Abstract
Papillary thyroid carcinoma (PTC) is the most common thyroid malignancy, accounting for 70%–80% of all thyroid malignancies. It is biologically indolent and has an excellent prognosis. Variations in histopathologic patterns are known to influence prognosis and often result in a diagnostic dilemma. We report an unusual case of a 35-year-old female with papillary carcinoma of a thyroid isthmus showing anastomosing channels on histopathology, a distinctive pattern that has not been described in PTC. Similar to tumor cells, the lining cells of these channels were also positive for thyroid transcription factor 1, thyroglobulin, and cytokeratin-19 and negative for CD34 and CD31. The diagnosis of PTC should rely on nuclear morphology rather than architecture. Pathologists should be aware of different variants because some of these variants show aggressive behavior and poor outcome. The present report highlights the distinctive pattern of PTC, recognition of which is important to avoid any diagnostic pitfall.
Keywords: Anastomosing channels, papillary carcinoma, thyroid carcinoma
Introduction
Papillary thyroid carcinoma (PTC) is the most common thyroid malignancy, accounting for 70%–80% of all thyroid malignancies. It typically occurs in the middle age with a peak incidence in the third and fourth decades of life. It is the predominant thyroid cancer in children and individuals exposed to external radiation.[1] The prognosis of this tumor is strongly associated with various clinical variables such as the age of the patient, tumor size, and histologic parameters such as extracapsular extension, extrathyroidal extension, lymph node invasion, distant metastasis, and histologic variants.[2,3,4] We report an unusual case of a 35-year-old female with papillary carcinoma of thyroid isthmus showing anastomosing channels on histomorphology. The nature of these channels was confirmed by positivity for cytokeratin 19 (CK19) and negativity for CD34. We report the present case for this distinctive pattern that has not been described in PTC before.
Case Report
