Abstract
Peripheral neuropathy is a major toxicity of vincristine, yet no strategies exist for identifying at-risk adult patients. We used a case-control design of 48 adults receiving protocol therapy for acute lymphoblastic leukemia (ALL) who developed vincristine-induced neuropathy (NCI Grade 2-4) during treatment, and 48 matched controls who did not develop grade 2-4 neuropathy. Peripheral neuropathy was prospectively graded by NCI criteria. CEP72 promoter genotype (rs924607) was determined using PCR based SNP genotyping. Frequency of the CEP72 T/T genotype was higher in cases (31% vs 10%, p=0.0221) and the incidence of vincristine-induced neuropathy (grades 2-4) was significantly higher in patients homozygous for the CEP72 T/T genotype. 75% of the 20 patients homozygous for the CEP72 T allele developed grade 2-4 neuropathy, compared to 44% of patients with CEP72 CC or CT genotype (p=0.0221). The CEP72 polymorphism can identify adults at increased risk of vincristine-induced peripheral neuropathy.
Keywords: Inherited genetic variant, CEP72, vincristine neuropathy, acute lymphoblastic leukemia, adults
Introduction
Vincristine is one of the most widely prescribed anticancer agents for treating leukemia and solid tumors in adults and children,(1, 2) with acute peripheral neuropathy being a common dose-limiting toxicity that can disrupt treatment and compromise quality of life.
Vincristine exerts its cytotoxic effects by interfering with microtubule formation and mitotic spindle dynamics, leading to mitotic arrest and cell death (3-5). Vincristine-induced peripheral neuropathy (6, 7) is characterized by neuropathic pain and sensory and motor dysfunction. A substantial number of patients with ALL develop clinically symptomatic vincristine neuropathy (8, 9), causing considerable morbidity and often resulting in dose reductions or drug discontinuation (10) and thus potentially reducing the efficacy of curative treatment strategies. There are a number of variables that can influence the incidence of vincristine-induced peripheral neuropathy, including the amount of vincristine given with each dose (11), the total cumulative dose given (12), the frequency of vincristine administration (13), interactions with concomitant medications (e.g., CYP3A inhibitors such as some azole antifungals), (14) patient ancestry (15) and the methods used to assess motor and sensory neuropathy (11, 12). Recently, an inherited polymorphism in the promoter of the CEP72 gene was associated with an increased risk and severity of vincristine-induced peripheral neuropathy in children with ALL (16). Because it is not known whether this polymorphism predisposes to vincristine-induced peripheral neuropathy in adults, we performed a blinded case-control study to determine whether the CEP72 high-risk genotype (T/T at rs924607) is associated with a higher-risk of vincristine-induced peripheral neuropathy in adults receiving vincristine as part of protocol-directed combination chemotherapy for ALL.
Results
A total of 48 cases and 48 controls were identified as outlined in the CONSORT diagram (Supplemental Figure S1). There were no significant differences in demographic or clinical characteristics of the cases and controls, as summarized in Table 1. The BSA trended higher among the cases (median, 2.01 m2 vs 1.89 m2; p=0.06), but the vincristine dose was capped at a maximum of 2 mg for everyone above a BSA of 1.5 m2. The grades of peripheral neuropathy for the 48 cases are also provided in Table 1. The median cumulative vincristine dose received for the 48 cases at the time of onset of at least grade 2 neuropathy was 5.74 mg/m2 (range 1.27-30.35), whereas the control group received a median vincristine cumulative dose of 10.43 mg/m2 (2.07 -46.79) without developing grade 2 or greater neuropathy.
Table 1.
