Abstract
For centuries, spices have been consumed as food additives or medicinal agents. However, there is increasing evidence indicating the plant-based foods in regular diet may lower the risk of neurodegenerative diseases including Alzheimer disease. Spices, as one of the most commonly used plant-based food additives may provide more than just flavors, but as agents that may prevent or even halt neurodegenerative processes associated with aging. In this article, we review the role and application of five commonly used dietary spices including saffron turmeric, pepper family, zingiber, and cinnamon. Besides suppressing inflammatory pathways, these spices may act as antioxidant and inhibit acetyl cholinesterase and amyloid β aggregation. We summarized how spice-derived nutraceuticals mediate such different effects and what their molecular targets might be. Finally, some directions for future research are briefly discussed.
Keywords: Alzheimer's disease, dementia, spice
INTRODUCTION
Alzheimer disease (AD), the most common type of dementia, is a progressive and irreversible neurodegenerative disease that affects more 27 million people worldwide.[1] The neuropathological hallmarks of AD comprise neuritic plaques, neurofibrillary tangles, and neuronal loss.[2] Over the past two decades, a significant number of research projects have been conducted to farther our knowledge of the underlying mechanisms of AD and to investigate novel intervention and preventive approaches. However, current therapeutic approaches to manage AD have temporary symptom relief effects and do not inhibit or converse the underlying disease mechanisms.[3,4] Therefore, treatment of AD still remains a great challenge and the development of novel strategies is an active field of research. For centuries, spices have been used in various forms including flavoring agents, colorants, and preservatives. The importance of plant-based foods in regular diet to decrease the risk of chronic diseases is increasingly examined and spices are now considered to add more than flavors;[5] that is, they are agents that may delay, prevent or even treat age-related diseases such as AD. Some of the most widely investigated spices are presented in Tables 1 and 2.
Table 1.
List of spices with potential against cognitive decline
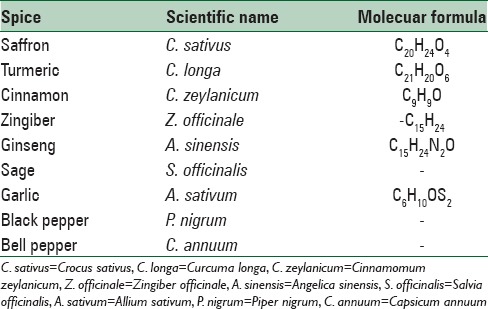
Table 2.
Clinical studies evaluating effects of common spices on Alzheimer's disease
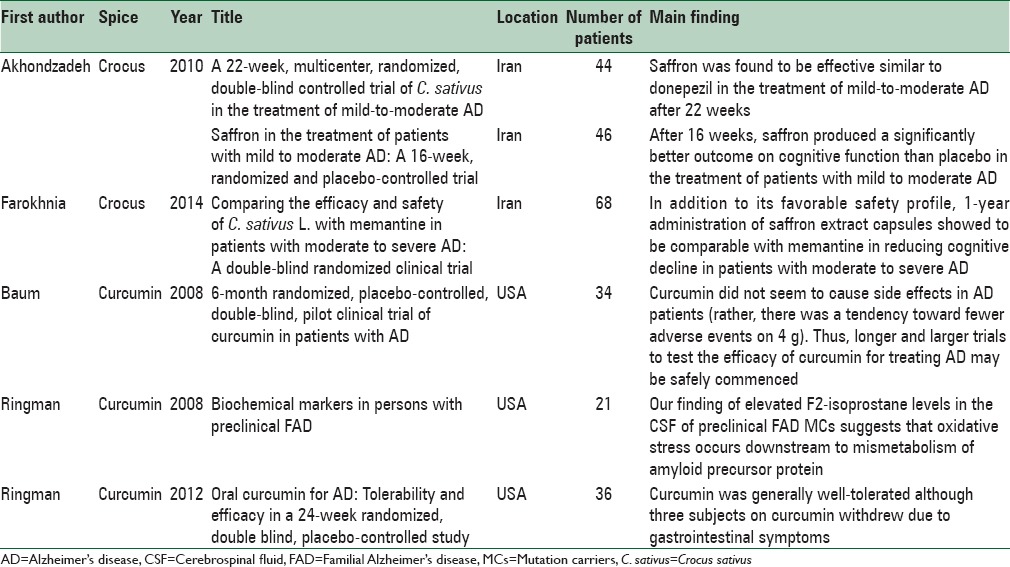
This review will focus on saffron as a promising spice for future trials in AD in addition to four other prominent spices used in routine diets worldwide including curcumin, pepper, ginger, and cinnamon. Furthermore, the properties of these five spices against different pathogenesis of AD are explained [Table 2].
METHODS
We performed a narrative review of all studies that assessed clinical and molecular of common spices on AD. Medline-PubMed, web of sciences, EMbase, and Cochrane databases up to June 2016 included in our searches. In addition, references lists from articles identified by search as well as a key review article to identify additional articles included.
