Abstract
Background & objectives:
Sepsis due to multidrug-resistant Gram-negative pathogens is a challenge for clinicians and microbiologists and has led to use of parenteral colistin. There is a paucity of data regarding safety and efficacy of intravenous colistin use in neonates. The objective of this retrospective analysis was to study the efficacy and safety of intravenous colistin in the treatment of neonatal sepsis.
Methods:
An audit of the data from neonates, admitted to a neonatal intensive care unit of a tertiary care hospital during January 2012 to December 2012, and who received intravenous colistin was carried out.
Results:
Sixty two neonates received intravenous colistin (52 preterm and 10 term) for the treatment of pneumonia, bloodstream infections and meningitis. The isolated pathogens in decreasing order of frequency were Acinetobacter baumannii, Klebsiella pneumonia and Pseudomonas aeruginosa. Of the total 62 neonates, 41 (66.12%) survived and 21 (33.87%) died. Significantly higher mortality was observed in neonates with lower body weights (P < 0.05). A significant association of mortality was found in those with sepsis due to Klebsiella species. Only one of seven with this infection survived as against 15 of the 23 who grew other organisms [P = 0.03; crude odds ratio = 11.25 (1.2, 110.5)]. None of the neonates developed neurotoxicity or nephrotoxicity.
Interpretation & conclusions:
This retrospective study in neonates with sepsis showed that intravenous colistin was safe and effective in the treatment of neonatal sepsis. Further, well–controlled, prospective clinical trials need to be done to corroborate these findings.
Keywords: Antibiotic, colistin, Gram-negative bacterial infection, neonatal intensive care, neonates
The worldwide incidence of neonatal sepsis has been described to range from 3.5 to 38 per 1000 live births1,2, with India reporting an incidence of approximately three per cent3. Sepsis in neonates is associated with a high mortality ranging from 19 to 38 per cent in the country4. The most common microorganisms associated with neonatal sepsis are Klebsiella pneumonia and Staphylococcus aureus4,5,6. Further, due to an increasing incidence of multidrug-resistant (MDR) Gram-negative infection, the availability of effective antimicrobials has become limited7.
A decline in the discovery and development of newer antibiotics creates a real daunting challenge for the clinicians and microbiologists to treat these MDR Gram-negative pathogens, especially Acinetobacter, Pseudomonas and Klebsiella species. There has also been a resurgence of interest in an old antibiotic, colistin8,9,10. Although effective, colistin never became popular because of the associated risks of nephrotoxicity and neurotoxicity11. However, some reports have belied these fears suggesting that the drug may be safer than earlier perceived and effective in MDR Gram-negative infections12,13,14 leading to its clinical use in adults, children and neonates. The data related to safety and efficacy of intravenous colistin in neonatal sepsis are limited. We therefore conducted this retrospective analysis to study the efficacy and safety of colistin use in the treatment of sepsis in critically sick term and preterm neonates.
Material & Methods
This was a retrospective analysis of data of neonates admitted in the neonatal intensive care unit (NICU) of KEM Hospital, Mumbai, India, between January and December 2012, with a diagnosis of sepsis who had been treated with intravenous colistin. The objective was to primarily assess outcomes (death/survival) and safety of the drug in these neonates. The Institutional Ethics Committee approved the protocol and a waiver for written informed consent was granted.
Culture negative/clinical sepsis4 was defined as the presence of any one of the following criteria:
-
(i)
Existence of predisposing factors - maternal fever or foul smelling liquor or prolonged rupture of membranes (>24 h) or gastric polymorphs (>5 per high power field).
-
(ii)
Positive sepsis screen two of the four parameters (namely, TLC <5000/µl, band to total polymorph ratio of >0.2, absolute neutrophil count <1800/µl and C-reactive protein >1 mg/dl).
-
(iii)
Radiological evidence of pneumonia.
Culture-positive sepsis4 was defined as a neonate having clinical picture suggestive of septicaemia, pneumonia or meningitis along with isolation of pathogens from blood or biological fluids. Clinical response was defined as resolving presenting signs and symptoms, tolerating enteral feeds and starting to gain weight. Failure of treatment was defined as persistence of signs and symptoms for 48-72 h or worsening or development of new infection or radiologic deterioration.
