Abstract
BACKGROUND AND OBJECTIVES:
Conventional tube technique (CTT) has been the mainstay for antibody detection in pretransfusion testing. There have been rapid technological advances in blood banking and methodology of crossmatch has been modified to improve the sensitivity of these tests and to enable automation. This study was done to compare the efficacy of three crossmatch techniques: CTT, tube low-ionic-strength-saline indirect antiglobulin test (tube LISS-IAT), and micro column technology (MCT) used in the blood bank serology laboratory.
MATERIALS AND METHODS:
In this prospective study, 150 samples from patients who had received two or more transfusions on two different occasions (with at least 72 h between two transfusions) were subjected to cross match by three different techniques – CTT, LISS-IAT, and MCT.
RESULTS:
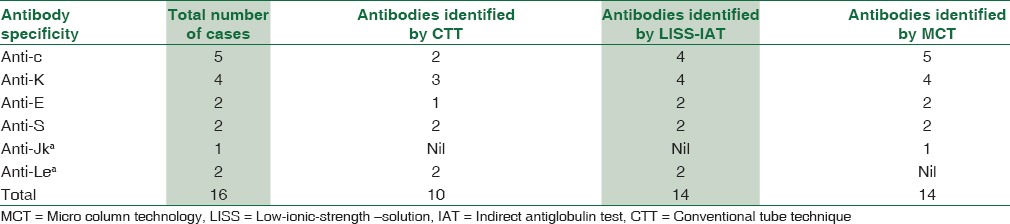
A total of 16 cases with antibodies were identified in 150 patients. Out of 16 cases, 14 were clinically significant (anti-c = 5, anti-K = 4, anti-E = 2, anti-S = 2, anti-Jka = 1) and 2 nonclinically significant antibody cases (anti-Lea). MCT detected all the 14 clinically significant antibody cases and no case of nonclinically significant antibody. Tube LISS-IAT detected 14 antibody cases including 2 cases of non-clinically significant antibody but failed to detect 1 case of anti-c and the only case of anti-Jka. CTT detected only 10 antibody cases including 2 cases of non-clinically significant antibody and but failed to detect 3 cases of anti-c, 1 case of anti-K, 1 case of anti-E, and the only case of anti-Jka.
CONCLUSION:
MCT was found to be most efficacious when compared to CTT and tube LISS-IAT in detecting clinically significant red cell antibodies; although MCT missed 2 cases of Lea antibody which were detected by CTT and LISS-IAT.
Keywords: Crossmatch, pretransfusion testing, antibody
Introduction
Crossmatch is an integral part of routine pretransfusion testing. It is done to prevent the incompatible red cell transfusions which may result in immune-mediated hemolytic transfusion reaction.[1] It ensures that transfused cells have an acceptable survival rate as well as there is no significant destruction of recipient's own red blood cells.[2] Conventional tube technique (CTT) has been the mainstay for antibody detection in pretransfusion testing for over 30 years.[3] Although this technique is believed to be the gold standard, it has got its own limitations.[4] The end-points of the reaction are unstable; reading and grading require a high level of expertise leading to interobserver variation.[5] In the last few decades, there has been rapid technological advancement in blood banking. In 1976, Low-ionic-strength–solution (LISS) based additives and tube LISS indirect antiglobulin test (tube LISS-IAT) was introduced which significantly increased sensitivity for antibody detection in a shorter duration of time.[6] In 1990's, the microcolumn technology (MCT) was introduced by Lapierre. MCT has an objective reading phase; its results are standardized and reproducible. The lack of washing phase in MCT decreases the potential for false weak or negative reactions and makes it ideal for automation. However, the incidence of false positives is more with MCT when compared to conventional tube methods.[7,8,9] With the aim to improve the efficiency, different laboratories select methods tailored to meet their needs. There are conflicting data in the literature about the relative sensitivities of various techniques being used for the serological crossmatch and in detection of clinically significant antibodies. This present study has been designed to compare the efficacy of three crossmatch techniques (CTT, LISS-IAT, and MCT) used in the blood bank serology laboratory.
Materials and Methods
This was a prospective study which was conducted in a tertiary care hospital from January 2011 to September 2012 after approval by the Institutional Ethics Committee. During the study period, we received request for cross match of 150 samples from patients who had received two or more transfusions on two different occasions (with at least 72 h between two transfusions). Detailed transfusion history, any relevant medical, surgical, and obstetric history was recorded. Blood grouping was performed using standard tube technique. Crossmatch was performed by – CTT, LISS-IAT, MCT.