Crocus
Crocus sativum L. (Crocus sativus), usually known as saffron,[6] C. sativus is a specie belonging to the Iridaceae family of Asparagales, a food additive with striking dye and pleasant aroma, also used as colorant and medical resolution, derived from dehydrated red stigmas of C. sativus. Saffron, also being used as dyeing and flavoring agent, is the most expensive spice worldwide.[7] Although saffron is spread in numerous regions, Iran and Spain produce more than 80% of the world's saffron and make lots of research projects into its potential medical applications.[8] Saffron derivatives are crocetin, crocin, di-methyl crocetin, and carotenoids. Di-gentiobioside ester of crocetin is called crocin.[9] Organoleptic characteristics and stigma tissue high concentration of apocarotenoids stigma tissue made this substance be widely studied about. The presence of three main compounds would determine the value of saffron: Flavor presented by picrocrocin, color produced by crocin and its derivatives; and odor presented by safranal. C. sativus has been mentioned as a memory enhancer, anticancer, and an intervention for AD as well as other neurodegenerative diseases with very low number of side effects.[10]
Mechanisms of effect
Anti-amyloid properties
Aβ1-40-fibrillogenesis has been affected by trans-crocin-4, saffron extract, and dimethyl crocetin. The extraction of C. sativus stigmas can inhibit amyloid fibrils formation depending on the time and concentration. For example, after 30 days, saffron extract, being incubated with Aβ1-40, may show decrease in efficacy probably because of oxidation or other chemical transformations lessening the activeness of saffron constituents. The main crocin constituent of stigmas is Trans-Crocin-4 that inhibits Aβ-fibrillogenesis. Also, in vitro amyloid aggregation has been inhibited by crocin constituents. In patients with mild-to-moderate AD, saffron plus donepezil had similar effectiveness.[11] Cognitive impairment can be prevented considerably by intracerebroventricular injection of crocin, as suggested by Khalili and Hamzehin an animal model study. In addition, Khalili and Hamzeh mentioned a potential therapeutic effect of crocin for age-related cognitive decline and neurodegenerative disorders.[12] In another animal study, saffron showed positive effect on cognitive behavior of adult rats exposed to amnestic agents (e.g., scopolamine, acetaldehyde, or ethanol).[13]
Antioxidant properties
Leaves and tepals of saffron have been proved as an antioxidant and metal chelating compound.[14] By in vitro measuring of free radicals scavenging and ferric ion reducing power, C. sativus stigmas extract antioxidant activity was determined. Saffron antioxidant power was compared with other vegetables rich in carotenoids antioxidant (carrots and tomatoes). All assays showed that the extract of C. sativus stigmas had stronger antioxidant activity than carrots and tomatoes.[15] Furthermore, a murine study suggested neuroprotective potentials of crocetin and crocin due to their antioxidant capacities.[16] In mice, crocin antioxidant capacity has proved to have some beneficial effects on memory processing because of malondialdehyde (MDA) reduction (an index of lipid peroxidation) levels.[14] Furthermore, anti-apoptotic characteristic of crocin demonstrated to have beneficial therapeutic actions in rat model of AD.
AChE inhibition activity
A mixed-type inhibition is presented by Kinetic analysis, which was confirmed by in silico docking studies. Safranal interaction is with the binding site of the AChE merely, but dimethylcrocetin and crocetin can bind to both the catalytic and peripheral anionic sites concurrently. Valuable finding has been focused for using novel carotenoid therapeutic agents based on their action on AChE inhibition.[17]
Anti-inflammatory effects
Another probable application of saffron comes from its neuroprotective effects by reducing the inflammation resulted from chemokine (C-C motif) ligand 2 down regulation. Both crocetin and crocin can suppress lipopolysaccharide-induced (LPS-induced) nitrite from microglial cells, and hence, LPS-induced cytotoxicity can be protected by them at the high concentrations.[18]
Human studies
Three clinical trial studies have examined the effects of saffron on AD. The first study was a double-blind study of patients resenting from AD. These patients with mild to moderate AD were divided into two 46-patient groups who received capsule of saffron (15 mg twice/day, Impiran, Tehran, Iran) or placebo in a 1:1 ratio randomly for 16 weeks. To monitor the clinical and global cognitive profile of these subjects, psychometric measures, including clinical dementia rating scale–sums of boxes (CDR-SB), the Mini-Mental State Examination (MMSE) and the AD Assessment Scale-cognitive subscale (ADAS-cog), were conducted. The ADAS-cog rating scale change significantly at 16th week as it was compared with week 0 in two groups. A significant difference was observed on the decrement of scores at week 16 comparison to week 0 in the two groups. The significant indication of this study was to show saffron short –term use effectiveness and safety in mild to moderate AD.[11]
The second study - a multicenter, double-blind randomized clinical trial - examined 54 AD patients. In this prospective 22-week study, patients, in parallel groups, received either saffron (15 mg twice/day, Impiran; Tehran, Iran) or a capsule of donepezil (5 mg twice/day) in a 1:1 ratio using a computer generated code. In both groups, there was not any considerable change in scores of the ADAS-cog and scores of the CDR-SB at 22nd week as it as compared with baseline. No severe side effect was reported by individuals who used saffron capsules.[11]