Summary of demographic and clinical characteristics of 48 adult patients with ALL who developed peripheral neuropathy after combination chemotherapy that included vncristine (cases) and 48 adult patients matched for race, sex and time on protocol treatment who did not develop grade 2-4 neuropathy (controls).
| Demographic and clinical characteristics | Cases (48) | Controls (48) | P value | |
|---|---|---|---|---|
| Age (Years) Median | 36.5 (17.2-71.2) | 34.5 (18.2-76.1) | 0.4479 | |
| Sex | Female | 22 (45.8%) | 22 (45.8%) | 1.000 |
| Male | 26 (54.2%) | 26 (54.2%) | ||
| Ethnicity | Hispanic | 3 (6.3%) | 4 (8.3%) | 0.4553 |
| Non-Hispanic | 38 (79.2%) | 41 (85.4%) | ||
| Unknown | 7 (14.6%) | 3 (6.3%) | ||
| Race | Hispanic American | 1(2.1%) | 2 (4.2%) | 1.000 |
| Indian subcontinent | 1(2.1%) | 0 (0.0%) | ||
| Unknown | 1(2.1%) | 1 (2.1%) | ||
| White | 45 (93.8%) | 45 (93.8%) | ||
| BSA (m2) Median (range) | 2.0 (1.5- 2.5) | 1.89 (1.5- 2.4) | 0.0648 | |
| Alliance ALL Protocol | 10102 | 25 (52.1%) | 25 (52.1%) | 1.000 |
| 10403 | 9 (18.8%) | 9 (18.8%) | ||
| 19802 | 14 (29.2%) | 14 (29.2%) | ||
| Highest Neuropathy Grade | 0 | 0 | 11 | |
| 1 | 0 | 37 | ||
| 2 | 29 | 0 | ||
| 3 | 17 | 0 | ||
| 4 | 2 | 0 | ||
| Vincristine Cumulative Dose (mg/m2)* Median (range) | 5.7 (1.3- 30.4) | 10.4 (2.1- 46.8) | 0.0008 | |
There was no difference in the cumulative dosage of vincristine in cases and controls, when assessed at the time when neuropathy was documented in the cases.
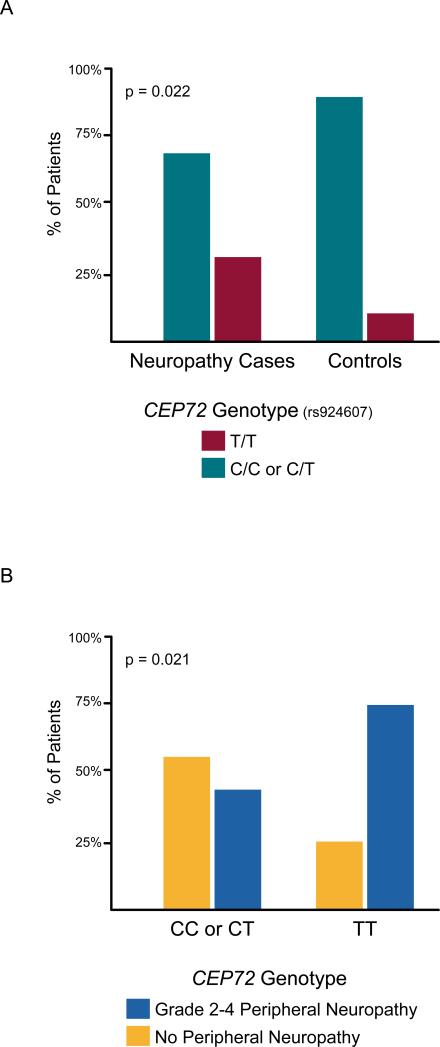
As depicted in Figure 1A and in Table 2, among the 48 patients developing grade 2-4 peripheral neuropathy, 15 (31%) had the CEP72 T/T genotype at rs924607, compared to only 5 of 48 matched controls (10.4%) (McNemar's test p=0.02). The odds ratio for developing vincristine-induced neuropathy was 4.33 (95% confidence interval 1.19 to 23.71; p=0.02). The T allele frequency was 44% in white patients (Table 3). There was not an adequate number of patients with African or Asian ancestry to estimate the allele frequencies in those groups. Overall, as depicted in Figure 1B, of 20 patients with the CEP72 T/T genotype (diplotype at rs924607), 75% developed grade 2-4 peripheral neuropathy, compared to 44% of patients with the CEP72 CC or CT promoter genotypes (Fisher's exact test p=0.02), comparable to that reported in children with ALL who developed vincristine Induced neuropathy (16) (Supplemental Figure 2). By design, the overall frequency of vincristine neuropathy was 50% in our selected case-control cohort, thus enriched for neuropathy cases compared to what would be expected in an unselected population.