Test procedures
Conventional tube technique-indirect antiglobulin test
Standard protocol for performing crossmatch by CTT was followed as per DGHS technical manual.[10] The grading system of CTT reactions was followed according to the American Association of Blood Banking [Table 1].[11]
Table 1.
Grading of agglutination reaction in conventional tube technique
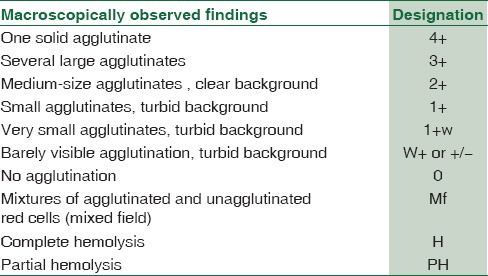
Tube low-ionic strength solution additive-indirect antiglobulin test
Standard procedure for the preparation of LISS was followed as per DGHS technical manual.[10] To prepare 1 L of LISS, 18 g of glycine was dissolved in 500 ml of distilled water. Phosphate buffer (0.15 M) pH 6.7 was prepared by adding 0.15 M NaH2PO4.2H2O (23.4 g/L) to 25 ml of 0.15 M Na2HPO4 (21.3 g/L). A volume of 20 ml of phosphate buffer (0.15 M) pH 6.7 was added to the above-prepared glycine solution. 1.79 g of NaCl dissolved in 100 ml of distilled water was added to this solution. The solution was made up to 1 L with distilled water. The LISS prepared was stored at 4°C. The standard procedure for performing LISS-IAT was followed as per DGHS technical manual.[10] The reaction strength was graded in the same way as CTT.[11]
Micro column technology
The Diamed-ID Microtyping Gel System (EU Patent 0305337) was used for cross matching. The tests using MCT were performed in accordance with the manufacturer's instructions. The reaction in gel microtube was graded with naked eye.[10] Depending on the intensity of the reaction, erythrocytes penetrate the gel to varying degrees and reactions are graded as either 4+ (reaction is represented by a solid band of agglutinated red cells at the top of the gel column)/3 + (reaction is represented by a predominant amount of agglutinated red cells toward the top of the gel column with a few agglutinates staggered below the thicker band)/2 + (reaction is characterized by red cell agglutinates dispersed throughout the gel column with few agglutinates at the bottom of the microtube)/1 + (reaction is characterized by red cell agglutinates predominantly observed in the lower half of the gel column with red cells also at the bottom)/weak + (few agglutinates remaining in the gel area just above the red cell pellet at the bottom of the microtube) or negative (red cells forming a well-delineated pellet in the bottom of the microtube). Mixed field reaction is recognized as a layer of red cell agglutinates at the top of the gel accompanied by pellet of unagglutinated cells at the bottom of the microtube.
Samples showing positive result by any of the technique were further evaluated using antibody screen (ID-DiaCell I-II-III, DiaMed GmbH 1785 Cressier, Switzerland) and identification (ID-DiaCell Panel, DiaMed GmbH 1785 Cressier, Switzerland) by gel technique (ID-Micro Typing System, DiaMed AG 1785 Cressier, Switzerland) on IgG + C3d LISS/Coombs cards (DiaMed GmbH 1785 Cressier, Switzerland). The strength of agglutination reaction observed with three techniques was compared to determine the efficacy of these techniques.
Statistical analysis
The statistical analysis was carried out using Statistical Package for Social Sciences (SPSS version 15.0 for Windows Inc., Chicago, IL, USA). For age, we calculated mean and standard deviation. Categorical variables were described as frequencies and percentages. Proportions were compared using Chi-square or Fisher's exact test whichever was applicable. To see the agreement between two methods, Kappa test of agreement was applied. All statistical tests were two-sided and performed at a significance level of α = 0.05.
Results
This prospective study included 150 patients out of which 86 patients were male and 64 were female. Study group included multitransfused patients, i.e., thalassemic children, patients with chronic renal failure, radiotherapy patients, patients admitted to ICU and miscellaneous group [Figure 1]. The mean age was 29.73 years with standard deviation of 22.30 years. A total of 16 cases with antibodies were identified in 150 patients. Over all incidence of alloimmunization was 10.6%. Antibody specificity did not show any statistically significant association with sex and age of the patient. Out of 16 cases, 14 were clinically significant (anti-c = 5, anti-K = 4, anti-E = 2, anti-S = 2, anti-Jka = 1) and 2 nonclinically significant antibody cases (anti-Lea). MCT detected all the 14 clinically significant antibody cases and no case of nonclinically significant antibody. Tube LISS-IAT detected 14 antibody cases including 2 cases of non-clinically significant antibody but failed to detect 1 case of anti-c and the only case of anti-Jkaa. CTT detected only 10 antibody cases including 2 cases of nonclinically significant antibody and but failed to detect 3 cases of anti-c, 1 case of anti-K, 1 case of anti-E, and the only case of anti-Jka [Table 2].