A 35-year-old female presented with swelling in midline neck. On examination, the swelling moved with deglutition but not on the protrusion of the tongue. It had well-defined contours, firm consistency and measured 6 cm × 4 cm. Contrast-enhanced computed tomography (CT) scan revealed a predominantly cystic, well-defined mass arising from isthmus with calcific foci and irregular enhancing component [Figure 1a].
Figure 1.
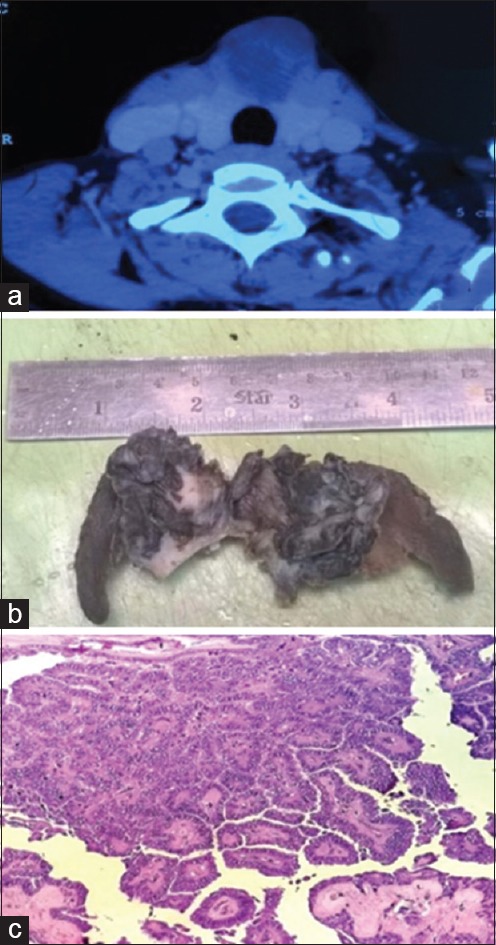
(a) Contrast-enhanced computed tomography - Predominantly cystic well-defined mass in isthmus with calcific foci and irregular enhancing component. (b) Grossly cystic mass in the isthmus with focal solid areas. (c) Sections from isthmus mass showed papillary carcinoma thyroid (H and E, ×200)
Peroperatively, a solid cystic lesion was identified in midline neck with thinning of the overlying skin. The lesion was densely adhered to both the lobes of the thyroid. Complete thyroidectomy was thus done, and the specimen sent for morphologic examination. Gross examination revealed a cystic mass in the isthmus measuring 4 cm × 4.5 cm × 3.5 cm. Focal solid areas were also seen within the mass [Figure 1b]. Both the thyroid lobes were unremarkable. Histopathologic examination of the lobes of thyroid revealed features of chronic lymphocytic thyroiditis. Sections from isthmus mass showed a tumor with features of papillary carcinoma with an intact capsule [Figure 1c]. Several foci of anastomosing channels lined by atypical cells with occasional blood in some were noted [Figure 2a]. Possibilities of vascular invasion and a coexisting angiosarcoma were kept in mind for these channels. This was followed by immunohistochemistry to establish the nature of the atypical lining cells. These cells exhibited positivity for thyroid transcription factor 1 (TTF1), thyroglobulin (TG) and CK19 [Figures 2b and 3a, b], and negativity for CD34 and CD31. This highlighted the thyroid origin of the lining cells and ruled out vascular invasion or angiosarcoma. Therefore, a final diagnosis of papillary carcinoma thyroid isthmus with a distinctive pattern of anastomosing channels was made. On 6 months follow-up, the patient is doing well.
Figure 2.
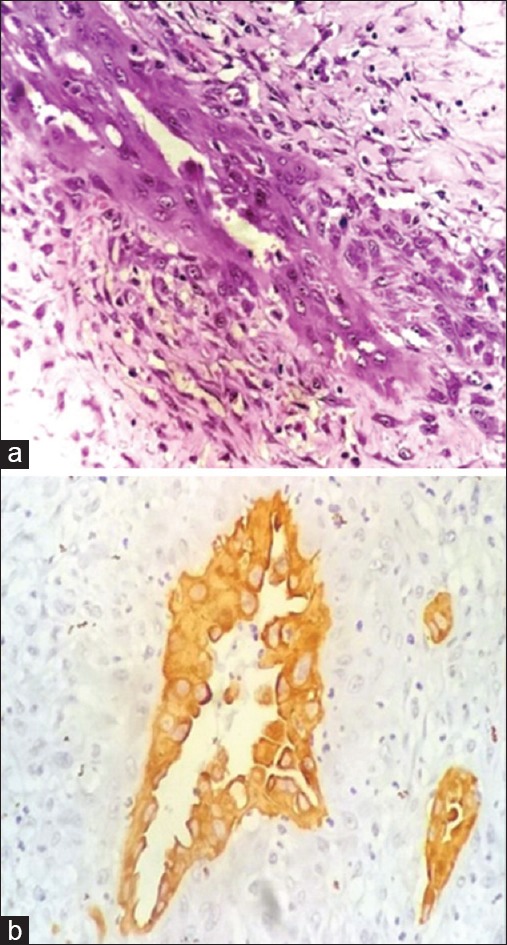
(a) Tumor showed foci of anastomosing channels lined by atypical cells. (b) Cytokeratin 19 positivity in the cells lining the anastomosing channels (×400)
Figure 3.