Figure 1.
(Panel A) Percentage of patients who developed grade 2-4 vincristine-induced neuropathy [left] and percentage of matched controls [right] who had the CEP72 T/T promoter genotype (at rs924607) [red bars] or the CEP72 C/C or C/T genotype (at rs924607) [blue bars].(Panel B) Percentage of patients who developed grade 2-4 vincristine-induced neuropathy [red bars] among patients with the CEP72 promoter C/C or C/T genotype (left) or with the CEP72 T/T promoter genotype at rs924607.
Table 2.
Patients with CEP72 rs924607 genotype. The number of patients for each genotype is presented for the controls and the cases with peripheral neuropathy.
| Diplotypes | TT | CC/CT | Total |
|---|---|---|---|
| Controls | 5 | 43 | 48 |
| Cases | 15 | 33 | 48 |
| Total | 20 | 76 | 96 |
Table 3.
Allele frequencies of CEP72 rs924607. The allele frequencies (in %) of the C allele and the T allele at rs924607 are presented for the combined case/control cohort.
| Frequency | White | Other Ancestry |
|---|---|---|
| C allele frequency (%) | 101 (56%) | 6 (75%) |
| T allele frequency (%) | 79 (44%) | 2 (25%) |
| Total number of patients | 90 | 4 |
| Total alleles | 180 | 8 |
Ancestry was not documented for 2 patients.
Discussion
This study is the first to show that an inherited polymorphism in the CEP72 gene is associated with an increased risk of vincristine-induced peripheral neuropathy in adults. This case-control study has documented that a higher percentage of adults who develop grade 2-4 vincristine-induced peripheral neuropathy have inherited the CEP72 promoter genotype (TT at SNP rs924607) that is associated with lower CEP72 expression. A significantly higher percentage of cases had the high-risk CEP72 TT genotype and developed more neuropathy despite receiving a lower cumulative dosage of vincristine (vincristine treatment was stopped or the dosage was reduced after patients developed neuropathy). Interestingly, by chance cases in the current study tended to have a larger body surface area (Table 1), and therefore received a lower mg/m2 dosage of vincristine than controls, yet developed neuropathy.
In the current study, we did not assess the pharmacokinetics of vincristine, and it is possible that changes in the metabolism of vincristine, due to drug interactions or liver dysfunction, could influence the risk of neuropathy by increasing or decreasing systemic exposure to vincristine. There is no mechanistic reason to anticipate that the CEP72 promoter polymorphism would influence vincristine pharmacokinetics, but this has not been directly assessed.
The current findings are consistent with a genome-wide association study that identified the association of this CEP72 polymorphism with a higher incidence of vincristine-induced peripheral neuropathy in children with ALL (16). Although patients who were homozygous for the high-risk CEP72 promoter SNP (TT at rs924607) had a significantly higher risk of developing grade 2-4 vincristine-induced peripheral neuropathy in the current study of adults and in a prior study of children (16), approximately 25% of patients with the high-risk genotype did not develop vincristine neuropathy (5 of 20 CEP72 TT patients did not develop neuropathy in the current study). Thus, penetrance is not 100% in high-risk patients, some of whom received vincristine treatment without developing neuropathy. Furthermore, grade 2-4 peripheral neuropathy developed in approximately 44% of adults with the lower-risk CEP72 genotypes in the current case-control study (43 of 76 CC or CT patients) and 29% of children in a prior cohort study (16). This is consistent with other variables influencing the development of vincristine neuropathy, some of which may be genetic (e.g., other genes or other variants in CEP72) and some of which are likely non-genetic (e.g., drug interactions, concomitant diseases). It will be important for the future studies to build upon the current findings by identifying additional genetic and non-genetic variables that influence the incidence and severity of vincristine induced peripheral neuropathy.