Figure 1.
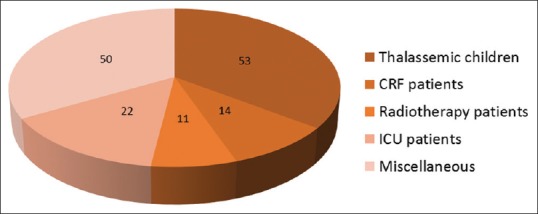
Distribution of patients according to groups in our study
Table 2.
Antibodies identified by different crossmatch techniques
Discussion
Crossmatch in transfusion medicine is a complex testing which is performed before blood transfusion for the detection of red cell antibodies. Its main aim is to detect maximum number of clinically significant antibodies, keeping clinically insignificant antibodies to minimum in a timely manner.[2] Red cell antibodies play an important role in perinatal immunohematology and are important factors contributing to the risks of immune-mediated hemolytic transfusion reactions. Some of the hemolytic transfusion reactions may have serious consequences including hemoglobinemia, disseminated intravascular coagulation, and death.[1,12] The techniques commonly employed to detect these antibodies in blood banks include CTT, LISS-IAT, MCT, polyethylene glycol tube test, solid phase red cell adherence assay, etc.[12]
Our study compared three techniques (CTT, LISS-IAT, and MCT) in terms of strength of agglutination reaction observed with the specific antibody [Table 2]. In our study, MCT was found to be most sensitive in the detection of clinically relevant antibodies of Rh, Kell, Kidd, and anti-S specificity as compared to LISS-IAT and CTT. MCT detected all the 14 clinically significant antibodies; tube LISS-IAT detected 12 of them and CTT detected only 8 antibodies. Similar set of antibodies have been identified in various studies conducted in the past in multitransfused patients [Table 3].[13,14,15,16] On extensive review of literature, no study comparing three techniques was found. However, the results of our study are similar to few other studies where two techniques have been compared [Table 4].[8,9,17,18,19,20,21,22,23] Few studies have contradictory findings in detection of Kell antibody. In our study, CTT was less sensitive than tube LISS-IAT and MCT in detecting anti-K while studies by various authors have shown CTT to be more sensitive in detecting Kell antibody when compared to LISS-IAT and MCT.[21,24,25] This may be due to intense hydrophilic nature of Kell antigen due to which anti-Kell binds less efficiently in low-ionic solutions when compared to normal saline.[24,26] Our study has shown MCT to be more sensitive in detection of anti-Jka and anti-S when compared with tube LISS-IAT while study by Phillips et al. has shown opposite results, which was attributed to unfamiliarity with the technique by the concerned personnel.[4] In our study, two cases of Lea were identified which were detected in immediate spin phase by CTT and LISS-IAT. This antibody was not detected by MCT. Lea is a cold reacting antibody, usually IgM type. Our result is contradictory to the studies conducted in the past which have shown gel technique to be more sensitive in detecting cold-reactive antibodies.[7,8,9] It may be due to incubation of patient's serum at 37°C with donor cells in MCT without immediate spin which might affect the reactivity of Lea at that temperature.
Table 3.
Antibodies identified in multitransfused patients
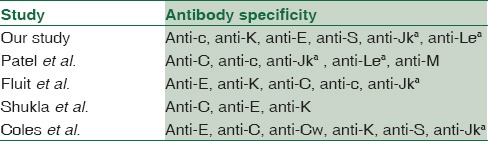
Table 4.
