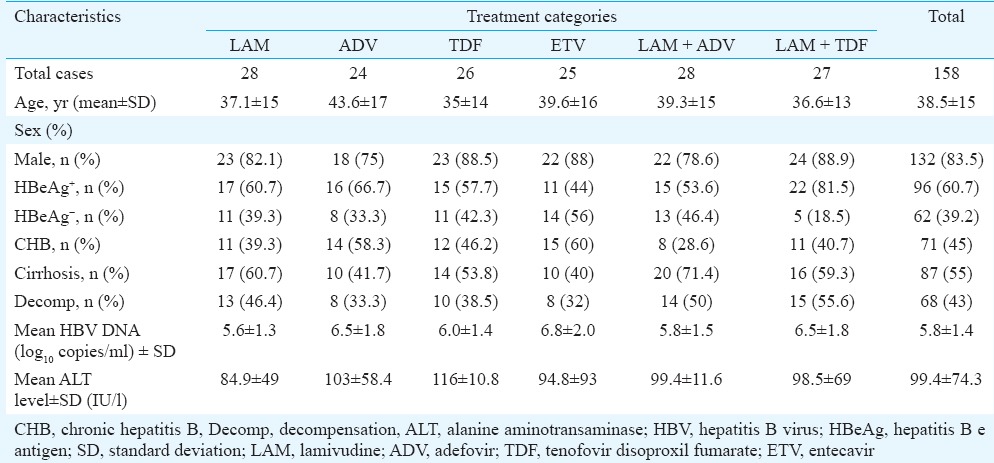
The mean age of 158 patients with HBV-related CLD included in this study was of 38.5 ± 14.9 yr, with male preponderance by more than four times. Of the 40 lost cases who were excluded from the study, 62.5 per cent (n = 25) were lost to follow up while the remaining 15 succumbed to disease. The cause of death was hepatic encephalopathy in eight (53.3%), variceal bleed in four (26.6%) and hepatorenal syndrome (HRS) in three (20%). CHB without any feature of decompensation was observed in 45 per cent and the remaining 55 per cent had cirrhosis, of whom 78 per cent were decompensated. HBeAg negativity was found in 39.2 per cent of our patients (Table I). The patients allocated for six different categories of therapy (LAM, ADV, TDF, ETV, LAM + ADV & LAM + TDF) had almost similar clinical, biochemical and virological characteristics (Table I). These patients were prospectively followed up for 24 months.
Table I.
Baseline (virological, biochemical and clinical) characteristics of patients with different antiviral therapies
Clinical response: Antiviral therapy resulted in clinical improvement in majority of patients (n = 113, 71.3%). Ascites was noted in 43 per cent of patients at baseline. The MELD and CTP scores were calculated. These scores were significantly improved with all arms of the therapies used except LAM (CTP: 9.6 vs. 8.9, P>0.01; MELD: 13.9 vs. 12.8, P<0.09) at 24 months. MELD (but not CTP) score significantly improved with ADV (13.9 vs. 11.0, P<0.04). The remaining therapeutic options (TDF, ETV, LAM + ADV and LAM + TDF) were found to significantly improve the CTP and MELD scores. Respective CTP and MELD were as follows: (TDF: 9.9 vs. 6.3; 13.9 vs. 7.8, P<0.05), (ETV: 9.1 vs. 6.2; 13.3 vs. 7.2, P<0.05), (LAM + ADV: 8.6 vs. 6.8; 13.4 vs. 10.9, P<0.05) and (LAM + TDF: 9.9 vs. 6.2; 16.2 vs. 8.3, P <0.05).