Renal impairment13 or nephrotoxicity was defined as an increase of more than 0.5 mg/dl in the serum creatinine value above baseline. Neurotoxicity14 was defined as onset of seizures, change in level of consciousness any time during the course of drug administration.
All neonates with clinical or culture-proven sepsis and treated continuously with intravenous colistin for more than 48 h or a minimum of 6 doses, were identified from the NICU data record sheets during the period of January to December 2012. The details of information collected from each neonate are given in Table I. In addition, information on predisposing factors such as maternal fever, foul smelling liquor, prolonged rupture of membranes (>24 h), urinary tract infection and preterm premature rupture of membranes was also collected.
Table I.
Information obtained from each neonate

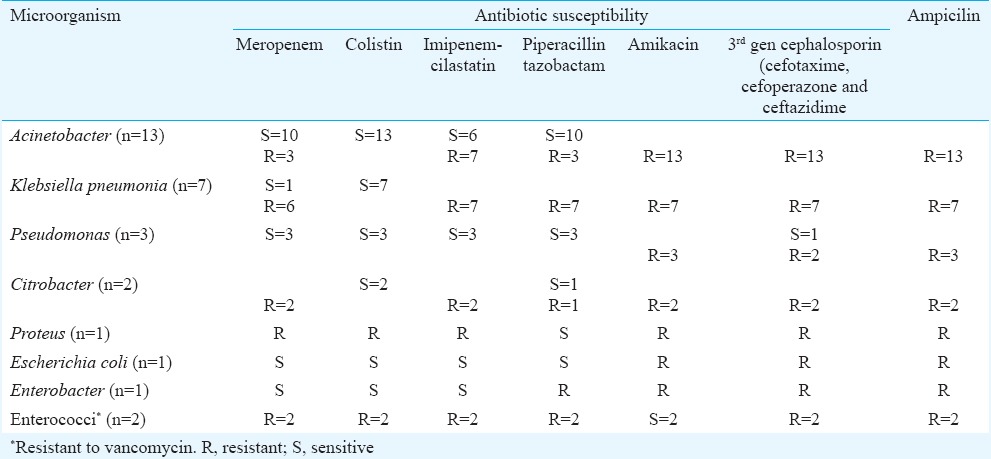
Microbiologic methods: All the clinical samples were inoculated on routine culture media, and identification of the isolated organism was performed by biochemical tests and Vitek-2 compact system (Biomerieux system, France). Antimicrobial susceptibility to colistin was tested with the validated disk diffusion method following Clinical Laboratory Standards Institute guidelines15.
Prevailing antibiotic policy: Neonates with evidence of clinical sepsis were started on a combination of intravenous ampicillin-sulbactam and amikacin. Second-line antibiotics were intravenous piperacillin-tazobactam and amikacin. Third-line antibiotics included carbapenems, colistin depending on the culture report and clinical condition of the neonate. Discontinuation of antibiotics was done on documentation of a negative culture report and/or normal sepsis screen with abatement of symptoms.
Route of colistin administration and dosage: All the neonates were administered colistin intravenously in 5 ml of normal saline over a period of 30 min at the dose of 75,000 IU/kg/day administered in three divided doses as per product label. Colistin formulation consisted of 1 million international units per vial.
The renal function tests (blood urea nitrogen, serum creatinine and 24 hourly urine output) were performed at baseline before initiation of intravenous colistin. Thereafter, the parameters were repeated 24 hourly if the neonate had manifestations of acute kidney injury and continued till normalization of abnormal results. Thereafter tests were repeated weekly till completion of therapy. A complete detailed neurological examination and neuroimaging were done at the time of neurologic deterioration, completion of antibiotic therapy and at discharge to ascertain evidence of neurotoxicity.
Outcomes included survival of neonates with sepsis and occurrence of adverse events during colistin therapy.