The current study provides an independent replication of the original association of this CEP72 promoter genotype with vincristine-induced peripheral neuropathy in children and extends these findings to adults, which comprise a much larger population of patients treated with vincristine. A previous study (17) that assessed peripheral neuropathy after only 4 doses of vincristine did not find an association with the CEP72 polymorphism, as was also true in our pediatric patients (18). The current study of adults and the original GWAS of children examined vincristine-induced peripheral neuropathy after chronic vincristine treatment (up to 39 doses over two years); it is plausible that predisposition to neuropathy after prolonged treatment may differ from variables that predispose to acute neuropathy after short-term treatment.
It has been shown that CEP72 mRNA expression is significantly lower with the risk allele (T) than with the wild-type allele (C) (16). The T nucleotide at rs924607 creates a binding site for the NKX-6.3 transcriptional inhibitor, and molecular modeling revealed that the C to T transition changes the flexibility of the target DNA duplex and markedly enhances binding affinity of NKX-6.3 with the T-allele. We previously used electrophoretic mobility shift analysis to document substantially greater binding of NKX-6.3 with the CEP72 risk allele, consistent with the observed lower expression of CEP72 with the risk (T) allele. This was corroborated by rescue of lower CEP72 expression with the T-allele following reduction of NKX-6.3 expression (16).
Using human induced pluripotent stem cells that were differentiated into neurons, we showed that knock down of CEP72 (~35%) significantly enhanced their sensitivity to vincristine, which was also evident when CEP72 was knocked down in human ALL cells. Further, it was documented that primary leukemia cells (ALL) from patients with the CEP72 T/T germline genotype (at rs924607) were significantly more sensitive to vincristine than primary ALL cells from patients with either the C/C or C/T CEP72 genotype (16). Collectively, these laboratory findings are consistent with lower expression and greater sensitivity to vincristine in cells homozygous for the CEP72 risk allele (T at rs924607).
CEP72 is a centrosomal protein involved in spindle pole and microtubule formation and in chromosome alignment at metaphase. Vincristine interferes with microtubule formation by interacting with β-tubulin, to prevent tubulin polymerization, leading to cell death. Thus, lower CEP72 expression and treatment with vincristine impair microtubule formation by inhibiting different components of the process. It has been shown that reduction in CEP72 expression in human neurons derived from induced pluripotent stem cells enhances their sensitivity to vincristine (16). Furthermore, reduced expression of CEP72 in human leukemia cells increased their sensitivity to vincristine, and primary leukemia cells from patients with the CEP72 TT genotype (lower CEP72 expression) are more sensitive to vincristine (16).
These findings, taken together, suggest that it may be possible to treat both adults and children with ALL who have inherited the CEP72 TT genotype (rs924607) more safely and effectively with a lower dosage of vincristine, as these findings indicate that both their leukemia cells and neurons are more sensitive to vincristine. This strategy could reduce treatment-related morbidity and potentially improve survival rates by increasing the ability to complete protocol directed therapies without further reducing or eliminating subsequent vincristine doses; thus, this approach merits further study in prospective clinical trials in adults and children.