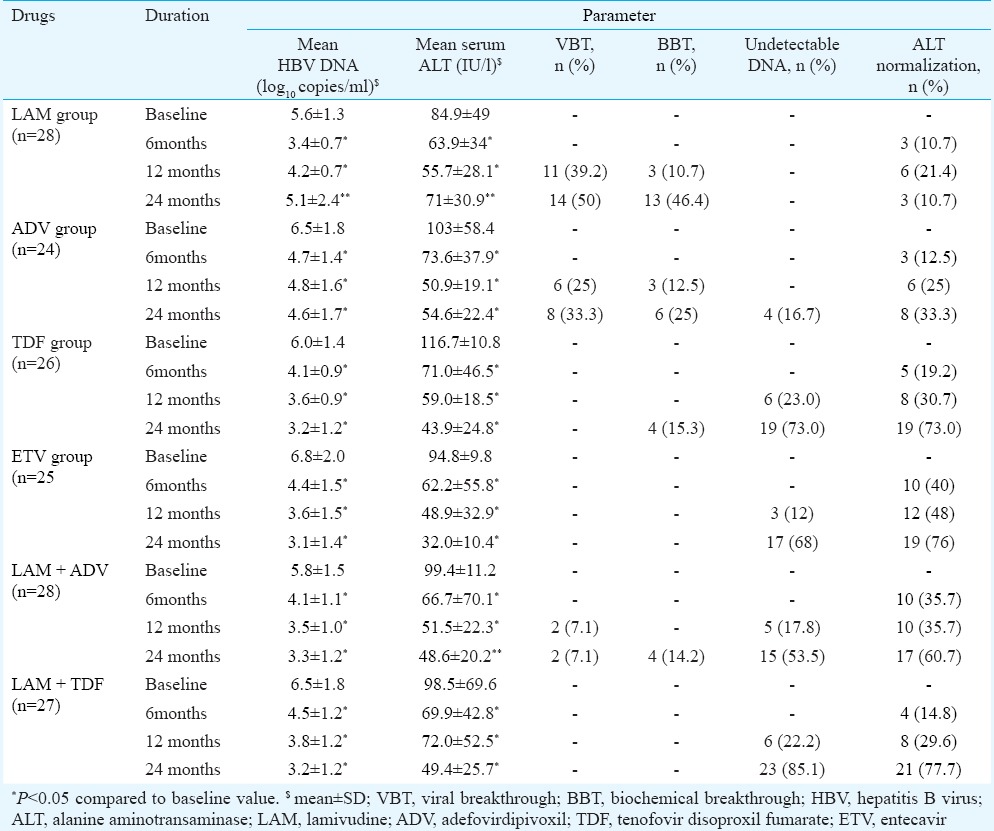
Biochemical response: Progressive improvement of ALT level was observed in all the therapeutic regimens with increasing duration of therapy except LAM and ADV regimens where rise in median ALT level was noted at 24 months and was associated with BBT. Median (IQR) changes in serum ALT level are shown in Fig. 1. ALT normalization achieved at 24 months was highest with LAM + TDF (n = 21; 78%), ETV (n = 19; 76%) and TDF (n = 19; 73%) (Table II). Patients with decompensated cirrhosis and CHB with compensated liver disease on different therapeutic regimens had similar reduction in ALT level by the end of second year. Noticeable ALT normalization was achieved at 12 and 24 months with TDF in 30.7 and 73 per cent, with ETV in 48 and 76 per cent, with LAM + ADV in 36 and 61 per cent and with LAM + TDF in 29.6 and 78 per cent patients (Table II). ALT normalization was more frequent in HBeAg-negative patients than HBeAg-positive patients with ADV (58 vs. 10.4%, P <0.05) and LAM + TDF (100 vs. 57.2%) therapies, while with remaining therapeutic regimens, ALT response did not differ in HBeAg-positive and HBeAg-negative groups.
Fig. 1.
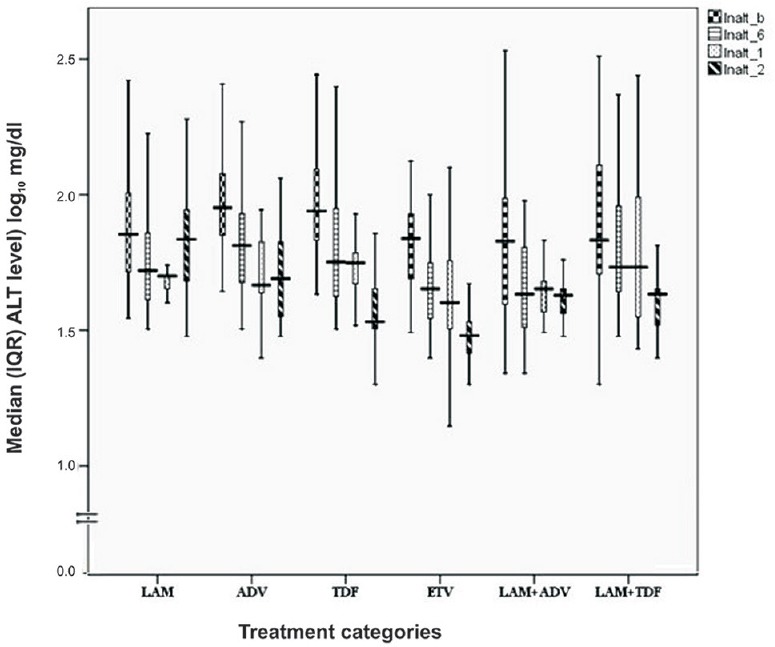
Median (interquartile range, IQR) alanine aminotransaminase (ALT) level (IU/l converted into log10) after antiviral therapy in due course of time. The boxes represent IQR (25th to 75th percentile). The horizontal lines within boxes represent the medians. The T-shaped lines that extend upwards from each box represent the highest value that is less than 1.5 times the 75th percentile plus 1.5 times interquartile range. The T-shaped lines that extend downwards from each box represent the lowest value that is greater than 1.5 times the 25th percentile minus 1.5 times interquartile range. (lnalt_b: Baseline, lnalt_6: 6months, lnalt_1: 12months, lnalt_2: 24 months).