Statistical analysis: Chi-square test for association or Fisher's exact probability test was used for analysis of categorical data and a crude odds ratio with 95 per cent confidence interval (95% CI) was calculated. Mann–Whitney U-test was applied to find out significance in the duration of colistin administration between the categories. GraphPad Instat 3.0 version (GraphPad Instat version 3.0 for Windows, GraphPad Software, San Diego, California USA, www.graphpad.com) was used for the statistical analysis.
Results
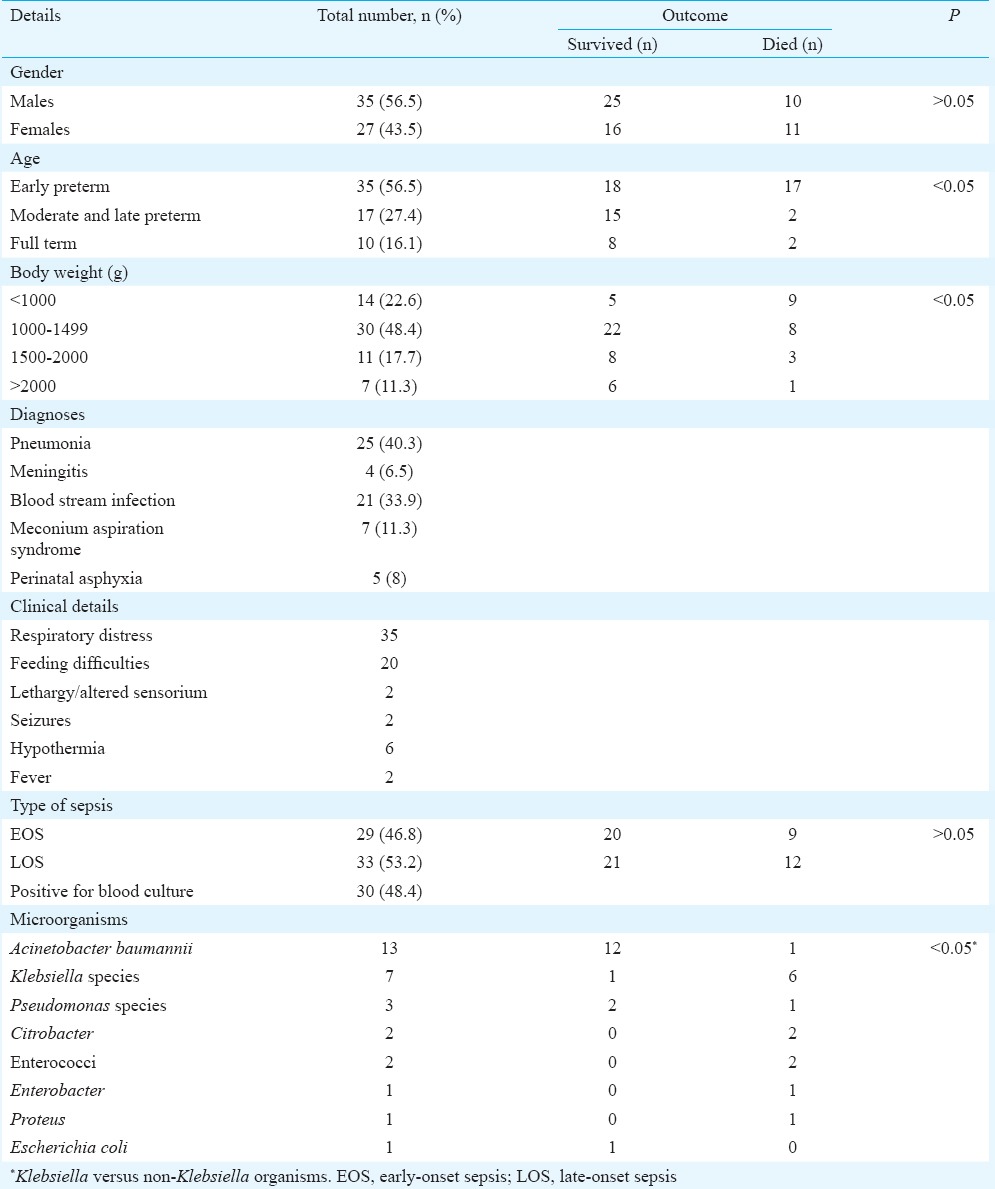
A total of 1794 neonates were admitted during the study period, of whom 269 (15%) were diagnosed to have neonatal sepsis. Sixty two (23%) of these neonates received intravenous colistin and formed the study group. The median body weight was 1207.5 g (range 680-3210 g). A summary of other demographic details is mentioned in Table II.