Methods
Patients and protocols
All patients (cases and controls) had a diagnosis of previously untreated ALL and were enrolled on one of three sequential NCI-sponsored institutional review board-approved cooperative group trials from 1998-2012 (see CONSORT Diagram, Supplemental Figure S1) in Cancer and Leukemia Group B (CALGB): CALGB 19802 (19), CALGB 10102 (20), and C10403 (21) and the correlative science companion protocol CALGB 9862 protocol (NCT0003700, NCT0061945, NCT00558519, NCT00003861, respectively). CALGB is now part of the Alliance for Clinical Trials in Oncology. Written IRB-approved, protocol-specific informed consent was obtained from all patients. All patients were > 17 years old; for protocols 19802 and 10102, there was no maximum age; for C10403 patients were enrolled up to the age of 40 years. The protocol-specified vincristine dose was 1.5 mg/m2/dose but was capped at a maximum of 2 mg for each dose administered. For CALGB 19802 and 10102, the prescribed total cumulative dose of vincristine during protocol treatment was approximately 64 mg; for C10403, a trial for young adults with ALL, the protocol- defined cumulative dose of vincristine was higher and differed by gender (78 mg for women and 102 mg for men) because the maintenance treatment extended for a longer period and men received an additional year of maintenance therapy. Cases were patients with ALL who developed grades 2, 3, or 4 motor and/or sensory neuropathy while receiving combination chemotherapy for ALL; controls were matched patients who did not develop grade 2-4 motor or sensory neuropathy while receiving the same protocol-defined combination chemotherapy that included vincristine. Cases and controls were matched for self-reported race, sex, protocol, protocol treatment arm and protocol-planned vincristine dose (mg/m2).
Neuropathy Phenotype
As part of required clinical trial adverse event monitoring, patients were prospectively assessed for the presence of peripheral neuropathy via physical examination by the treating physician at each clinic visit during treatment. Neuropathy was graded for each patient according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4 (22). This scale classifies neuropathy events as mild (grade 1), moderate (grade 2), serious/disabling (grade 3), or life threatening (grade 4). There were no neuropathy-related deaths (grade 5). In general, clinicians reduced the dose of vincristine or deleted it after moderate or worse treatment-related toxicity developed.
CEP72 Genotyping
Leukocytes isolated from bone marrow aspirates when patients were in complete remission or from leukemia cells from bone marrow aspirates at diagnosis were provided from the Alliance Leukemia Tissue Bank (The Ohio State University). DNA was extracted from leukocytes or leukemia cells. Samples were genotyped for the CEP72 promoter polymorphism (rs924607) by investigators (BD, WE) who were blinded to whether the DNA was from cases or controls. The rs924607 SNP (either C or T) genotype (diplotype) was determined using the TapMan SNP Genotyping Assay (C_8292459_20) from Life Technologies (Durham, NC). Forward and reverse primers were used to amplify the polymorphic sequence of interest and two dye-labeled probes for allele specific detection. One probe is labeled with VIC dye, which detects the “Allele C” sequence, while the other one is labeled with FAM dye (6-carboxyfluorescein), which detects the “Allele T” sequence. Assays were performed in accordance with manufacturer's protocols. Briefly DNA was amplified in the presence of the TaqMan genotyping master mix, the primers and the probes following a PCR protocol of 95°C 10 min, 40 cycles of 15 sec at 92°C and 1 min at 60°C. Fluorescence was quantified using a 7900HT fast real time PCR system. The data were analyzed by the software which makes an automatic call of either AlleleY (homozygous T/T), AlleleX (homozygous C/C) or heterozygous genotype (C/T).
Statistical Analysis
Statistical analyses were conducted by the Alliance Statistics and Data Center (KL, SJM) and by biostatisticians at St. Jude Children's Research Hospital (DP, CC). A case-control design using a 1:1 Greedy algorithm was used; specifically, eligible patients were matched for self-reported race, sex, and time on protocol treatment (+/− one month) within each protocol. Regarding the genotype status (T/T or non-T/T) of each individual in cases and matched controls as paired binary data, McNemar's test (23) was applied to compare the TT genotype frequency between cases and controls. Additionally, Fisher's exact test (23) was applied to compare overall the frequency of neuropathy between subjects with the T/T genotype and those with the C/C or C/T genotype. In Table 1 comparisons between cases and controls were done by chi-square test for categorical variables (23) and by Wilcoxon rank-sum test for continuous variables (24). All analyses were done by SAS version 9.3 (Cary, NC).
