Abstract
Background & objectives:
Ventilator-associated pneumonia (VAP) is an important hospital-acquired infection with substantial mortality. Only a few studies are available from India addressing the microbiological aspects of VAP, which have been done with small study populations. This study was carried out in the intensive care units (ICUs) of a tertiary care hospital to assess the profile of pathogens and to determine the pattern of antimicrobial resistance.
Methods:
This was a retrospective study of clinically suspected cases of VAP. Over a three year period, a total of 247 cases in 2011, 297 in 2012 and 303 in 2013 admitted in ICUs on mechanical ventilation with clinical evidence of VAP were included in our study. The endotracheal aspirate samples from these suspected cases were subjected to quantitative culture technique, and colony count of ≥105 colony forming units/ml was considered significant. Antimicrobial susceptibility test for the isolates was done.
Results:
VAP rates of 44.1, 43.8 and 26.3 were seen in 2011, 2012 and 2013, respectively. In all the three years, non-fermentative Gram-negative bacilli were the predominant organisms, followed by Pseudomonas spp. and Klebsiella spp. Staphylococcus aureus exhibited a downwards trend in prevalence from 50.0 per cent in 2011 to 34.9 per cent in 2013. An increase in vancomycin-resistant enterococci was seen from 4.3 per cent in 2012 to 8.3 per cent in 2013, while methicillin resistance amongst the S. aureus crossed the 50 per cent mark in 2013. An increasing trend in resistance was shown by Pseudomonas spp. for piperacillin-tazobactam (PTZ), amikacin and imipenem (IPM). For the non-fermenters, resistance frequency remained very high except for IPM (33.1%) and polymyxin-B (2.4%).
Interpretation & conclusions:
Our findings show VAP as an important problem in the ICU setting. The incidence of multidrug-resistant pathogens was on the rise. The resistance pattern of these pathogens can help an institution to formulate effective antimicrobial policy. To have a comprehensive pan-India picture, multicentric studies are needed.
Keywords: Antimicrobial resistance, multidrug resistance, pathogens, ventilator-associated pneumonia
Ventilator-associated pneumonia (VAP) is considered the second most common hospital-acquired infection associated with higher mortality and morbidity1, and it develops when a patient is on mechanical ventilation for ≥48 h2. It has been found that 10-20 per cent of patients requiring mechanical ventilation develop associated pneumonia with a high mortality3. The VAP rate is measured as episodes per 1000 ventilator days and it varies widely in different regions, ranging from 4.4 cases/1000 ventilator days in the USA4 to as high as 13-51/100 ventilator days in other parts of the world5. While the incidence is low in developed countries of the world, it continues to be unacceptably high in less-developed nations6. Thus, in a study from India, the incidence was found to be 30.7 and 15.8 per 1000 days in two different ICUs7, while another report found it to be 53.2 per 1000 days8.
The aetiologic agents of VAP include some of the common hospital pathogens such as Pseudomonas spp., Acinetobacter and other non-fermenters, members of the Enterobacteriaceae family, as well as Gram-positive pathogens such as staphylococci, and the fungal agent Candida9,10. The emergence of multidrug-resistant strains associated with VAP is linked to the excessive use of broad-spectrum antibiotics early in the intensive care settings in the economically developing countries6.
Therefore, this study was undertaken to find the spectrum of pathogens and their antimicrobial resistance patterns over a period of three years in the intensive care units (ICUs) of a tertiary care hospital, and to determine the trends of infection.
Material & Methods
This was a retrospective, cross-sectional, descriptive study using data from laboratory records for the three consecutive years 2011, 2012 and 2013. The study was conducted at Sri Venkateswara Institute of Medical Sciences, Tirupati, India, which is a tertiary care referral hospital having a dedicated Respiratory Intensive Care Unit (ICU) and Medical ICU, apart from other ICUs for various medical and surgical subspecialities.
Only those patients who were on mechanical ventilation for more than 48 h and clinically suspected of having pneumonia were included. The total number of patients for the three years were 1159 in 2011, 903 in 2012 and 1022 in 2013, of whom 247, 297 and 303 had clinical and microbiological evidence of VAP, respectively, as per the modified Clinical Pulmonary Infection Score (CPIS)11 of more than six.
The study protocol was approved by the institutional ethics committee.