The third one was a double-blind randomized clinical trial studied 60 patients with moderate to mild AD. In this 1-year of administration study, patients divided in two parallel groups. Thirty patients received memantine (Ebixa®, Ebixa, Valby, Denmark, 20 mg/day) and 30 ones received saffron extract (Saffrotin®, Impiran, Tehran, Iran, 30 mg/day) in a 1:1 ratio by means of the random distribution method. The efficacy assessment measures used in this study were Severe Cognitive Impairment Rating Scale, Functional Assessment Staging and MMSE. In conclusion, 1-year management with saffron was comparable with memantine for decreasing cognitive decline in patients suffering from moderate to severe AD.[19]
The adverse effects of treating AD with crocin included dizziness, hypomania, dry mouth (the main side effect), vomiting, fatigue and nausea. In addition, saffron extract was suggested to be generally well tolerated by the study participants. The bioavailability of the saffron is unknown and whether crocetin, crosses the blood–brain barrier after saffron administration is yet to be established.[20]
Curcumin
Curcumin (turmeric), a constituent of the spice turmeric, is one of the ingredients of Indian curry powder. Turmeric, a phytochemical extract of Curcuma longa rhizome is widely used for its flavoring and medicinal properties in Indian Ayurveda medicine and other traditional medicinal approaches in Middle East and in East and South East Asian countries for 1000s years.[21] Turmeric extracts are usually standardized to 95% of total curcuminoids. Curcumin is the principal curcuminoid which found in commercially-available turmeric extracts. However, the curcuminoid mixtures also contain two minor demethylated curcuminoids which are coextracted with curcumin – demethoxycurcumin and bisdemethoxycurcumin.[22] The association between biological effects of these curcuminoids and their molecular or stoichiometric properties need further study. Of note, cell type, function, disease system, and organism in question can affect the efficacy of these analogues noticeably. Thus, there is no unanimous agreement about the most effective preparation method for human applications. However, the promising herbal drug-curcuminlonga (turmeric), utilized widely for treating many medical conditions and is being investigated for the treatment of dementia and brain injuries including AD.[23]
Mechanisms of effect
Antioxidant properties
Both in vitro and in vivo studies have proved curcumin as a powerful antioxidant.[24,25,26] In male Wistar rats, curcumin and demethoxy-curcumin, but not bisdemethoxycurcumin, prevented the decrease of glutathione level and protein oxidation.[27] Park et al. found increment in the level of antioxidant enzymes and DNA damage and attenuated the elevation of intracellular calcium levels and tau hyperphosphorylation-induced by Aβ due to administration of 10 μg/mL curcumin.[28]
Anti-Inflammatory effects
Giri et al. reported curcumin (12.5–25 μM) suppression on early growth response-1 (Egr-1) activation using peripheral blood monocytes and THP-1 cells. In the above-mentioned study, increase in expression of cytokines (tumor necrosis factor-α [TNF-α] and interleukin-1β [IL-1β]) and chemokines (macrophage inflammatory protein-1β, monocyte chemotactic protein-1, and IL-8) in monocytes by the interaction of Aβ1-40 or Fibrillar Aβ1-42 and reduction in the expression of these cytokines and chemokines was found. The inhibition of Egr-1 by curcumin may represents a potential therapeutic approach for AD.[29]
Anti-amyloid properties
In numerous in vitro studies curcumin binding potential to Aβ and restriction of Aβ aggregation, has been indicated. The strongest inhibition effect on formation of Aβ fibrils was reported for curcumin among 214 antioxidant compounds.[30] The effect of curcumin on Aβ oligomers and Aβ-derived diffusible ligand has been recently studied.[31] Interestingly, both Aβ levels and amyloid-beta precursor protein maturation decreased in mouse primary cortical neurons by curcumin demonstrated the decrement of Aβ deposition and plaque burden in an AD transgenic mice model after. Furthermore, a recent study of screening and molecular docking studies for curcumin has shown that curcumin can be considered as the most probable inhibitors of the Alzheimer's Precursor Protein.[32]
AChE inhibition activity
Curcumin has shown to inhibit the AChE, but no significant effect on ex vivo AChE activity has been reported yet.[33]
Cholesterol-lowering properties
High-fat diets and increased blood cholesterol can lead to increased amyloid plaques by intracellular accumulation of cholestrylesters.[34] Researchers believe that curcumin beneficial effects on AD is somehow the result of cholesterol formation restriction and decrease in serum peroxides.[35]
Metal chelation effects
The interaction between heavy metals such as cadmium and curcumin, can prevent neurotoxicity caused by these metals. Using spectrophotometry, it was shown that curcumin effectively binds to copper, iron, and zinc. These detections suggest curcumin inflammatory damage suppression by preventing metal induction of nuclear factor kappa (NF-κ).
Human studies
Three main clinical trials with curcumin in AD have been reported so far. A pilot study conducted in Hong Kong (China) included the consumption of combined standardised Ginkgo biloba (120 mg/day) extraction and curcumin (1 g/day) by 34 patients with probable AD for 6 months; no plasma Aβ level change or score improvement on the MMSE shown in comparison to the control group.[36] In a study on mild to moderate AD patients received curcumin (2–10 mg) for 6 months but no cognitive function improvement, or noticeable changes in the level of Aβ, total and phosphorylated tau in plasma and in cerebrospinal fluid (CSF) were found.[3,32] In another study, 36 patients with mild-to-moderate AD received placebo, oral curcumin in 2 g/day, or 4 g/day dose randomly for 24 weeks. From week 24 to 48, patients on curcumin treatment continued with the same dose, whereas others started randomized use of 2 g/day or 4 g/day in a 1:1 ratio. In addition to measuring the incidence of side effects, changes in clinical laboratory tests and the ADAS-cog, the Neuropsychiatric Inventory, the AD Cooperative Study-Activities of Daily Living scale, levels of Aβ1-40 and Aβ1-42 in plasma and levels of Aβ1-42, t-tau, p-tau181 and F2-isoprostanes in CSF were examined. No significant clinical or biomarker differences were found between the two groups. Although, the compound's bioavailability was limited primarily.[3]