Comparison of different crossmatch techniques
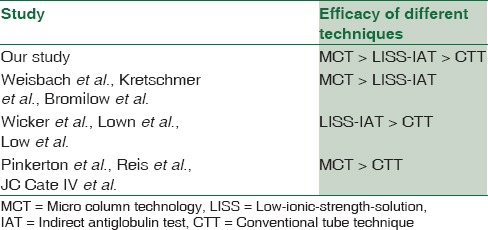
Different methods have their own merits and demerits when compared to each other. CTT crossmatch has been the mainstay for antibody detection for a long period. The sensitivity and specificity of properly performed CTT crossmatch have been estimated to be 100% with anti-human globulin phase included in the procedure.[27] However, it requires skilled expertise of hand and eye. Over-vigorous agitation to dislodge the cell button can cause false-negative results.[3] The washing step often causes elution of weakly bound antibodies. There is often variation in cell concentration used in cell-serum ratio. The end-points of the reaction are unstable; reading and grading require a high level of expertise.[5] The behavior of Kidd antibodies is shown to be poor with CTT.[21] Moreover, CTT also requires prolonged incubation phase which can delay the release of blood in emergency situations. LISS medium increases the rate and amount of alloantibody uptake and decreases the incubation time to approximately 15 min, thus preventing the unnecessary delay in releasing blood in emergency situations.[12,18] However, it also increases the uptake of gamma globulins and complement which leads to increased incidence of false positive reactions.[17,26] The reactivity of certain antibodies, especially Kell antibodies is known to decrease in LISS medium.[24,26] LISS-IAT is still the most frequently used test tube method for identification of alloantibodies.[12] MCT has many advantages over conventional methods. MCT has an objective reading phase; its results are standardized and reproducible. The lack of washing phase does not cause elution of antibodies and thus contributes to the improved sensitivity of the test which makes it ideal for automation. The reactions are stable for several days and can be photocopied or photographed for future reference.[8] Although gel technology is known to be more sensitive in detection of clinically significant antibodies; it also shows increased detection rates for benign cold-reacting antibodies which can cause undue hindrance in releasing blood in emergency situations. In addition, the incidence of false positives was more with MCT in certain studies when compared to conventional tube methods.[8,19,28]
Conclusion
The results of the previous studies comparing the sensitivity of different crossmatch techniques have been inconsistent. Certain studies found CTT to be a better option while certain studies concluded LISS-IAT and MCT to be a better substitute for detecting clinically significant antibodies. The present study compared the efficacy of three techniques (CTT, LISS-IAT, MCT) in terms of strength of agglutination reaction observed with the specific antibody and found MCT to be most efficacious when compared to CTT and tube LISS-IAT in detecting clinically significant red cell antibodies; although MCT missed 2 cases of Lea antibody, which were detected by CTT and LISS-IAT. The present study also found the rate of alloimmunization in multitransfused patients which came out to be 10.6%. This suggests that this group of patients is at constant risk of alloimmunization and needs antigen-matched blood by appropriate crossmatch technique which can detect clinically significant antibodies in these patients.
The old saying still holds true that “no one method will detect all antibodies of clinical relevance”. The variables which influence antigen-antibody reactions have not changed. The answer to the question which of these systems be employed in blood banks is influenced by the cost of the procedure, technical skills of the concerned personnel, sensitivity and specificity of the method as well as the possibility of automation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Shulman IA, Downes KA, Sazama K, Maffei LM. Pretransfusion compatibility testing for red blood cell administration. Curr Opin Hematol. 2001;8:397–404. doi: 10.1097/00062752-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Downes KA, Shulman IA. Pretransfusion testing. In: Roback JD, Grossman BJ, Harris T, Hillyer CD, editors. American Association of Blood Banking (AABB) Technical Manual. 17th ed. AABB, Bethesda: 2011. pp. 437–61. [Google Scholar]
- 3.Voak D. Validation of new technology for antibody detection by antiglobulin tests. Transfus Med. 1992;2:177–9. doi: 10.1111/j.1365-3148.1992.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 4.Phillips PK, Whitton CM, Lavin F. The use of the antiglobulin ‘gel-test’ for antibody detection. Transfus Med. 1992;2:111–3. doi: 10.1111/j.1365-3148.1992.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 5.Bajpai M, Kaur R, Gupta E. Automation in immunohematology. Asian J Transfus Sci. 2012;6:140–4. doi: 10.4103/0973-6247.98914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westhoff CM. Red cell immunology and compatibility testing. In: Simon TL, McCullough J, Snyder EL, Solheim BG, Strauss RG, editors. Rossi's Principles of Transfusion Medicine. 5th ed. Ch. 16. West Sussex: John Wiley & Sons, Ltd; 2016. p. 200. [Google Scholar]