Sample collection and microbiological analysis: The endotracheal aspirate samples were subjected to quantitative culture technique. A colony count of ≥105 colony forming units (cfu)/ml was considered significant12. Any growth below this was considered as colonization or contamination. Identification of the isolates was done by standard biochemical tests13, and antimicrobial susceptibility test was performed and interpreted as per the Clinical and Laboratory Standards Institute (CLSI) guidelines14. For the Enterobacteriaceae members and non-fermenters, the antibiotics used were amikacin (AK), ampicillin (AMP), amoxicillin-clavulanate (AXV), aztreonam (AZT), cefotaxime (CTX), cefepime (CPM), ceftazidime (CTZ), cefoperazone-sulbactam (CFS), chloramphenicol (CHL), ciprofloxacin (CIP), co-trimoxazole (COT), gentamicin (GEN), imipenem (IPM), piperacillin-tazobactam (PTZ), netilmicin (NET), polymyxin-B (PB) and tigecycline (TGC). For Pseudomonas isolates, AMP, AXV, cefoxitin (CXT), CHL, COT and TGC were excluded from the panel. For the Gram-positive pathogens, the panel included AMP, AXV, penicillin (PEN), CXT, COT, CIP, GEN, erythromycin (ERY), vancomycin (VAN) and linezolid (LIZ). CXT (30 µg) disc was used as a surrogate marker for determining methicillin resistance amongst the staphylococci. Control strains of Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923 and Pseudomonas aeruginosa ATCC 27853 were used for antimicrobial susceptibility tests. In selected instances, where CLSI guidelines for disc diffusion technique are not available, the following strategies were adopted: (i)for CFS, the CLSI interpretative guideline for cefoperazone was used; (ii)for PB, the recommendation of Galani et al15 was adopted; (iii)and VAN susceptibility for Staphylococcus spp. and Enterococcus spp. were interpreted as per the British Society for Antimicrobial Chemotherapy guidelines16.
Data analysis: All laboratory and clinical data were extracted from infection control committee records and database. The clinical data concerning the number of ventilated days, CPIS score and various clinical and radiological findings were obtained from the VAP registers kept and maintained at all the ICUs by the infection control committee and updated daily for surveillance records. Simple counts and percentages were analyzed.
Results
During the study period significant growth of pathogens (mono or polybacterial) was found in 247 of 1159 (21.3%) patients in 2011, 297 of 903 (32.9%) in 2012 and 303 of 1022 (29.6%) in 2013. The VAP rates were 44.1, 43.8 and 26.3 per 1000 ventilator days in the three years, respectively.
The total numbers of Gram-negative organisms isolated in the three years from 2011 to 2013 were 578, 737 and 512, respectively. In all the three years, non-fermentative Gram-negative bacilli were the predominant organisms, followed by Pseudomonas spp. and Klebsiella spp. Although the relative frequency of most of these organisms remained more or less the same over the years, a small incremental decrease in the number of Klebsiella spp. (23.7% in 2011 to19.3% in 2013) and E. coli (14.9-11.5% during the same period) were noted over the years. Amongst the Gram-positive isolates, S. aureus exhibited a downward trend in prevalence from 50.0 per cent in 2011 to 34.9 per cent in 2013 (Table I).
Table I.
Distribution of ventilator-associated pneumonia pathogens during the study period (2011-2013)
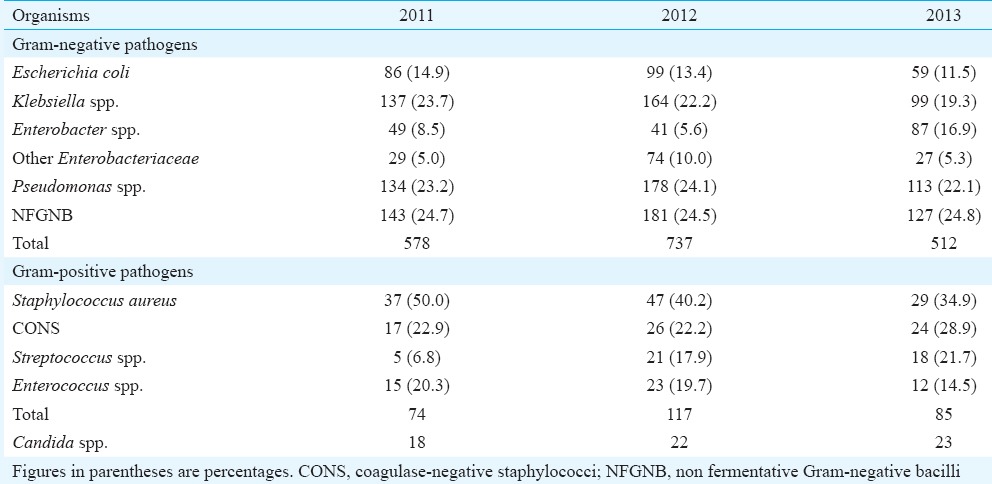
The percentages of resistant strains of the members of Enterobacteriaceae family are shown in Table II. Very high frequency of resistance ranging from 45 to 100 per cent was exhibited by these organisms for AMP, AXV, CIP and for CTX throughout the study period. AK resistance showed a steady rise for E. coli and other Enterobacteriaceae, but for Klebsiella spp., a fall was seen initially. Except for Enterobacter spp., most others showed an increasing resistance for IPM, as well as PTZ and CFS; the three drugs most commonly used in ICU settings. Sporadic resistant strains were seen appearing for PB, which, apart from TGC, remained the last line of agents for IPM-resistant isolates. There was no isolate of Enterobacteriaceae family and non-fermenters resistant to TGC.