Supplementary Material
Study Highlights.
What is the current Knowledge on the topic?
Peripheral neuropathy is the major dose-limiting toxicity of vincristine, and there are currently no precision medicine strategies for identifying adults at high-risk of vincristine-induced neuropathy.
What question did this study address?
To determine whether the incidence of vincristine-induced peripheral neuropathy in adults is influenced by an inherited genetic variant in the CEP72 gene promoter.
What this study adds to our knowledge?
This study is the first to show that an inherited polymorphism in the CEP72 gene is associated with an increased risk of vincristine-induced peripheral neuropathy in adults.
How this might change clinical pharmacology or translational science?
The current pharmacogenomic study provides a genetic test for identifying adults who are predisposed to vincristine-induced peripheral neuropathy, offering the first precision medicine strategy for identifying adults who have a higher risk of developing the major dose limiting toxicity of this widely prescribed anticancer agent. Because ALL cells of patients with the CEP72 T/T genotype are more sensitive to vincristine, it may be feasible to treat these patients with a lower dosage of vincristine.
Acknowledgements
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA031946, U10CA033601, U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), and U10CA004919, U10CA015488, U10CA021115, U10CA032102, U10CA180836, U10CA180820, and U10CA180888. Also supported in part by funds from the NIH Grants R01 CA36401 (W.E.E), P50 GM115279 (W.E.E.), U01 GM92666 (W.E.E.), St. Jude Comprehensive Cancer Center grant CA21765 from the National Cancer Institute, and by the American Lebanese Syrian Associated Charities. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author Contributions
W.S., B.D., K.R.C., R.A.L., and W.E.E. wrote the manuscript; W.S., B.D., K.R.C., and W.E.E. designed the research; W.S., B.D., K.R.C., D.P., C.C., K.L., S.L.M., S.L., A.A., R.M.S., R.A.L., and W.E.E. performed the research; W.S., B.D., D.P., C.C., and W.E.E. analyzed the data.
Conflict of Interest/Disclosures: The authors declare no competing financial interests.
REFERENCES
- 1.Pui CH, Evans WE. Acute lymphoblastic leukemia. The New England journal of medicine. 1998;339(9):605–15. doi: 10.1056/NEJM199808273390907. [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. The New England journal of medicine. 2006;354(2):166–78. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 3.Jordan MA, Toso RJ, Thrower D, Wilson L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(20):9552–6. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nature reviews Cancer. 2004;4(4):253–65. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 5.Larson RA, Dodge RK, Burns CP, Lee EJ, Stone RM, Schulman P, et al. A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: cancer and leukemia group B study 8811. Blood. 1995;85(8):2025–37. [PubMed] [Google Scholar]
- 6.Bradley WG, Lassman LP, Pearce GW, Walton JN. The neuromyopathy of vincristine in man. Clinical, electrophysiological and pathological studies. Journal of the neurological sciences. 1970;10(2):107–31. doi: 10.1016/0022-510x(70)90013-4. [DOI] [PubMed] [Google Scholar]
- 7.Gidding CE, Meeuwsen-de Boer GJ, Koopmans P, Uges DR, Kamps WA, de Graaf SS. Vincristine pharmacokinetics after repetitive dosing in children. Cancer chemotherapy and pharmacology. 1999;44(3):203–9. doi: 10.1007/s002800050968. [DOI] [PubMed] [Google Scholar]
- 8.Toh HC, Sun L, Koh CH, Aw SE. Vinorelbine induces apoptosis and caspase-3 (CPP32) expression in leukemia and lymphoma cells: a comparison with vincristine. Leukemia & lymphoma. 1998;31(1-2):195–208. doi: 10.3109/10428199809057599. [DOI] [PubMed] [Google Scholar]