Table II.
Virological and biochemical response during follow up treatment (i.e., 6, 12 and 24 months) within different antiviral therapies
Virological response: Median serum HBV DNA level was lower at 24 months (than at baseline) with all the therapeutic regimens. Reduction was progressive and >2 logs (2.2 to 3.8 logs) at 24 months with all the regimens except LAM (Table II and Fig. 2). Undetectable DNA was achieved in four (16.7%) in ADV, 19 (73 %) in TDF, 17 (68%) in ETV, 15 (53.5%) in LAM + ADV and 23 (85%) in LAM + TDF group patients. ADV resulted in higher HBV DNA reduction in patients with CHB than decompensated cirrhosis (3.6 vs. 1.3 log10; P<0.05); however, with other therapeutic regimens, the response was similar in CHB and decompensated cirrhosis patients. On the basis of HBeAg serostatus, a significant reduction in HBV DNA was observed in HBeAg-positive than HBeAg-negative patients with ADV (7.5 vs. 4.6 log10; P<0.02) and ETV regimen (8.5 vs. 3.8 log10; P <0.02). Other therapeutic regimens did not show a significant difference between the serostatus. HBeAg seroconversion at 24 months was seen in one-third of the patients with all the therapeutic regimens, except LAM (28%) and LAM+TDF (20%).
Fig. 2.
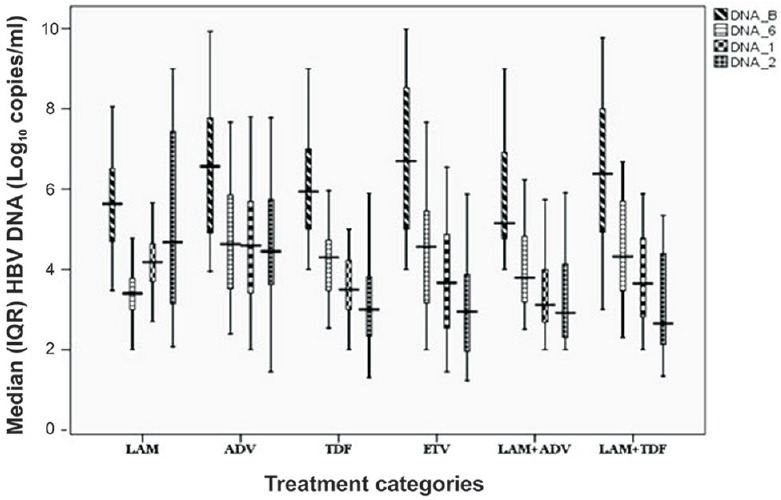
Median (interquartile range, IQR) hepatitis B virus DNA level (log10copies/ml) after antiviral therapy in due course of time. The boxes represent interquartile ranges (25th to 75th percentile). The horizontal lines within boxes represent the medians. The T-shaped lines that extend upwards from each box represent the highest value that is less than 1.5 times the 75th percentile plus 1.5 times interquartile range. The T-shaped lines that extend downwards from each box represent the lowest value that is greater than 1.5 times the 25th percentile minus 1.5 times interquartile range (DNA_B: Baseline, DNA_6: 6 months, DNA_1: 12 months, DNA_2: 24 months).