Table II.
Antimicrobial resistance (in %) of members of Enterobacteriaceae family
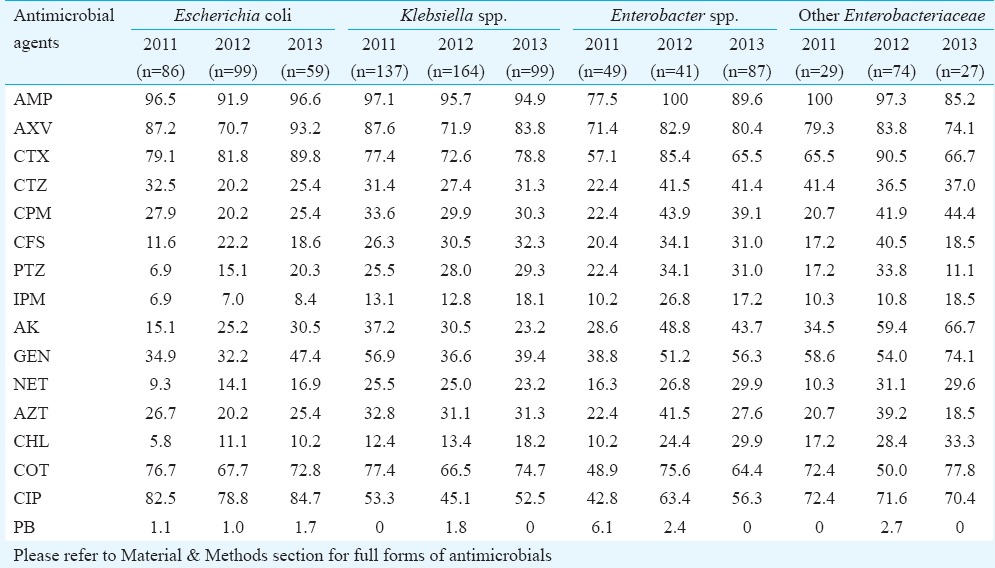
The resistance patterns of two important hospital pathogens, namely Pseudomonas spp. and non-fermentative Gram-negative bacilli (NFGNB, primarily Acinetobacter spp.) are shown in Table III. An increase in resistance was shown by Pseudomonas spp. for CFS, PTZ, AK and IPM. For the non-fermenters, resistance frequency remains very high for most of the antimicrobials, ranging from 40 per cent to >80 per cent, except for IPM (33.1%) and PB (2.4%).
Table III.