Safety and dose
Because of insolubility in water, systemic bioavailability of curcumin is poor specifically through oral administration. Some evidence suggest that <4 g of oral curcumin is hardly detectable in serum; however, some researchers have reported to detect curcumin in serum and urine at lower doses.[37] Therefore, a pharmacological challenge is to enhance solubility and stability of curcumin to optimize its oral bioavailability for therapeutic applications and some methods have been proposed including using piperine as an adjuvant for blocking metabolic pathways. Probably intestinal and hepatic glucuronidation is the reason for curcumin bioavailability limitation. Administration of curcumin concomitantly with piperine, an inhibitor of glucuronidation, may increase oral curcumin bioavailability. Gupta et al. have experimented this approach in both rats and humans and reported that using 20 mg of piperine with 2 mg of curcumin orally, increased the curcumin bioavailability by 20-folds.[38] None of the 10 participants of this study reported toxicity.[39] Curcumin bioavailability has also been enhanced by cooking probably because of dissolution in oil or cooking itself. Some studies have suggested that using a combination of curcuminoids increases curcumin bioavailability because of the potential synergistic activity. Even very high doses of curcumin, as much as 500–8000 mg/day for 3 months can be well tolerated without toxicity.[40] The world Health Organization has been suggested 0–1 mg/kg body weight for daily curcumin intake. In human trials, diarrhea has been recorded as the most commonly seen side effect of curcumin.[37]
Cinnamon
Cinnamon (Cinnamomum zeylanicum Nees) (CZ) is a spice used commonly in different areas of the world. It is a tropical evergreen tree which grows in Sri Lanka, India, Madagascar, and Indochina countries. The inner bark of the tree has been used in ethno-medicine and as flavoring agent in food. It has been used in a broad range of health uses and for treatment of different conditions such as boosting cognitive function.[41] Several clinical studies have been performed for evaluating the potential effectiveness of cinnamon against different complications such as plasma lipids and diabetes. Cinnamon has been reported to be safe for a wide age range.[42]
Mechanisms of effect
Antioxidant and anti-inflammatory effects
Antioxidant properties of cinnamon have been identified. There are several reports using cell culture models and an experiment in rat model representing that cinnamon and cinnamaldehyde have antioxidant effect and scavenge free radicals. Various studies have showed that the use of cinnamon provided protection against the oxidative disorder by increasing antioxidants enzymes activities and lowering the human MDA levels.[43,44] Mitochondrial dysfunction, decline in ATP formation, free radical and oxidation generation can fasten the aging process. Usta et al. have proposed mitochondria as another potential target of the actions or toxicity of spices whereby deranging mitochondrial functions would lower ATP levels, which then may be effective on viability, aging process, and cell growth.[45] Treatment with C. zeylanicum 100 mg/30ml added to the tea daily in 54 healthy volunteers were significantly effective in decrease of lipid peroxidation and growing. Total antioxidant power (TAP) and total thiol molecules compared to the controls. The amount of increase in plasma TAP for the CZ group was significantly upper than in those given regular tea only.[46] There is very limited evidence that shows cinnamon and cinnamaldehyde have immunomodulatory effects and may suppress inflammatory processes.
Anti-amyloid properties
An aqueous extract of CZ is identified to inhibit tau aggregation and filament formation. In addition, it stimulates disassembly of recombinant tau filaments and changes the paired helical filaments morphology, isolated from brains of those with AD; however, it was not destructive to the normal cellular function of tau, as reported by Peterson et al. An A-linked proanthocyanidintrimer molecule derived from the CZ extract has revealed to contain a significant proportion of this inhibitory effect.[4] Furthermore, another study by binding of fluorescence-tagged Aβ1-42 showed that the Aβ1-42 deposits in AD or transgenic brain tissue might have a marginal affinity of ginger, cinnamon, and turmeric.[47]
Anti-insulin resistance effect
Accumulating studies demonstrate that insulin and insulin signaling mechanisms are essential for neuronal survival. A reduced expression of the insulin receptors and related members of the insulin signaling pathways has also been shown in patients and animals with impaired brain function and AD.[48] On the other hand, cinnamon extracts were shown to have anti diabetic effects as a number of studies demonstrated an insulin-like action. Furthermore, cinnamaldehyde promoted glucose uptake into skeletal muscle over glucose transporter 4 translocation.[49] Application of cinnamon therapy for patients with diabetes was investigated in several clinical trials and some studies demonstrated improvement of fasting blood glucose concentrations and insulin sensitivity; especially its insulin-like effects were presented in type 2 diabetic patients.[50]
Pepper