- 9.Bukowinski AJ, Burns KC, Parsons K, Perentesis JP, O'Brien MM. Toxicity of Cancer Therapy in Adolescents and Young Adults (AYAs). Seminars in oncology nursing. 2015;31(3):216–26. doi: 10.1016/j.soncn.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Liew E, Thyagu S, Atenafu EG, Alibhai SM, Brandwein JM. Quality of life following completion of treatment for adult acute lymphoblastic leukemia with a pediatric-based protocol. Leuk Res. 2013;37(12):1632–5. doi: 10.1016/j.leukres.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013;63(6):419–37. doi: 10.3322/caac.21204. [DOI] [PubMed] [Google Scholar]
- 12.Pal PK. Clinical and electrophysiological studies in vincristine induced neuropathy. Electromyogr Clin Neurophysiol. 1999;39(6):323–30. [PubMed] [Google Scholar]
- 13.Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat Rev. 2014;40(7):872–82. doi: 10.1016/j.ctrv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Bohme A, Ganser A, Hoelzer D. Aggravation of vincristine-induced neurotoxicity by itraconazole in the treatment of adult ALL. Annals of hematology. 1995;71(6):311–2. doi: 10.1007/BF01697985. [DOI] [PubMed] [Google Scholar]
- 15.Renbarger JL, McCammack KC, Rouse CE, Hall SD. Effect of race on vincristine-associated neurotoxicity in pediatric acute lymphoblastic leukemia patients. Pediatric blood & cancer. 2008;50(4):769–71. doi: 10.1002/pbc.21435. [DOI] [PubMed] [Google Scholar]
- 16.Diouf B, Crews KR, Lew G, Pei D, Cheng C, Bao J, et al. Association of an inherited genetic variant with vincristine-related peripheral neuropathy in children with acute lymphoblastic leukemia. JAMA. 2015;313(8):815–23. doi: 10.1001/jama.2015.0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutierrez-Camino A, Martin-Guerrero I, Lopez-Lopez E, Echebarria-Barona A, Zabalza I, Ruiz I, et al. Lack of association of the CEP72 rs924607 TT genotype with vincristine-related peripheral neuropathy during the early phase of pediatric acute lymphoblastic leukemia treatment in a Spanish population. Pharmacogenetics and genomics. 2016;26(2):100–2. doi: 10.1097/FPC.0000000000000191. [DOI] [PubMed] [Google Scholar]
- 18.Diouf B, Crews KR, Evans WE. Vincristine pharmacogenomics: 'winner's curse' or a different phenotype? Pharmacogenetics and genomics. 2016;26(2):51–2. doi: 10.1097/FPC.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 19.Stock W, Johnson JL, Stone RM, Kolitz JE, Powell BL, Wetzler M, et al. Dose intensification of daunorubicin and cytarabine during treatment of adult acute lymphoblastic leukemia: results of Cancer and Leukemia Group B Study 19802. Cancer. 2013;119(1):90–8. doi: 10.1002/cncr.27617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stock W, Yu D, Sanford B, Lozanski G, Cataland S, Vik R, et al. Incorporation of alemtuzumab into front-line therapy of adult acute lymphoblastic leukemia is feasible(A phase I/II study from the Cancer and leukemia Group B (CALGB 10102). Blood. 2005;106(11):145. [Google Scholar]
- 21.Stock W, Luger S, Advani A, et al. Favorable Outcomes for Older Adolescents and Young Adults (AYA) with Acute Lymphoblastic Leukemia (ALL): Early Results of U.S. Intergroup Trial C10403. Blood. 2014;124(21):79620. [Google Scholar]
- 22.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–81. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 23.Agresti A. Categorical Data Analysis. 2nd. Edition. Wiley-Interscience; New York: 2002. p. 721. [Google Scholar]
- 24.Lehman E. Nonparametrics: Statistical methods based on ranks. Prentice-Hall; New Jersey: 2006. p. 464. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.