Antimicrobial resistance (in %) of Pseudomonas spp. and non-fermentative Gram-negative Bacilli
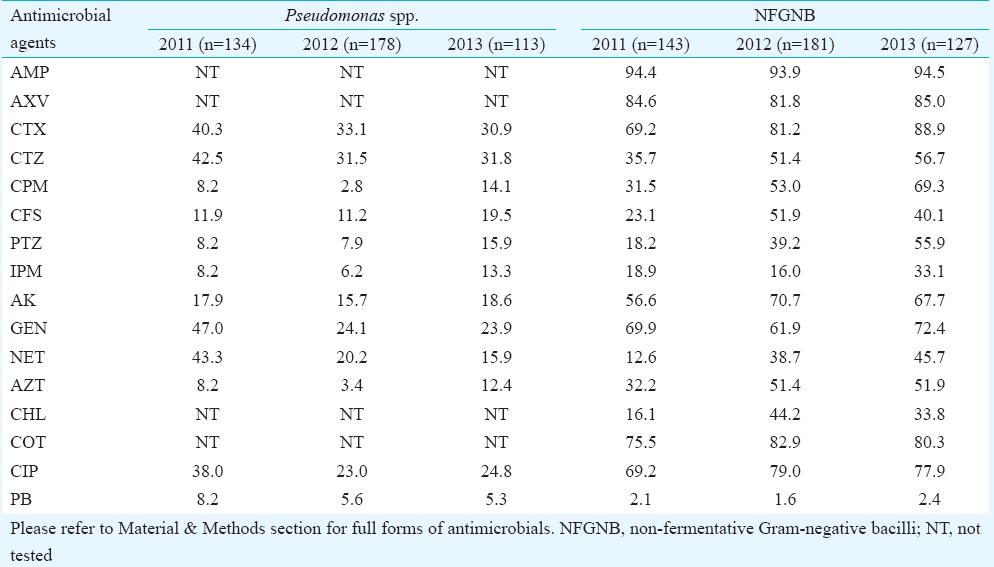
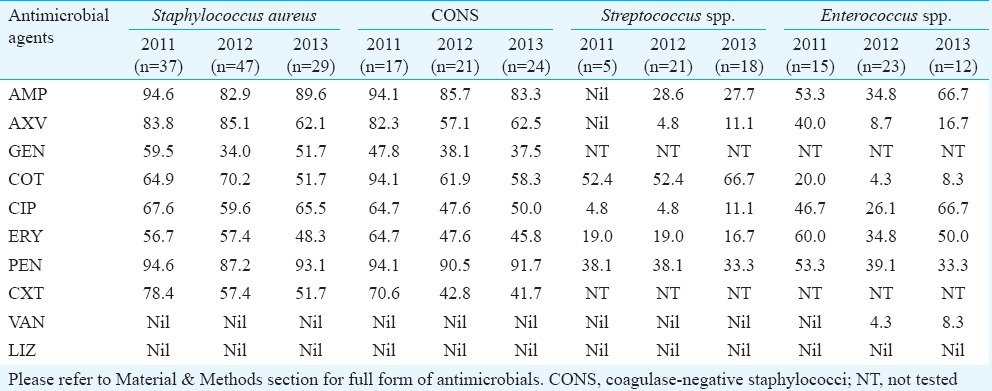
Table IV gives the resistance pattern for the Gram-positive isolates. Methicillin resistance (using the surrogate marker CXT) amongst the S. aureus crossed the 50 per cent mark while for coagulase-negative staphylococci (CONS), it was >40 per cent. However, a small decrease in the frequency of resistance was observed for both S. aureus and CONS over the years. From 2012, a rise in VAN-resistant enterococci (VRE) was observed (from 4.3 to 8.3 per cent in 2013) although all the other Gram-positive isolates remained susceptible to VAN as well as LIZ.
Table IV.
Antimicrobial resistance (in %) of Gram-positive pathogens
Discussion
VAP remains a serious complication amongst critical care patients in any healthcare setting and its incidence has been found to be highly variable not only amongst hospitals, but also in different ICUs in the same hospital. The infection rates seen in the present study were comparable to the figures of 35.1 and 37 per cent in two different studies from India17,18. The VAP rate per 1000 ventilator days is a commonly used parameter worldwide, and although its rate was quite low in the USA (4.4)4, in various other countries, it was found to be high in the range of 13-51 per 1000 ventilator days5. In various studies from India, the VAP rates were 22.9 from Puducherry7 and 26 and 27.77, respectively, from two separate studies from Mumbai19,20. In the initial two years of our study, the VAP rates were high (44.1 and 43.8 per 1000 ventilator days), but could be brought down to 26.3 per 1000 days in 2013.