Piperine (1-piperoylpiperidine), a nitrogenous pungent substance, is the active principle of black pepper (Piper nigrum L.), long pepper (Piper longum L), and other piper species (family: Piperaceae). The piperine content is 3%–9% and 3%–5% (on dry weight basis) in P. nigrum and and P. longum, respectively.[51] In traditional Middle Eastern medicine, black pepper has been used as a nerve tonic. Recently, the analeptic properties of piperine, the main alkaloid phytochemical compound found in plants of the family Piperaceae, have been studied. Piperine has been used in folk medicine for treating various diseases such as cognitive deficit conditions including AD condition. One study demonstrates that receiving piperine in any dose can improve memory deficit. Furthermore, protective role of other subtypes of pepper family such as Piper methysticum, Piper submultinerve and piper betel against neurodegenerative disease has been evaluated.[52]
Mechanisms of effect
Anti-inflammatory effects
Piperine may facilitate nutrient absorption by alleviating inflammatory conditions at the site of absorption. Piper sub multi nerve as one of the piperaceae family, has been shown to protect neurons from Aβ1-42-induced neurotoxicity through its anti-apoptotic, anti-oxidative, and anti-inflammatory properties.[53]
Antioxidant properties
Several in vitro studies have shown the powerful antioxidant role of peperine. Reduction of oxidative stress is one of the most therapeutic effects of piperine found in the study utilizing the piperine in solid lipid nano formulation in an experimentally induced AD model.[54]
AChE inhibition activity
The role of pepper in inhibition of AChE has been investigated in several studies. At a dose between 5 and 20 mg/kg body weight given to cholinergic-deficient rats, piperine could attenuate the increase in lipid peroxidation and AChE activity. A phenolic compound derived from piper betel, administrated orally in a rat model of AD, significantly reversed the decline in AChE and glutathione activity induced by streptozotocin injection.[55]
Anti-amyloid properties
Another mechanism which makes pepper a potential herbal medicine against AD, is its role against amyloid-beta peptide. It has been reported that after intake of KSOP1009 (consisting of a ethanol extract of eight herbs including fructus of P. longum L) Aβ-induced memory impairment improved, and Aβ levels and plaque deposition suppressed in the brain of a transgenic mice to a rate similar to donepezil treatment. The appropriate dose of black pepper to use for ameliorative purposes depends on several factors including age, physical health, and several other conditions. However, piperine was failed to induce mutations in germ cells of mouse. Therefore, it can be concluded that piperine is a nongenotoxic chemical.[56]
The effect of P. nigrum L, used in Thai traditional rejuvenating and neurotonic remedies on activity of AChE, showed 50%–65% inhibitory activity on AChE.[57]
Tissue distribution profiles of three alkaloids from P. longum L. in rats have been shown that they could cross the blood–brain barrier (BBB). There is no human study evidence indicating that these alkaloids pass the BBB.[56,58]
Zingiber officinale
Zingiber, best known for its rhizome (ginger), is a valuable source of phytonutrients, and is characterized as having an aromatic aura and a pungent taste. The root of the ginger is the part of the plant that is harvested for food, beverage and medical industries. The flat surfaces of the rhizome are removed, leaving the remains of the underground stem. Gingerol and zingiberene are essential oils originated from ginger. It also contains pungent principles such as zingerone, gingerol and shogaol. Ginger is generally considered safe but a few studies about toxicological or safety data from clinical trials in humans are available.[59]
Mechanisms of effect
Lee et al. observed the protective role of pretreatment with gingerol against Aβ25-35-which results from its potentiality in inducing cytotoxicity and apoptotic cell death such as DNA fragmentation, elevated Bax/Bcl-2 ratio, and activation of caspase-3. Furthermore, gingerol effectively suppressed Aβ25-35-induced intracellular accumulation of reactive oxygen and/or nitrogen species and restored Aβ25-35. Up-regulation of mRNA and protein expression of antioxidant enzymes after treatment with gingerol is probable.[60] Another study designated to investigate the antioxidant activity of zingiber has been performed through using the Alpinia galanga (L.) Willd (Zingiberaceae) in the AD models (mice) and found that Na+/K+-ATPase and antioxidant activity increasing, depicts brain membrane integrity improvement and free radical scavenging property.[61] Moreover, it was found that increased habituation memory and decreased escape latency in behavioral characteristics are the indicative of the cognitive enhancement after treatment with A. galanga fractions and the AChE level reduction may improve cognition by enhancing cholinergic transmission.[62]
Inhibition of pro-inflammatory mediators IL-1β and inflammatory factors (nitric oxide [NO], prostaglandin E2 [PGE2], TNF-α) has been found as a probable therapeutic effect of ginger hexan extract. Suppression of NF-κB activation in aged rat founded as a probable mechanism for its properties against inflammation.[63] Furthermore, ginger is able to inhibit the activation of human monocytic THP-1 cells by different proinflammatory stimuli and reduction of the expression of a wide range of inflammatory-related genes in microglial-like cells.[64]