Multiple comparisons were done between different regimens to determine change in HBV DNA load at 24 months compared to the longitudinal data (base line, 6 and 12 months). The change observed was not significant with LAM as well as ADV monotherapy. The other monotherapies (TDF, ETV) or combination therapies (LAM + ADV, LAM + TDF) showed a significant (P<0.05) difference with LAM regimen. ADV revealed non-significant change in viral load when compared to LAM but borderline significance with LAM + ADV (P = 0.056), while the association was significant with TDF, ETV and LAM + TDF.
BBT was noted with LAM and ADV regimens in 10.7 and 12.5 per cent, and 46.4 and 25 per cent at 12 and 24 months, respectively. The BBT was non-significant between the two HBeAg serostatus (HBeAg +ve and −ve) (LAM: 53 vs. 46%, and ADV: 31 vs. 23%, respectively). Fewer patients were noted with rise in ALT among TDF (15%) and LAM + ADV groups (14%) by the end of second year, but on further analysis, these cases were not associated with VBT. None of the patients in ETV or LAM + TDF groups showed a rise in ALT level during therapy (Table II).
Virological breakthrough (VBT) in LAM and ADV therapies was observed in 39.2 and 25 per cent at 12 months while 50 per cent and 33.3 per cent at 24 months (Table II). VBT was higher in HBeAg-positive patients than in HBeAg-negative patients (LAM: 66.7 vs. 33%, P<0.05, ADV: 41 vs.26%, P>0.10). VBT was similar whether liver disease was compensated or decompensated i.e., LAM 51and 50 per cent and ADV 34 and 32 per cent by the end of second year.
Genotypic mutations were demonstrated at 24 months of therapy. Fifteen patients (53%) on LAM therapy revealed mutation [14(50%) with VBT and 1(3.5%) without any VBT] (Table II). Mutation in the YMDD, M204V (methionine to valine) in 39.3 per cent and along with L180M (leucine to methionine) in 10.7 per cent was detected in the conserved regions C and B of reverse transcriptase (rt) domain of HBV polymerase, respectively, after 24 months of LAM therapy.
VBT on ADV monotherapy was noted in eight patients (33.3%), rtA181T and rtN236T mutants were detected alone in 8.1 and 8.3 per cent in the conserved regions B and D of rt region of HBV polymerase gene, respectively. Other four (16.7%) patients showed VBT, but no genotypic mutations were observed after sequencing.
Combination therapy in LAM + ADV group also revealed VBT in 7 per cent (all HBeAg+ve) of patients, but no genotypic mutation was observed after sequencing. None of the patients on TDF and ETV monotherapy and LAM + TDF combination therapy showed any VBT or genotypic mutation during 24 months of therapy.
Discussion
Over the past two decades, treatment of CHB has greatly improved with the availability of NAs. The first-generation NAs, namely, LAM and ADV therapies though had fine initial response in clinical parameters both in CHB and decompensated cirrhosis patients, failed to obtain any benefit by the end of second year. Significant improvement was noted in the form of lower CTP and MELD scores at the end of study period with TDF, ETV and combination therapies (LAM + ADV, LAM + TDF).
LAM therapy has been shown to be associated with improvement in clinical parameters with improvement in CTP by 2-5 points during the median duration of 12-20 months9,10,11,12,13. However, if the LAM therapy was continued for 24 months or beyond, deterioration in decompensation has been noted in many studies, and lack of any improvement in CTP and MELD score was revealed14,15. Our findings were in concurrence with these observations. In earlier reports, ADV was found to improve CTP by 2 or 3 points and MELD by 3-6 points at 12 months, but this improvement lacked significance at 24 months6,7,16,17 as also seen in the present study. Therapies with newer molecules such as TDF and ETV showed improvement in CTP score by >2 points and MELD score by 4 points with no adverse events or noted decompensation along with lower rate of progression towards cirrhosis after 96 weeks of therapy18,19,20,21,22,23.
Combination therapies showed a significant decrease (of 2 points) in CTP score and (>3 points) in MELD score at 24 months along with resolved ascites24,25,26. In our study during the second year, marked elevation in median ALT level was observed due to the emergence of biochemical BT in about 2/3rd (with LAM) and 2/5th (with ADV) of patients. In contrast, newer drugs (TDF and ETV) and combination therapies showed progressive improvement with normalization of ALT by the end of second year in 2/3rd to 3/4th of the patients. Previous studies also reported improvement in ALT levels and its normalization in 2/5th to 3/4th of the patients on short term i.e., 12 months of LAM therapy10,11. When the therapy was continued beyond 12 months, biochemical BT started appearing and was noted in 2/3rd of the cases by the end of second year15,27.