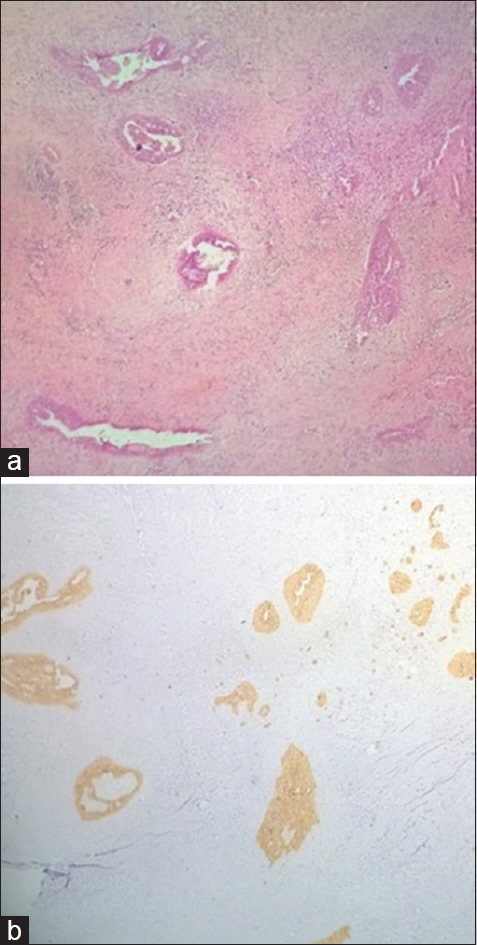
(a) Tumor showed several similar foci (H and E, ×40). (b) Cytokeratin 19 positivity in these foci (×40)
Discussion
PTC is the most common type of malignant thyroid tumor accounting for more than 70% thyroid malignancies. The incidence of PTC has been on a rise worldwide for the past few decades. Environmental, genetic and hormonal factors, as well as radiation, play a role in the pathogenesis of PTC.[1,3,4]
Imaging of the neck of a patient with PTC is done for visualization of both thyroids as well as regional lymph nodes to detect metastases. Patients with PTC usually present with a cold nodule on radioactive iodine scan.[1,2] A few patients may present with cervical lymphadenopathy. These lymph node metastases have a tendency to cavitate and turn cystic with occasional septations, calcification, and thick walls.[5] The CT scan in the present case revealed similar findings in the thyroid isthmus pointing toward a malignant etiology.
Papillary carcinoma has varied gross appearance. It may be solid or cystic with papillary excrescences in the classic variant. Solid nodules usually have a tan color and a firm consistency, oncocytic variant exhibits mahogany color. The borders of the nodule may be infiltrative or well circumscribed, with or without a capsule, and occasional sclerosis and calcification. Focal degeneration such as cystic change, hemorrhage, and necrosis may occur spontaneously or owing to previous fine-needle aspiration.[6] In the present case, the tumor was located in the isthmus and was predominantly cystic macroscopically. Literature reports that PTC located in the isthmus is more likely to be associated with multiple foci in bilateral lobes and higher rates of capsular invasion than tumors in other regions of the thyroid gland.[7]
The diagnosis of papillary carcinoma is based on the nuclear morphology of a thyroid neoplasm.[1,6] The architectural spectrum is, however, wide encompassing patterns such as follicular, insular, columnar, tall cell, diffuse sclerosing type, and cribriform-morular.[1] The tall cell variant makes up to 10% of papillary cancers. The tall cells are twice as tall as are wide, and should represent 50% of the tumor. This variant is usually seen in the elderly and is associated with a less favorable prognosis. The diffuse sclerosis variant accounts for 3% of papillary cancers and is commonly seen in children and young adults. These lesions are typically associated with regional nodal metastases, worse prognosis and decreased disease-free survival. When the solid pattern occupies more than 50% of the tumor, a diagnosis of solid variant is made. This variant has a propensity for both lymphatic and venous invasion. The follicular variant is composed of follicles of varying size. The diagnosis of this entity often creates a dilemma because of an overlap with follicular carcinoma. The prognosis of this entity is similar to that of classical PTC.[8]
Our case exhibited features of classical papillary carcinoma with areas of numerous anastomosing channels lined by plump tumor cells with the presence of blood in some. The morphologic picture of angiosarcoma reveals extensive areas of necrosis, hemorrhage with the presence of anastomosing channels lined by endothelial cells. Coexistence of angiosarcoma with papillary carcinoma has been reported in literature, so this diagnosis was kept a possibility in the present case.[8,9,10] The endothelial differentiation can be demonstrated by the expression of vascular markers such as CD31 (the most sensitive and specific marker), CD34 and factor VIII-related antigen.[8] However, immunohistochemical positivity for TTF1, TG, and CK19 and negativity for CD34 and CD31 highlighted the thyroid origin of the lining cells thus ruling out angiosarcoma in our case. The possibility of vascular invasion was ruled out on histomorphology as these channels were present within the tumor; the lining cells were atypical and negative for vascular markers. Other markers that can be used to demonstrate PTC include HBME1 (Hector Battifora mesothelial - 1) and galectin-3. Molecular markers such as RET/PTC, NTRK1, RAS, and BRAF mutations also play an important role in understanding the pathogenesis and the clinicopathologic behavior of this tumor.[1,4,6]