Ha et al. investigated the effects of 6-shogaol, on microglia activation. They found significant inhibition in releasing of NO and the expression of inducible nitric oxide synthase induced by LPS thus it suppressed the microglial activation induced by LPS both in primary cortical neuron-glia culture and in an in vivo neuroinflammatory model. Also, 6-Shogaol leads to anti-inflammatory effects by inhibiting the production of PGE2 and proinflammatory cytokines, such as IL-1β and TNF-α, and by down regulating cyclooxygenase-2, p38 mitogen-activated protein kinase, and NF-κB expression.[61] Presence of phytochemicals such as flavonoids, tannins, alkaloids, and terpenoids in ginger extracts lead to inhibitory effects on AChE activities in rat's brain (in vitro).[65,66] Another mechanisms of ginger and its extracts is to prevent lipid proxidation induced by Fe2+. This protective property could be attributed to their ability to scavenge free radicals thus preventing overstimulation of NMDA receptors.[67] Oxidative stress in the brain could be prevented by dietary intake of ginger varieties (red ginger and white ginger rhizomes) through inhibiting the MDA end-product of lipid peroxidation and the production of this aldehyde. Another part of the mechanisms through which the extractable phytochemicals in ginger (red and white) protect the brain may be through their antioxidant properties, Fe2+ chelating and OH• scavenging ability.[65]
CONCLUSIONS
Spices have been used for more than 2000 years; however, most of their biological activities have been examined recently. As the positive effects of natural agents against age related conditions including neurodegenerative diseases receive more research support, some of these nutraceuticals could be examined as novel ameliorative agents against AD. Clinical trials on these spices should further our understanding of their efficacy in the treatment and prevention of AD. There is a need for large clinical trials to examine the applications and side effects of these spices and their potential contribution to maintain our cognitive functions as we age.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sohrabi HR, Weinborn M, Badcock J, Bates KA, Clarnette R, Trivedi D, et al. New lexicon and criteria for the diagnosis of Alzheimer's disease. Lancet Neurol. 2011;10:299–300. doi: 10.1016/S1474-4422(11)70056-4. [DOI] [PubMed] [Google Scholar]
- 2.Markesbery WR. Neuropathological criteria for the diagnosis of Alzheimer's disease. Neurobiol Aging. 1997;18(4 Suppl):S13–9. doi: 10.1016/s0197-4580(97)00064-x. [DOI] [PubMed] [Google Scholar]
- 3.Ringman JM, Frautschy SA, Teng E, Begum AN, Bardens J, Beigi M, et al. Oral curcumin for Alzheimer's disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res Ther. 2012;4:43. doi: 10.1186/alzrt146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peterson DW, George RC, Scaramozzino F, LaPointe NE, Anderson RA, Graves DJ, et al. Cinnamon extract inhibits tau aggregation associated with Alzheimer's disease in vitro. J Alzheimers Dis. 2009;17:585–97. doi: 10.3233/JAD-2009-1083. [DOI] [PubMed] [Google Scholar]
- 5.Food labeling: Health claims; plant sterol/stanol esters and coronary heart disease. Food and Drug Administration, HHS. Interim final rule. Fed Regist. 2000;65:54686–739. [PubMed] [Google Scholar]
- 6.Shati AA, Elsaid FG, Hafez EE. Biochemical and molecular aspects of aluminium chloride-induced neurotoxicity in mice and the protective role of Crocus sativus L. extraction and honey syrup. Neuroscience. 2011;175:66–74. doi: 10.1016/j.neuroscience.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 7.Imenshahidi M, Razavi BM, Faal A, Gholampoor A, Mousavi SM, Hosseinzadeh H. The effect of chronic administration of saffron (Crocus sativus) stigma aqueous extract on systolic blood pressure in rats. Jundishapur J Nat Pharm Prod. 2013;8:175–9. doi: 10.17795/jjnpp-12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hausenblas HA, Saha D, Dubyak PJ, Anton SD. Saffron (Crocus sativus L.) and major depressive disorder: A meta-analysis of randomized clinical trials. J Integr Med. 2013;11:377–83. doi: 10.3736/jintegrmed2013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zarei Jaliani H, Riazi GH, Ghaffari SM, Karima O, Rahmani A. The effect of the Crocus sativus L. Carotenoid, crocin, on the polymerization of microtubules, in vitro. Iran J Basic Med Sci. 2013;16:101–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Moshiri E, Basti AA, Noorbala AA, Jamshidi AH, Hesameddin Abbasi S, Akhondzadeh S. Crocus sativus L. (petal) in the treatment of mild-to-moderate depression: A double-blind, randomized and placebo-controlled trial. Phytomedicine. 2006;13:607–11. doi: 10.1016/j.phymed.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Akhondzadeh S, Sabet MS, Harirchian MH, Togha M, Cheraghmakani H, Razeghi S, et al. Saffron in the treatment of patients with mild to moderate Alzheimer's disease: A 16-week, randomized and placebo-controlled trial. J Clin Pharm Ther. 2010;35:581–8. doi: 10.1111/j.1365-2710.2009.01133.x. [DOI] [PubMed] [Google Scholar]
- 12.Khalili M, Hamzeh F. Effects of active constituents of Crocus sativus L. crocin on streptozocin-induced model of sporadic Alzheimer's disease in male rats. Iran Biomed J. 2010;14:59–65. [PMC free article] [PubMed] [Google Scholar]
- 13.Pitsikas N, Zisopoulou S, Tarantilis PA, Kanakis CD, Polissiou MG, Sakellaridis N. Effects of the active constituents of Crocus sativus L. crocins on recognition and spatial rats’ memory. Behav Brain Res. 2007;183:141–6. doi: 10.1016/j.bbr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Naghizadeh B, Mansouri MT, Ghorbanzadeh B, Farbood Y, Sarkaki A. Protective effects of oral crocin against intracerebroventricular streptozotocin-induced spatial memory deficit and oxidative stress in rats. Phytomedicine. 2013;20:537–42. doi: 10.1016/j.phymed.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Papandreou MA, Kanakis CD, Polissiou MG, Efthimiopoulos S, Cordopatis P, Margarity M, et al. Inhibitory activity on amyloid-beta aggregation and antioxidant properties of Crocus sativus stigmas extract and its crocin constituents. J Agric Food Chem. 2006;54:8762–8. doi: 10.1021/jf061932a. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad AS, Ansari MA, Ahmad M, Saleem S, Yousuf S, Hoda MN, et al. Neuroprotection by crocetin in a hemi-parkinsonian rat model. Pharmacol Biochem Behav. 2005;81:805–13. doi: 10.1016/j.pbb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Geromichalos GD, Lamari FN, Papandreou MA, Trafalis DT, Margarity M, Papageorgiou A, et al. Saffron as a source of novel acetylcholinesterase inhibitors: Molecular docking and in vitro enzymatic studies. J Agric Food Chem. 2012;60:6131–8. doi: 10.1021/jf300589c. [DOI] [PubMed] [Google Scholar]