ALT normalization has been reported in 50-80 per cent of the cases at the end of two years16,17,27 compared to 33 per cent in the present study. However, low normalization similar to the present study was also reported17. Similar ALT normalization has been noted with TDF and ETV regimens as found in the present study (70-80%) with minimal or no BT18,19,20,28. Combination therapies in the past have shown mean reduction in ALT level along with normalization of ALT in 45-56% cases at a median duration of 20 months and persisted further with no serious events25, which was in conformity to our results.
LAM and ADV therapies failed to produce undetectable DNA in the first 12 months in the present study. However, 16.7 per cent achieved DNA negativity after two years of ADV therapy, whereas newer drugs (TDF and ETV) were more effective in achieving DNA negativity. In contrast to our observation, earlier groups achieved relatively higher DNA negativity i.e., 16-44 per cent at the first year and >50 per cent at the second year10,11 with LAM; and with ADV 20-35 per cent and 46-55 per cent at the first and second years, respectively6,7,29. In concurrence, due to poor clinical and virological outcome, it was suggested that there was no benefit to continue LAM14,15. Compared to our findings, TDF and ETV had relatively higher (55-85%) viral DNA negativity at 12-15 months18,19,20,28, but at 24 months, it was comparable to our observation18,21,24.
Lower virological response in our study was not limited to only LAM and ADV but with other drugs also at the first year of therapy, reasons for which largely remain unclear. The factors for poor response such as presence of decompensation, HBeAg serostatus, higher ALT levels and high DNA loads were evaluated. The outcome in decompensated as well as compensated CHB cases was similar with different drugs. It was by and large similar in HBeAg-positive and HBeAg-negative cases and in patients with low or high ALT levels. Hence, these factors could not be ascribed to have any relation with low virological response in the present study.
Several investigators found no significant relationship of decompensation and serostatus as predictors of virological response and showed similar outcome in both patients with CHB and decompensation with LAM and ADV therapy10,16. However, in a few studies HBeAg-positive cases were found to be associated with poor DNA negativity14,17 with LAM and ADV therapy. Both newer agents (TDF and ETV) showed non-significant association of decompensation or serostatus with DNA negativity18,19. It is likely that these agents have very high efficacy; therefore, these predictors fail to modify their effects on viral suppression. Viral suppression with combination therapy showed no relationship with hepatic functional status25,26, but HBeAg negativity was associated with higher DNA suppression than HBeAg positivity at the second year25.
VBT was uncommon in the first year of therapy, and only a few cases with LAM and ADV experienced it, but by the end of the second year, it was quite frequent with these drugs which resulted in overall poor virological response as well as biochemical flares. VBT was noted with LAM + ADV therapy by the end of second year but was minimal. With other therapeutic regimens (TDF, ETV and LAM + TDF), VBT was almost absent. A few previous studies have shown similar results with LAM13,15,25 and ADV7,17,27,29.
TDF and ETV therapies in earlier studies were shown to be infrequently associated with VBT as noted by us while maintained high virological suppression with minimal occurrence of VBT19,20,23,26 at 24 months but these were not associated with any genotypic mutation, clinical or biochemical flares. Combination therapies showed impeccable response and long-lasting effects without any association to VBT whereas occurrence of VBT was higher than ours i.e., 15-43.6 per cent at two years of LAM + ADV therapy25.
Polymerase gene sequencing revealed genotypic substitutions in all the patients with VBT during LAM and ADV therapies. In all patients on LAM therapy, the site of mutation was methionine in the YMDD motif for valine i.e., M204V at the end of second year. In addition, there was another compensatory mutation i.e., L180M + M204V. M204V substitution was already reported earlier13,15,27. Of the total 16.4 per cent with ADV mutation, sites detected were adenine substitution to threonine i.e., A181V (8.1%) and asparagine substitution to threonine (N236T: 8.3%). These mutational sites were similar to the earlier reports in 34.9-50 per cent of the cases12,14,15,29 while 22-60 per cent with ADV among VBT cases17,18. Combination therapy (LAM + ADV) revealed VBT in seven per cent of cases, but no specific mutations were observed in our study. None of the patients on TDF and ETV therapy and LAM + TDF combination therapy showed any VBT or genotypic mutation during the 24 months of therapy. This showed that newer NAs were not associated with any mutation and this was corroborated by the absence of clinical and biochemical flares in patients with VBT. Similar results have been reported earlier18,19,21,23. A LAM + ADV combination study showed YMDD mutation in 27-35 per cent of the cases while ADV-related mutation (A181V) was shown only in 5-7 per cent of the cases. Similar to our findings, LAM + TDF did not reveal any genotypic resistance after two years of therapy and patients with decompensated cirrhosis revealed significant efficacy26.