Table II.
Summary of demographic details of neonates included in the study (n=62)
Colistin administration and concomitant antimicrobials: All neonates were administered colistin intravenously in 5 ml of normal saline over a period of 30 min at the dose of 75,000 IU/kg/day administered in three divided doses as per product labelling16. Median (range) of time taken to initiate colistin was 2 (1-4) days. The median (range) of duration (in days) of colistin was 10 (4-21). All neonates received antimicrobials [meropenem (41), piperacillin-tazobactam (16) and imipenem-cilastatin (5)] either concomitantly or preceding colistin. In all, 34 neonates received concomitant antimicrobials, of whom nine (26.5%) died while 12 of the 28 (42.9%) who received antimicrobials before colistin was initiated, died.
Outcomes: Of the total 62 neonates, 41 (66.1%) survived. All deaths were attributed to severe sepsis with multiorgan dysfunction syndrome. The analysis of various variables with the outcome is given below.
Body weight and gestational age of the neonates: A greater mortality was observed in neonates with lower body weights (P<0.05). There was significant association (P<0.05) between gestational age and mortality (Table II).
Onset of sepsis: Nine of the 29 (31%) neonates with early-onset sepsis (EOS) and 12 of the 33 (36.4%) neonates with late-onset sepsis died (Table II).
Time of initiation and duration of colistin: Colistin was initiated on day 2 (1-4) [median (range)] in all the neonates irrespective of outcome. The median (range) duration (in days) of colistin was 10 (4-21). Among those who died the median (range) duration of colistin was 7 (4-15) days and it was 10 (10-21) days among the survivors (P<0.05).
Microorganisms: Of the total 30 neonates whose culture showed growth, majority of them grew Acinetobacter species (n = 13). Table III enlists the antimicrobial sensitivity of the isolated organisms. When the neonates were classified according to the infection they had, a significant association of mortality was found in those with sepsis due to Klebsiella species. Only one of seven with this infection survived as against 15 of the 23 who grew other organisms (Table I) [P = 0.03; crude odds ratio = 11.25 (1.2, 110.5)].
Table III.
Antibiogram of the isolated microorganisms
Nephrotoxicity and neurotoxicity: None of the neonates developed either neurotoxicity or nephrotoxicity. Blood urea nitrogen and serum creatinine were normal in all patients before starting, during and on completion of colistin therapy.
Maternal risk factors: A total of 10 neonates (four in the early preterm and two each in the mid, late preterm and term) were found to have one of the maternal high-risk factors. Of these, two of the early preterm neonates died and the rest survived. Only three neonates of these showed a growth of microorganisms (two had grown Acinetobacter species and one Enterobacter).
Discussion
The present study was a retrospective audit of 62 neonates with sepsis who received intravenous colistin along with other antimicrobial therapy; 66.12 per cent of the neonates survived with a significant mortality due to Klebsiella species and no drug-related renal or neurotoxicity was found.
The overall efficacy of intravenous colistin was found to be similar to that of reported studies in neonates13, children14,16,17 and adults18. The only other study from India on the use of colistin in neonates reported an efficacy of 72 per cent13. This study had a smaller sample of 18 patients and a larger number of full-term neonates which could be the reason for the difference. One of the factors that influences the outcome is the dose of colistin used18. In the Indian study13 slightly lower doses (50 to 69,500 IU/kg/day) were used. A previous report in Caucasians used colistin in infants up to 200,000 IU/kg/day for a period of almost three months although there was only one neonate in the study who received the drug at a dose of 170,000 IU/kg/day but died a day following drug administration making comparisons difficult14. Although there were no pharmacokinetic studies done with colistin in neonates, a study from adults reported similar pharmacokinetic behaviour of colistin between Asians and Caucasians18.