- 18.Nam KN, Park YM, Jung HJ, Lee JY, Min BD, Park SU, et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol. 2010;648:110–6. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Farokhnia M, Shafiee Sabet M, Iranpour N, Gougol A, Yekehtaz H, Alimardani R, et al. Comparing the efficacy and safety of Crocus sativus L. with memantine in patients with moderate to severe Alzheimer's disease: A double-blind randomized clinical trial. Hum Psychopharmacol. 2014;29:351–9. doi: 10.1002/hup.2412. [DOI] [PubMed] [Google Scholar]
- 20.Linardaki ZI, Orkoula MG, Kokkosis AG, Lamari FN, Margarity M. Investigation of the neuroprotective action of saffron (Crocus sativus L.) in aluminum-exposed adult mice through behavioral and neurobiochemical assessment. Food Chem Toxicol. 2013;52:163–70. doi: 10.1016/j.fct.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: From kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Anand P, Murali KY, Tandon V, Murthy PS, Chandra R. Insulinotropic effect of cinnamaldehyde on transcriptional regulation of pyruvate kinase, phosphoenolpyruvate carboxykinase, and GLUT4 translocation in experimental diabetic rats. Chem Biol Interact. 2010;186:72–81. doi: 10.1016/j.cbi.2010.03.044. [DOI] [PubMed] [Google Scholar]
- 23.Mishra S, Palanivelu K. The effect of curcumin (turmeric) on Alzheimer's disease: An overview. Ann Indian Acad Neurol. 2008;11:13–9. doi: 10.4103/0972-2327.40220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strimpakos AS, Sharma RA. Curcumin: Preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal. 2008;10:511–45. doi: 10.1089/ars.2007.1769. [DOI] [PubMed] [Google Scholar]
- 25.Venkatesan N, Punithavathi D, Arumugam V. Curcumin prevents adriamycin nephrotoxicity in rats. Br J Pharmacol. 2000;129:231–4. doi: 10.1038/sj.bjp.0703067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Demerdash FM, Yousef MI, Radwan FM. Ameliorating effect of curcumin on sodium arsenite-induced oxidative damage and lipid peroxidation in different rat organs. Food Chem Toxicol. 2009;47:249–54. doi: 10.1016/j.fct.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Dairam A, Limson JL, Watkins GM, Antunes E, Daya S. Curcuminoids, curcumin, and demethoxycurcumin reduce lead-induced memory deficits in male Wistar rats. J Agric Food Chem. 2007;55:1039–44. doi: 10.1021/jf063446t. [DOI] [PubMed] [Google Scholar]
- 28.Park SY, Kim HS, Cho EK, Kwon BY, Phark S, Hwang KW, et al. Curcumin protected PC12 cells against beta-amyloid-induced toxicity through the inhibition of oxidative damage and tau hyperphosphorylation. Food Chem Toxicol. 2008;46:2881–7. doi: 10.1016/j.fct.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 29.Venigalla M, Sonego S, Gyengesi E, Sharman MJ, Münch G. Novel promising therapeutics against chronic neuroinflammation and neurodegeneration in Alzheimer's disease. Neurochem Int. 2016;95:63–74. doi: 10.1016/j.neuint.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Kim H, Park BS, Lee KG, Choi CY, Jang SS, Kim YH, et al. Effects of naturally occurring compounds on fibril formation and oxidative stress of beta-amyloid. J Agric Food Chem. 2005;53:8537–41. doi: 10.1021/jf051985c. [DOI] [PubMed] [Google Scholar]
- 31.Yanagisawa D, Taguchi H, Yamamoto A, Shirai N, Hirao K, Tooyama I. Curcuminoid binds to amyloid-ß1-42 oligomer and fibril. J Alzheimers Dis. 2011;24(Suppl 2):33–42. doi: 10.3233/JAD-2011-102100. [DOI] [PubMed] [Google Scholar]
- 32.Ringman JM, Frautschy SA, Cole GM, Masterman DL, Cummings JL. A potential role of the curry spice curcumin in Alzheimer's disease. Curr Alzheimer Res. 2005;2:131–6. doi: 10.2174/1567205053585882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed HH, Salem AM, Sabry GM, Husein AA, Kotob SE. Possible therapeutic uses of Salvia triloba and Piper nigrum in Alzheimer's disease-induced rats. J Med Food. 2013;16:437–46. doi: 10.1089/jmf.2012.0165. [DOI] [PubMed] [Google Scholar]
- 34.Puglielli L, Tanzi RE, Kovacs DM. Alzheimer's disease: The cholesterol connection. Nat Neurosci. 2003;6:345–51. doi: 10.1038/nn0403-345. [DOI] [PubMed] [Google Scholar]
- 35.Canevari L, Clark JB. Alzheimer's disease and cholesterol: The fat connection. Neurochem Res. 2007;32:739–50. doi: 10.1007/s11064-006-9200-1. [DOI] [PubMed] [Google Scholar]
- 36.Baum L, Lam CW, Cheung SK, Kwok T, Lui V, Tsoh J, et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol. 2008;28:110–3. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- 37.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–54. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 38.Gupta B, Kulshrestha VK, Srivastava RK, Prasad DN. Mechanisms of curcumin induced gastric ulcer in rats. Indian J Med Res. 1980;71:806–14. [PubMed] [Google Scholar]
- 39.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–6. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 40.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–900. [PubMed] [Google Scholar]
- 41.Jayaprakasha GK, Rao LJ. Chemistry, biogenesis, and biological activities of Cinnamomum zeylanicum. Crit Rev Food Sci Nutr. 2011;51:547–62. doi: 10.1080/10408391003699550. [DOI] [PubMed] [Google Scholar]
- 42.Rao PV, Gan SH. Cinnamon: A multifaceted medicinal plant. Evid Based Complement Alternat Med 2014. 2014:642942. doi: 10.1155/2014/642942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moselhy SS, Ali HK. Hepatoprotective effect of cinnamon extracts against carbon tetrachloride induced oxidative stress and liver injury in rats. Biol Res. 2009;42:93–8. [PubMed] [Google Scholar]
- 44.Amin KA, Abd El-Twab TM. Oxidative markers, nitric oxide and homocysteine alteration in hypercholesterolimic rats: Role of atorvastatine and cinnamon. Int J Clin Exp Med. 2009;2:254–65. [PMC free article] [PubMed] [Google Scholar]