In conclusion, LAM was found to be the least effective drug followed by ADV in patients with CHB. Addition of newer generation molecule (TDF) to LAM monotherapy provided better efficacy and delayed the onset of resistance and mutations. However, long-term therapy may be needed to evaluate potential benefits of first-line combination therapy for CHB.
Acknowledgment
The authors acknowledge the Department of Science & Technology, Government of India, New Delhi, for financial support (DST-PURSE grant Dev. Scheme number ‘4157’).
Footnotes
Conflicts of Interest: None.
References
- 1.Papatheodoridis G, Buti M, Cornberg M, Janssen H, Mutimer D, Pol S, et al. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–85. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Hadziyannis SJ, Vassilopoulos D. Hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2001;34:617–24. doi: 10.1053/jhep.2001.27834. [DOI] [PubMed] [Google Scholar]
- 3.Bartholomeusz A, Locarnini S. Hepatitis B virus mutations associated with antiviral therapy. J Med Virol. 2006;78(Suppl 1):S52–5. doi: 10.1002/jmv.20608. [DOI] [PubMed] [Google Scholar]
- 4.Zoulim F. Mechanism of viral persistence and resistance to nucleoside and nucleotide analogs in chronic hepatitis B virus infection. Antiviral Res. 2004;64:1–15. doi: 10.1016/j.antiviral.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology. 2009;49:1503–14. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 6.Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology. 2007;45:307–13. doi: 10.1002/hep.21534. [DOI] [PubMed] [Google Scholar]
- 7.Lampertico P, Viganò M, Manenti E, Iavarone M, Lunghi G, Colombo M. Adefovir rapidly suppresses hepatitis B in HBeAg-negative patients developing genotypic resistance to lamivudine. Hepatology. 2005;42:1414–9. doi: 10.1002/hep.20939. [DOI] [PubMed] [Google Scholar]
- 8.Yim HJ, Hussain M, Liu Y, Wong SN, Fung SK, Lok AS. Evolution of multi-drug resistant hepatitis B virus during sequential therapy. Hepatology. 2006;44:703–12. doi: 10.1002/hep.21290. [DOI] [PubMed] [Google Scholar]
- 9.Chayama K, Suzuki Y, Kobayashi M, Kobayashi M, Tsubota A, Hashimoto M, et al. Emergence and takeover of YMDD motif mutant hepatitis B virus during long-term lamivudine therapy and re-takeover by wild type after cessation of therapy. Hepatology. 1998;27:1711–6. doi: 10.1002/hep.510270634. [DOI] [PubMed] [Google Scholar]
- 10.Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61–8. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 11.Dienstag JL, Schiff ER, Wright TL, Perrillo RP, Hann HW, Goodman Z, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341:1256–63. doi: 10.1056/NEJM199910213411702. [DOI] [PubMed] [Google Scholar]
- 12.Kapoor D, Guptan RC, Wakil SM, Kazim SN, Kaul R, Agarwal SR, et al. Beneficial effects of lamivudine in hepatitis B virus-related decompensated cirrhosis. J Hepatol. 2000;33:308–12. doi: 10.1016/s0168-8278(00)80372-4. [DOI] [PubMed] [Google Scholar]
- 13.Xu H, Chen Y, He LL, Lei BJ, Lei XZ. Lamivudine-resistant analysis and management for chronic hepatitis B patients with initial lamivudine therapy. Zhonghua Gan Zang Bing ZaZhi. 2011;19:427–30. [PubMed] [Google Scholar]
- 14.Yuen MF, Seto WK, Chow DH, Tsui K, Wong DK, Ngai VW, et al. Long-term lamivudine therapy reduces the risk of long-term complications of chronic hepatitis B infection even in patients without advanced disease. Antivir Ther. 2007;12:1295–303. [PubMed] [Google Scholar]
- 15.Liaw YF, Chien RN, Yeh CT. No benefit to continue lamivudine therapy after emergence of YMDD mutations. Antivir Ther. 2004;9:257–62. [PubMed] [Google Scholar]