Both Gram-positive and Gram-negative bacteria are implicated in VAPs, and ESKAPE organisms (Enterococcus faecium, S. aureus, Klebsiella pneumoniae, Acinetobacter baumannii, P. aeruginosa and Enterobacter spp.) constitute 80 per cent of the VAP epoisodes21. Contrary to this, in a large international study of the 606 cases of VAP, Gram-positive pathogens were the predominant isolates (72.8%) with methicillin-resistant S. aureus (MRSA) constituting 42.7 per cent22. This finding was in contrast to other reports, where Gram-negative bacteria (particularly Pseudomonas spp., Acinetobacter spp., E. coli and Klebsiella spp.) were the predominant isolates from VAP23,24. In our study, the Gram-negative bacilli were the principal isolates, and Pseudomonas spp., non-fermenters and Klebsiella spp. were the most common pathogens with almost similar proportion of frequency, and these findings were corroborated by other Indian studies17,25.
The introduction of extended spectrum third-generation cephalosporins about three decades back resulted in mutations in both blaTEM and blaSHV genes which were mainly reported amongst the Klebsiella spp26. However, in the last 10 years, there has been a rise in the prevalence of CTX-M phenotype which has spread to E. coli27. In our study the resistance to CTX rose to 78.8 per cent in Klebsiella spp. and almost 90 per cent in E. coli. Although these inactivating agents can be inhibited by β-lactamase inhibitors, non-susceptibility to PTZ in CTX-M-producing E. coli and Klebsiella spp. was 27.4 and 38.1 per cent, respectively, in a European study28. The situation was similar in our setting where resistance to piperacillin-tazobactam was on the rise not only for the Enterobacteriaceae members but also for non-fermenters. A similar trend was shown for cefoperazone-sulbactam, which is another potent agent widely used in the ICU settings.
The incidence of IPM-resistant Enterobacteriaceae was highest amongst Enterobacter spp., and other members such as Citrobacter spp. and Proteus group. In E. coli and Klebsiella spp., a rising trend could be observed in our study. In the multinational SENTRY study (2007-2009), the overall carbapenem resistance in Klebsiella spp. was 5.3 per cent, while it was 0.3 per cent in E. coli29. Increasing IPM resistance was also shown by Pseudomonas spp. in our study and amongst the non-fermenters, 33.1 per cent of the strains became IPM resistant by 2013. In one Indian study, the figures were 40 and 37.5 per cent for P. aeruginosa and Acinetobacter spp., respectively17. In Europe, the non-susceptibility rates for P. aeruginosa have been reported to increase to about 20 per cent for carbapenems, 25 per cent for aminoglycosides and about 8 per cent for PTZ30. In Spain, however, the situation was different for Acinetobacter spp., with resistance rates of 45 per cent for carbapenem, 70 per cent for PTZ, 35 per cent for AK and 40 per cent for CTZ31. These findings point to an increasing global emergence of highly resistant strains of Pseudomonas spp. and Acinetobacter spp. In such cases of carbapenem resistance, colistin/polymyxin B remains the last option, and in our centre, the polymyxin B resistance rates were 1.6-2.4 per cent for non-fermenters and 5.3-8.2 per cent for Pseudomonas spp. In Europe, 0.4-0.8 per cent of Pseudomonas spp. and Acinetobacter spp. has been found to be colistin resistant32.
MRSA is another global problem with prevalence reaching up to 25-50 per cent in America, Australia and South Europe33. This study showed >50 per cent of S. aureus and >40 per cent of CONS isolates being methicillin resistant. One important finding of our study was the emergence of VRE in 4.3 per cent in 2012 rising to 8.3 per cent in 2013. This is alarming since the high prevalence of VRE has been linked to the emergence of VAN-resistant S. aureus34.
In conclusion, the findings showed VAP as a problem in the ICU setting, with high percentage of multidrug-resistant pathogens. The resistance pattern of these pathogens along with their profile can help an institution to formulate effective antimicrobial policy for VAP based on evidence of the local scenario, along with the necessary infection control measures. Further, to have a comprehensive pan-India picture, multicentric studies with high number of patient population need to be initiated.
Footnotes
Conflicts of Interest: None.