- 45.Usta J, Kreydiyyeh S, Bajakian K, Nakkash-Chmaisse H. In vitro effect of eugenol and cinnamaldehyde on membrane potential and respiratory chain complexes in isolated rat liver mitochondria. Food Chem Toxicol. 2002;40:935–40. doi: 10.1016/s0278-6915(02)00071-6. [DOI] [PubMed] [Google Scholar]
- 46.Ranasinghe P, Pigera S, Premakumara GA, Galappaththy P, Constantine GR, Katulanda P. Medicinal properties of ‘true’ cinnamon (Cinnamomum zeylanicum): A systematic review. BMC Complement Altern Med. 2013;13:275. doi: 10.1186/1472-6882-13-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo JP, Yu S, McGeer PL. Simple in vitro assays to identify amyloid-beta aggregation blockers for Alzheimer's disease therapy. J Alzheimers Dis. 2010;19:1359–70. doi: 10.3233/JAD-2010-1331. [DOI] [PubMed] [Google Scholar]
- 48.Hoyer S, Lee SK, Löffler T, Schliebs R. Inhibition of the neuronal insulin receptor. An in vivo model for sporadic Alzheimer disease? Ann N Y Acad Sci. 2000;920:256–8. doi: 10.1111/j.1749-6632.2000.tb06932.x. [DOI] [PubMed] [Google Scholar]
- 49.Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol. 2008;76:1590–611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 50.Wang JG, Anderson RA, Graham GM, 3rd, Chu MC, Sauer MV, Guarnaccia MM, et al. The effect of cinnamon extract on insulin resistance parameters in polycystic ovary syndrome: A pilot study. Fertil Steril. 2007;88:240–3. doi: 10.1016/j.fertnstert.2006.11.082. [DOI] [PubMed] [Google Scholar]
- 51.Cao X, Ye X, Lu Y, Yu Y, Mo W. Ionic liquid-based ultrasonic-assisted extraction of piperine from white pepper. Anal Chim Acta. 2009;640:47–51. doi: 10.1016/j.aca.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 52.Balasubramanian S, Roselin P, Singh KK, Zachariah J, Saxena SN. Postharvest processing and benefits of black pepper, coriander, cinnamon, fenugreek, and turmeric spices. Crit Rev Food Sci Nutr. 2016;56:1585–607. doi: 10.1080/10408398.2012.759901. [DOI] [PubMed] [Google Scholar]
- 53.Thangnipon W, Puangmalai N, Chinchalongporn V, Jantrachotechatchawan C, Kitiyanant N, Soi-Ampornkul R, et al. N-benzylcinnamide protects rat cultured cortical neurons from ß-amyloid peptide-induced neurotoxicity. Neurosci Lett. 2013;556:20–5. doi: 10.1016/j.neulet.2013.09.071. [DOI] [PubMed] [Google Scholar]
- 54.Yusuf M, Khan M, Khan RA, Ahmed B. Preparation, characterization, in vivo and biochemical evaluation of brain targeted Piperine solid lipid nanoparticles in an experimentally induced Alzheimer's disease model. Journal of Drug Targeting. 2013;21:300–11. doi: 10.3109/1061186X.2012.747529. [DOI] [PubMed] [Google Scholar]
- 55.Pandey A, Bani S. Hydroxychavicol inhibits immune responses to mitigate cognitive dysfunction in rats. J Neuroimmunol. 2010;226:48–58. doi: 10.1016/j.jneuroim.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 56.Li S, Wang C, Li W, Koike K, Nikaido T, Wang MW. Antidepressant-like effects of piperine and its derivative, antiepilepsirine. J Asian Nat Prod Res. 2007;9:421–30. doi: 10.1080/10286020500384302. [DOI] [PubMed] [Google Scholar]
- 57.Ingkaninan K, Temkitthawon P, Chuenchom K, Yuyaem T, Thongnoi W. Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies. J Ethnopharmacol. 2003;89:261–4. doi: 10.1016/j.jep.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 58.Ali SK, Hamed AR, Soltan MM, Hegazy UM, Elgorashi EE, El-Garf IA, et al. In-vitro evaluation of selected Egyptian traditional herbal medicines for treatment of Alzheimer disease. BMC Complement Altern Med. 2013;13:121. doi: 10.1186/1472-6882-13-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Portnoi G, Chng LA, Karimi-Tabesh L, Koren G, Tan MP, Einarson A. Prospective comparative study of the safety and effectiveness of ginger for the treatment of nausea and vomiting in pregnancy. Am J Obstet Gynecol. 2003;189:1374–7. doi: 10.1067/s0002-9378(03)00649-5. [DOI] [PubMed] [Google Scholar]
- 60.Lee C, Park GH, Kim CY, Jang JH. [6]-Gingerol attenuates ß-amyloid-induced oxidative cell death via fortifying cellular antioxidant defense system. Food Chem Toxicol. 2011;49:1261–9. doi: 10.1016/j.fct.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 61.Ha SK, Moon E, Ju MS, Kim DH, Ryu JH, Oh MS, et al. 6-Shogaol, a ginger product, modulates neuroinflammation: A new approach to neuroprotection. Neuropharmacology. 2012;63:211–23. doi: 10.1016/j.neuropharm.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 62.Hanish Singh JC, Alagarsamy V, Diwan PV, Sathesh Kumar S, Nisha JC, Narsimha Reddy Y. Neuroprotective effect of Alpinia galanga (L.) fractions on Aß(25-35) induced amnesia in mice. J Ethnopharmacol. 2011;138:85–91. doi: 10.1016/j.jep.2011.08.048. [DOI] [PubMed] [Google Scholar]
- 63.Jung HW, Yoon CH, Park KM, Han HS, Park YK. Hexane fraction of Zingiberis Rhizoma Crudus extract inhibits the production of nitric oxide and proinflammatory cytokines in LPS-stimulated BV2 microglial cells via the NF-kappaB pathway. Food Chem Toxicol. 2009;47:1190–7. doi: 10.1016/j.fct.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 64.Ghayur MN, Gilani AH, Ahmed T, Khalid A, Nawaz SA, Agbedahunsi JM, et al. Muscarinic, Ca(++) antagonist and specific butyrylcholinesterase inhibitory activity of dried ginger extract might explain its use in dementia. J Pharm Pharmacol. 2008;60:1375–83. doi: 10.1211/jpp/60.10.0014. [DOI] [PubMed] [Google Scholar]
- 65.Oboh G, Ademiluyi AO, Akinyemi AJ. Inhibition of acetylcholinesterase activities and some pro-oxidant induced lipid peroxidation in rat brain by two varieties of ginger (Zingiber officinale) Exp Toxicol Pathol. 2012;64:315–9. doi: 10.1016/j.etp.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 66.Mathew M, Subramanian S. In vitro evaluation of anti-Alzheimer effects of dry ginger (Zingiber officinale Roscoe) extract. Indian J Exp Biol. 2014;52:606–12. [PubMed] [Google Scholar]
- 67.Bellé NA, Dalmolin GD, Fonini G, Rubin MA, Rocha JB. Polyamines reduces lipid peroxidation induced by different pro-oxidant agents. Brain Res. 2004;1008:245–51. doi: 10.1016/j.brainres.2004.02.036. [DOI] [PubMed] [Google Scholar]