An increased risk of mortality was observed among those infected with Klebsiella species as compared to other organisms regardless of gestational age/birth weight. All those neonates who died were on concomitant carbapenems. It has been suggested that K. pneumoniae has started developing resistance to colistin and carbapenems19,20. An in vitro study21 has concluded that the addition of doripenem with colistin is synergistic and suppresses those Klebsiella organisms that were resistant to colistin. A study conducted using a murine model for K. pneumoniae showed more rapid resolution with enhanced antibacterial activity of doripenem as compared to other carbapenems22. These studies including the present study warrant continuous surveillance of infection with Klebsiella organisms including the use of combination therapy with colistin. In our study, 31 per cent neonates with EOS died because of Gram-negative sepsis similar to a study from Eastern part of India that had revealed that around 27 per cent of neonates with EOS died mainly because of MDR Gram-negative bacilli infections23.
None of the 62 neonates showed any renal impairment or clinically evident renal dysfunction in the present study. The earlier study13 in neonates had two deaths attributable to nephrotoxicity although these were confounded with concomitant netilmicin therapy and multiorgan dysfunction syndrome. There was one death due to nephrotoxicity in the Caucasian study14 which was confounded with concomitant therapy with gentamicin. It thus appears from these three audits that the earlier fears of drug toxicity that lead to colistin to disrepute are unfounded. Limitations of this study included lack of follow up culture reports to confirm bacteriological outcomes, its retrospective design and lack of a control group, the concomitant use of other antibiotics with colistin, and a multivariate analysis of variables affecting the outcome could not be performed due to a small sample size.
In conclusion, intravenous colistin appears to be safe and efficacious in critically sick preterm and term neonates with sepsis. Additional prospective studies are needed to explore its pharmacokinetics and pharmacodynamics as well as safety and efficacy in neonatal sepsis.
Acknowledgment
Authors acknowledge Dr Sandhya Kamath, Dean, Seth GS Medical College and KEM Hospital, for giving permission to publish the study.
Footnotes
Conflicts of Interest: None.
References
- 1.Klein JO. Bacteriology of neonatal sepsis. Pediatr Infect Dis J. 1990;9:778. [PubMed] [Google Scholar]
- 2.Vergnano S, Sharland M, Kazembe P, Mwansambo C, Heath PT. Neonatal sepsis: an international perspective. Arch Dis Child Fetal Neonatal Ed. 2005;90:F220–4. doi: 10.1136/adc.2002.022863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tripathi S, Malik GK. Neonatal sepsis: past, present and future: a review article. Internet J Med Update. 2010;5:45–54. [Google Scholar]
- 4.NNPD Network. National Neonatal-Perinatal Database Report 2002-2003. [accessed on July 5, 2013]. Available from: http://www.newbornwhocc.org/pdf/nnpd_report_2002-03.PDF .