References
- 1.Kalanuria AA, Zai W, Mirski M. Ventilator associated pneumonia in the ICU. Crit Care. 2014;18:208. doi: 10.1186/cc13775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 3.Peleg AY, Hooper DC. Hospital-acquired infections due to Gram-negative bacteria. N Engl J Med. 2010;362:1804–13. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudeck MA, Weiner LM, Allen-Bridson K, Malpiedi PJ, Peterson KD, Pollock DA, et al. National Healthcare Safety Network (NHSN) report, data summary for 2012, Device-associated module. Am J Infect Control. 2013;41:1148–66. doi: 10.1016/j.ajic.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alp E, Voss A. Ventilator associated pneumonia and infection control. Ann Clin Microbiol Antimicrob. 2006;5:7. doi: 10.1186/1476-0711-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khilnani GC, Jain N. Ventilator-associated pneumonia: changing microbiology and implications. Indian J Crit Care Med. 2013;17:331–2. doi: 10.4103/0972-5229.123432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joseph NM, Sistla S, Dutta TK, Badhe AS, Parija SC. Ventilator-associated pneumonia in a tertiary care hospital in India: incidence and risk factors. J Infect Dev Ctries. 2009;3:771–7. doi: 10.3855/jidc.396. [DOI] [PubMed] [Google Scholar]
- 8.Charles MP, Easow JM, Joseph NM, Ravishankar M, Kumar S, Umadevi S. Incidence and risk factors of ventilator associated pneumonia in a tertiary care hospital. Australas Med J. 2013;6:178–82. doi: 10.4066/AMJ.2013.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dey A, Bairy I. Incidence of multidrug-resistant organisms causing ventilator-associated pneumonia in a tertiary care hospital: a nine months’ prospective study. Ann Thorac Med. 2007;2:52–7. doi: 10.4103/1817-1737.32230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medell M, Hart M, Marrero O, Espinosa F, Montes de Oca Z, Valdés R. Clinical and microbiological characterization of pneumonia in mechanically ventilated patients. Braz J Infect Dis. 2012;16:442–7. doi: 10.1016/j.bjid.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Fartoukh M, Maitre B, Honoré S, Cerf C, Zahar JR, Brun-Buisson C. Diagnosing pneumonia during mechanical ventilation: the clinical pulmonary infection score revisited. Am J Respir Crit Care Med. 2003;168:173–9. doi: 10.1164/rccm.200212-1449OC. [DOI] [PubMed] [Google Scholar]
- 12.Ioanas M, Ferrer R, Angrill J, Ferrer M, Torres A. Microbial investigation in ventilator-associated pneumonia. Eur Respir J. 2001;17:791–801. doi: 10.1183/09031936.01.17407910. [DOI] [PubMed] [Google Scholar]
- 13.Collee JG, Marmion BP, Fraser AG, Simmons A. Mackie and McCartney's practical medical microbiology. 14th ed. New York: Churchill Livingstone; 1996. p. 978. [Google Scholar]
- 14.Clinical and Laboratory Standards Institute 2012. Performance standards for antimicrobial susceptibility testing. 22nd international supplement. M100-S22. Wayne, PA, USA: CLSI; 2012. [Google Scholar]
- 15.Galani I, Kontopidou F, Souli M, Rekatsina PD, Koratzanis E, Deliolanis J, et al. Colistin susceptibility testing by Etest and disk diffusion methods. Int J Antimicrob Agents. 2008;31:434–9. doi: 10.1016/j.ijantimicag.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Andrews JM. BSAC Working Party on Susceptibility Testing. BSAC standardized disc susceptibility testing method (version 8) J Antimicrob Chemother. 2009;64:454–89. doi: 10.1093/jac/dkp244. [DOI] [PubMed] [Google Scholar]
- 17.Golia S, Sangeetha KT, Vasudha CL. Microbial profile of early and late onset ventilator-associated pneumonia in the Intensive Care Unit of a Tertiary Care Hospital in Bangalore, India. J Clin Diagn Res. 2013;7:462–6. doi: 10.7860/JCDR/2013/6344.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gadani H, Vyas A, Kar AK. A study of ventilator-associated pneumonia: incidence, outcome, risk factors and measures to be taken for prevention. Indian J Anaesth. 2010;54:535–40. doi: 10.4103/0019-5049.72643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rakshit P, Nagar VS, Deshpande AK. Incidence, clinical outcome, and risk stratification of ventilator-associated pneumonia - A prospective cohort study. Indian J Crit Care Med. 2005;9:211–6. [Google Scholar]