- 16.Manolakopoulos S, Bethanis S, Koutsounas S, Goulis J, Vlachogiannakos J, Christias E, et al. Long-term therapy with adefovir dipivoxil in hepatitis B e antigen-negative patients developing resistance to lamivudine. Aliment Pharmacol Ther. 2008;27:266–73. doi: 10.1111/j.1365-2036.2007.03567.x. [DOI] [PubMed] [Google Scholar]
- 17.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348:800–7. doi: 10.1056/NEJMoa021812. [DOI] [PubMed] [Google Scholar]
- 18.Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442–55. doi: 10.1056/NEJMoa0802878. [DOI] [PubMed] [Google Scholar]
- 19.Woo G, Tomlinson G, Nishikawa Y, Kowgier M, Sherman M, Wong DK, et al. Tenofovir and entecavir are the most effective antiviral agents for chronic hepatitis B: a systematic review and Bayesian meta-analyses. Gastroenterology. 2010;139:1218–29. doi: 10.1053/j.gastro.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 20.Liaw YF, Sheen IS, Lee CM, Akarca US, Papatheodoridis GV, Suet-Hing Wong F, et al. Tenofovir disoproxil fumarate (TDF), emtricitabine/TDF, and entecavir in patients with decompensated chronic hepatitis B liver disease. Hepatology. 2011;53:62–72. doi: 10.1002/hep.23952. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava M, Rungta S, Dixit VK, Shukla SK, Singh TB, Jain AK. Predictors of survival in hepatitis B virus related decompensated cirrhosis on tenofovir therapy: an Indian perspective. Antiviral Res. 2013;100:300–5. doi: 10.1016/j.antiviral.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–10. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 23.Schiff ER, Lee SS, Chao YC, Kew Yoon S, Bessone F, Wu SS, et al. Long-term treatment with entecavir induces reversal of advanced fibrosis or cirrhosis in patients with chronic hepatitis B. Clin Gastroenterol Hepatol. 2011;9:274–6. doi: 10.1016/j.cgh.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 24.Ghany MG, Feld JJ, Zhao X, Heller T, Doo E, Rotman Y, et al. Randomised clinical trial: the benefit of combination therapy with adefovir and lamivudine for chronic hepatitis B. Aliment Pharmacol Ther. 2012;26:1027–35. doi: 10.1111/j.1365-2036.2012.05059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sung JJ, Lai JY, Zeuzem S, Chow WC, Heathcote EJ, Perrillo RP, et al. Lamivudine compared with lamivudine and adefovir dipivoxil for the treatment of HBeAg-positive chronic hepatitis B. J Hepatol. 2008;48:728–35. doi: 10.1016/j.jhep.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 26.Schmutz G, Nelson M, Lutz T, Sheldon J, Bruno R, von Boemmel F, et al. Combination of tenofovir and lamivudine versus tenofovir after lamivudine failure for therapy of hepatitis B in HIV-coinfection. AIDS. 2006;20:1951–4. doi: 10.1097/01.aids.0000247116.89455.5d. [DOI] [PubMed] [Google Scholar]
- 27.Pradeep Kumar S, Medhi S, Asim M, Das BC, Gondal R, Kar P. Evaluation of adefovir & lamivudine in chronic hepatitis B: correlation with HBV viral kinetic, hepatic-necro inflammation & fibrosis. Indian J Med Res. 2011;133:50–6. [PMC free article] [PubMed] [Google Scholar]
- 28.Ismail AM, Sharma OP, Kumar MS, Eapen CE, Kannangai R, Abraham P. Virological response and antiviral resistance mutations in chronic hepatitis B subjects experiencing entecavir therapy: an Indian subcontinent perspective. Antiviral Res. 2013;98:209–16. doi: 10.1016/j.antiviral.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Ismail AM, Ramachandran J, Kannangai R, Abraham P. Antiviral efficacy of adefovir dipivoxil in the treatment of chronic hepatitis B subjects from Indian subcontinent. Indian J Med Microbiol. 2014;32:60–3. doi: 10.4103/0255-0857.124312. [DOI] [PubMed] [Google Scholar]