The management of PTC is variable and depends on the characteristics of each tumor. Total thyroidectomy is associated with better patient outcome as compared to lobectomy. Radioactive adjuvant therapy is used to treat microscopic foci and metastases and detect early recurrences. Some variants of PTC such as tall cell and columnar type are associated with poor prognosis.[1,4] Distant metastasis of PTC are rare and usually occur in advanced stages of the disease, especially in lung, bone, lymph nodes of the chest, pancreas, and breast.[4]
To summarize, the diagnosis of PTC should rely on nuclear morphology rather than architecture. Pathologists should be aware of different variants because some of these variants show aggressive behavior and poor outcome. Ancillary studies, including immunohistochemical stains and molecular detection of gene rearrangements and point mutation, are of value in elusive cases. Because this is the first case reporting the presence of anastomosing channels in PTC, the prognostic significance of this finding still remains to be elucidated.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Tallini G, Rosai J. Rosai and Ackerman's Surgical Pathology. 10th ed. Missouri: Elsevier; 2011. Thyroid gland; pp. 2077–8. [Google Scholar]
- 2.Lloyd RV, Buehler D, Khanafshar E. Papillary thyroid carcinoma variants. Head Neck Pathol. 2011;5:51–6. doi: 10.1007/s12105-010-0236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin JD, Hsueh C, Huang BY. Papillary thyroid carcinoma with different histological patterns. Chang Gung Med J. 2011;34:23–34. [PubMed] [Google Scholar]
- 4.Gonzalez-Gonzalez R, Bologna-Molina R, Carreon-Burciaga RG, Gómezpalacio-Gastelum M, Molina-Frechero N, Salazar-Rodríguez S. Papillary thyroid carcinoma: Differential diagnosis and prognostic values of its different variants: Review of the literature. ISRN Oncol 2011. 2011:915925. doi: 10.5402/2011/915925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wunderbaldinger P, Harisinghani MG, Hahn PF, Daniels GH, Turetschek K, Simeone J, et al. Cystic lymph node metastases in papillary thyroid carcinoma. AJR Am J Roentgenol. 2002;178:693–7. doi: 10.2214/ajr.178.3.1780693. [DOI] [PubMed] [Google Scholar]
- 6.Al-Brahim N, Asa SL. Papillary thyroid carcinoma: An overview. Arch Pathol Lab Med. 2006;130:1057–62. doi: 10.5858/2006-130-1057-PTCAO. [DOI] [PubMed] [Google Scholar]
- 7.Lee YS, Jeong JJ, Nam KH, Chung WY, Chang HS, Park CS. Papillary carcinoma located in the thyroid isthmus. World J Surg. 2010;34:36–9. doi: 10.1007/s00268-009-0298-6. [DOI] [PubMed] [Google Scholar]
- 8.LiVolsi VA. Papillary thyroid carcinoma: An update. Mod Pathol. 2011;24(Suppl 2):S1–9. doi: 10.1038/modpathol.2010.129. [DOI] [PubMed] [Google Scholar]
- 9.Kefeli M, Mete O. An unusual malignant thyroid nodule: Coexistence of epithelioid angiosarcoma and follicular variant papillary thyroid carcinoma. Endocr Pathol. 2014;25:350–2. doi: 10.1007/s12022-013-9243-1. [DOI] [PubMed] [Google Scholar]
- 10.Couto J, Martins RG, Santos AP, Matos J, Torres I. Invasive thyroid angiosarcoma with a favorable outcome. Int J Endocrinol Metab. 2014;12:e15806. doi: 10.5812/ijem.15806. [DOI] [PMC free article] [PubMed] [Google Scholar]