- 20.Set R, Bobade O, Shastri J. Bacteriology profile among patients with ventilator-associated pneumonia from a medical Intensive Care Unit at a Tertiary Care Center in Mumbai. Indian J Pathol Microbiol. 2011;54:432–3. doi: 10.4103/0377-4929.81622. [DOI] [PubMed] [Google Scholar]
- 21.Sandiumenge A, Rello J. Ventilator-associated pneumonia caused by ESKAPE organisms: cause, clinical features, and management. Curr Opin Pulm Med. 2012;18:187–93. doi: 10.1097/MCP.0b013e328351f974. [DOI] [PubMed] [Google Scholar]
- 22.Quartin AA, Scerpella EG, Puttagunta S, Kett DH. A comparison of microbiology and demographics among patients with healthcare-associated, hospital-acquired, and ventilator-associated pneumonia: a retrospective analysis of 1184 patients from a large, international study. BMC Infect Dis. 2013;13:561. doi: 10.1186/1471-2334-13-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medell M, Hart M, Duquesne A, Espinosa F, Valdés R. Nosocomial ventilator-associated pneumonia in Cuban Intensive Care units: bacterial species and antibiotic resistance. MEDICC Rev. 2013;15:26–9. doi: 10.37757/MR2013V15.N2.6. [DOI] [PubMed] [Google Scholar]
- 24.Restrepo MI, Peterson J, Fernandez JF, Qin Z, Fisher AC, Nicholson SC. Comparison of the bacterial etiology of early-onset and late-onset ventilator-associated pneumonia in subjects enrolled in 2 large clinical studies. Respir Care. 2013;58:1220–5. doi: 10.4187/respcare.02173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joseph NM, Sistla S, Dutta TK, Badhe AS, Rasitha D, Parija SC. Ventilator-associated pneumonia in a tertiary care hospital in India: role of multi-drug resistant pathogens. J Infect Dev Ctries. 2010;4:218–25. doi: 10.3855/jidc.634. [DOI] [PubMed] [Google Scholar]
- 26.Maseda E, Mensa J, Valía JC, Gomez-Herreras JI, Ramasco F, Samso E, et al. Bugs, hosts and ICU environment: countering pan-resistance in nosocomial microbiota and treating bacterial infections in the critical care setting. Rev Esp Quimioter. 2013;26:312–31. [PubMed] [Google Scholar]
- 27.Livermore DM. Fourteen years in resistance. Int J Antimicrob Agents. 2012;39:283–94. doi: 10.1016/j.ijantimicag.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Hombach M, Mouttet B, Bloemberg GV. Consequences of revised CLSI and EUCAST guidelines for antibiotic susceptibility patterns of ESBL- and AmpC ß-lactamase-producing clinical Enterobacteriaceae isolates. J Antimicrob Chemother. 2013;68:2092–8. doi: 10.1093/jac/dkt136. [DOI] [PubMed] [Google Scholar]
- 29.Castanheira M, Mendes RE, Woosley LN, Jones RN. Trends in carbapenemase-producing Escherichia coli and Klebsiella spp. from Europe and the Americas: report from the SENTRY antimicrobial surveillance programme (2007-09) J Antimicrob Chemother. 2011;66:1409–11. doi: 10.1093/jac/dkr081. [DOI] [PubMed] [Google Scholar]
- 30.Souli M, Galani I, Giamarellou H. Emergence of extensively drug-resistant and pandrug-resistant Gram-negative Bacilli in Europe. Euro Surveill. 2008;13:pii:19045. [PubMed] [Google Scholar]
- 31.Picazo JJ, Betriu C, Rodríguez-Avial I, Culebras E, Gómez M, López F. Grupo VIRA. Antimicrobial resistance surveillance: VIRA STUDY 2006. Enferm Infecc Microbiol Clin. 2006;24:617–28. doi: 10.1157/13095373. [DOI] [PubMed] [Google Scholar]
- 32.Jones RN, Flonta M, Gurler N, Cepparulo M, Mendes RE, Castanheira M. Resistance surveillance program report for selected European nations (2011) Diagn Microbiol Infect Dis. 2014;78:429–36. doi: 10.1016/j.diagmicrobio.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Grundmann H, Aires-de-Sousa M, Boyce J, Tiemersma E. Emergence and resurgence of methicillin-resistant Staphylococcus aureus as a public-health threat. Lancet. 2006;368:874–85. doi: 10.1016/S0140-6736(06)68853-3. [DOI] [PubMed] [Google Scholar]
- 34.Leclercq R. Epidemiological and resistance issues in multidrug-resistant staphylococci and enterococci. Clin Microbiol Infect. 2009;15:224–31. doi: 10.1111/j.1469-0691.2009.02739.x. [DOI] [PubMed] [Google Scholar]