- 5.Sundaram V, Kumar P, Dutta S, Mukhopadhyay K, Ray P, Gautam V, et al. Blood culture confirmed bacterial sepsis in neonates in a North Indian tertiary care center: changes over the last decade. Jpn J Infect Dis. 2009;62:46–50. [PubMed] [Google Scholar]
- 6.Hannan A, Qamar MU, Usman M, Ahmad K, Waheed I, Rauf K. Multidrug resistant microorganisms causing neonatal septicemia: in a tertiary care hospital Lahore, Pakistan. Afr J Microbiol Res. 2013;7:1896–902. [Google Scholar]
- 7.Engel LS. Multidrug-resistant Gram-negative bacteria: trends, risk factors, and treatment. Emerg Med. 2009;41:18–27. [Google Scholar]
- 8.Li J, Nation RL, Milne RW, Turnidge JD, Coulthard K. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int J Antimicrob Agents. 2005;25:11–25. doi: 10.1016/j.ijantimicag.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Livermore DM. The need for new antibiotics. Clin Microbiol Infect. 2004;10(Suppl 4):1–9. doi: 10.1111/j.1465-0691.2004.1004.x. [DOI] [PubMed] [Google Scholar]
- 10.Landman D, Georgescu C, Martin DA, Quale J. Polymyxins revisited. Clin Microbiol Rev. 2008;21:449–65. doi: 10.1128/CMR.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durakovic N, Radojcic V, Boban A, Mrsic M, Sertic D, Serventi-Seiwerth R, et al. Efficacy and safety of colistin in the treatment of infections caused by multi-drug resistant Pseudomonas aeruginosa in patients with hematologic malignancy: a matched pair analysis. Intern Med. 2011;50:1009–13. doi: 10.2169/internalmedicine.50.4270. [DOI] [PubMed] [Google Scholar]
- 12.Arnold TM, Forrest GN, Messmer KJ. Polymyxin antibiotics for Gram-negative infections. Am J Health Syst Pharm. 2007;64:819–26. doi: 10.2146/ajhp060473. [DOI] [PubMed] [Google Scholar]
- 13.Jajoo M, Kumar V, Jain M, Kumari S, Manchanda V. Intravenous colistin administration in neonates. Pediatr Infect Dis J. 2011;30:218–21. doi: 10.1097/INF.0b013e3182064bfe. [DOI] [PubMed] [Google Scholar]
- 14.Iosifidis E, Antachopoulos C, Ioannidou M, Mitroudi M, Sdougka M, Drossou-Agakidou V, et al. Colistin administration to pediatric and neonatal patients. Eur J Pediatr. 2010;169:867–74. doi: 10.1007/s00431-009-1137-3. [DOI] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 22nd informational supplement M100-S22. Wayne, PA, USA: CLSI; 2012. [Google Scholar]
- 16.Colomycin Injection – Summary of Product Characteristics. [accessed on October 21, 2013]. Available from: http://www.medicines.org.uk/emc/medicine/1590/SPC/Colomycin+Injection/
- 17.Falagas ME, Vouloumanou EK, Rafailidis PI. Systemic colistin use in children without cystic fibrosis: a systematic review of the literature. Int J Antimicrob Agents. 2009;33:503.e1–503.e13. doi: 10.1016/j.ijantimicag.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 18.Karnik ND, Sridharan K, Jadhav SP, Kadam PP, Naidu RK, Namjoshi RD, et al. Pharmacokinetics of colistin in critically ill patients with multidrug-resistant Gram-negative bacilli infection. Eur J Clin Pharmacol. 2013;69:1429–36. doi: 10.1007/s00228-013-1493-9. [DOI] [PubMed] [Google Scholar]
- 19.Mammina C, Bonura C, Di Bernardo F, Aleo A, Fasciana T, Sodano C, et al. Ongoing spread of colistin-resistant Klebsiella pneumoniae in different wards of an acute general hospital, Italy, June to December 2011. Euro Surveill. 2012;17 pii:20248. [PubMed] [Google Scholar]
- 20.Marchaim D, Chopra T, Pogue JM, Perez F, Hujer AM, Rudin S, et al. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob Agents Chemother. 2011;55:593–9. doi: 10.1128/AAC.01020-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deris ZZ, Yu HH, Davis K, Soon RL, Jacob J, Ku CK, et al. The combination of colistin and doripenem is synergistic against Klebsiella pneumoniae at multiple inocula and suppresses colistin resistance in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother. 2012;56:5103–12. doi: 10.1128/AAC.01064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hilliard JJ, Melton JL, Hall L, Abbanat D, Fernandez J, Ward CK, et al. Comparative effects of carbapenems on bacterial load and host immune response in a Klebsiella pneumoniae murine pneumonia model. Antimicrob Agents Chemother. 2011;55:836–44. doi: 10.1128/AAC.00670-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viswanathan R, Singh AK, Basu S, Chatterjee S, Sardar S, Isaacs D. Multi-drug resistant Gram negative bacilli causing early neonatal sepsis in India. Arch Dis Child Fetal Neonatal Ed. 2012;97:F182–7. doi: 10.1136/archdischild-2011-300097. [DOI] [PubMed] [Google Scholar]