- 7.Lapierre Y, Rigal D, Adam J, Josef D, Meyer F, Greber S, et al. The gel test: A new way to detect red cell antigen-antibody reactions. Transfusion. 1990;30:109–13. doi: 10.1046/j.1537-2995.1990.30290162894.x. [DOI] [PubMed] [Google Scholar]
- 8.Bromilow IM, Adams KE, Hope J, Eggington JA, Duguid JK. Evaluation of the ID-gel test for antibody screening and identification. Transfus Med. 1991;1:159–61. doi: 10.1111/j.1365-3148.1991.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 9.Kretschmer V, Heuckeroth A, Schulzki T, Dietrich G. Superiority of gel centrifugation in antibody screening and identification. Infusionsther Transfusionsmed. 1992;19:226–30. doi: 10.1159/000222633. [DOI] [PubMed] [Google Scholar]
- 10.Saran RK. Transfusion Medicine Technical Manual. 2nd ed. New Delhi: Directorate General of Services, Ministry of Health and Family Welfare, Government of India; 2003. Preparation of solution & methods; pp. 305–40. [Google Scholar]
- 11.Roback JD, Grossman BJ, Harris T, Hillyer CD. American Association of Blood Banking Technical Manual. 17th ed. AABB, Bethesda; 2011. General laboratory methods; pp. 863–74. [Google Scholar]
- 12.Casina TS. In search of the Holy Grail: Comparison of antibody screening methods. Immunohematology. 2006;22:196–202. [PubMed] [Google Scholar]
- 13.Patel J, Shukla R, Gupte S. Red cell alloimmunization in multitransfused patients and multiparous women. Indian J Hematol Blood Transfus. 2009;25:49–52. doi: 10.1007/s12288-009-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shukla JS, Chaudhary RK. Red cell alloimmunization in multi-transfused chronic renal failure patients undergoing hemodialysis. Indian J Pathol Microbiol. 1999;42:299–302. [PubMed] [Google Scholar]
- 15.Fluit CR, Kunst VA, Drenthe-Schonk AM. Incidence of red cell antibodies after multiple blood transfusion. Transfusion. 1990;30:532–5. doi: 10.1046/j.1537-2995.1990.30690333485.x. [DOI] [PubMed] [Google Scholar]
- 16.Coles SM, Klein HG, Holland PV. Red cell alloimmunization in a diverse population of transfused patients with thalassaemia. Transfusion. 1981;21:462–6. [Google Scholar]
- 17.Löw B, Messeter L. Antiglobulin test in low-ionic strength salt solution for rapid antibody screening and cross-matching. Vox Sang. 1974;26:53–61. doi: 10.1111/j.1423-0410.1974.tb02666.x. [DOI] [PubMed] [Google Scholar]
- 18.Wicker B, Wallas CH. A comparison of a low ionic strength saline medium with routine methods for antibody detection. Transfusion. 1976;16:469–72. doi: 10.1046/j.1537-2995.1976.16577039305.x. [DOI] [PubMed] [Google Scholar]
- 19.Reis KJ, Chachowski R, Cupido A, Davies D, Jakway J, Setcavage TM. Column agglutination technology: The antiglobulin test. Transfusion. 1993;33:639–43. doi: 10.1046/j.1537-2995.1993.33893342744.x. [DOI] [PubMed] [Google Scholar]
- 20.Lown JA, Barr AL, Davis RE. Use of low ionic strength saline for crossmatching and antibody screening. J Clin Pathol. 1979;32:1019–24. doi: 10.1136/jcp.32.10.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinkerton PH, Ward J, Chan R, Coovadia AS. An evaluation of a gel technique for antibody screening compared with a conventional tube method. Transfus Med. 1993;3:201–5. [Google Scholar]
- 22.Cate JC, 4th, Reilly N. Evaluation and implementation of the gel test for indirect antiglobulin testing in a community hospital laboratory. Arch Pathol Lab Med. 1999;123:693–7. doi: 10.5858/1999-123-0693-EAIOTG. [DOI] [PubMed] [Google Scholar]
- 23.Weisbach V, Kohnhäuser T, Zimmermann R, Ringwald J, Strasser E, Zingsem J, et al. Comparison of the performance of microtube column systems and solid-phase systems and the tube low-ionic-strength solution additive indirect antiglobulin test in the detection of red cell alloantibodies. Transfus Med. 2006;16:276–84. doi: 10.1111/j.1365-3148.2006.00674.x. [DOI] [PubMed] [Google Scholar]
- 24.Merry AH, Thomson EE, Lagar J, Howell P, Voak D, Downie M, et al. Quantitation of antibody binding to erythrocytes in LISS. Vox Sang. 1984;47:125–32. doi: 10.1111/j.1423-0410.1984.tb01574.x. [DOI] [PubMed] [Google Scholar]
- 25.Molthan L, Strohm PL. Hemolytic transfusion reaction due to anti-Kell undetectable in low-ionic-strength solutions. Am J Clin Pathol. 1981;75:629–31. doi: 10.1093/ajcp/75.4.629. [DOI] [PubMed] [Google Scholar]
- 26.Ahn JH, Rosenfield RE, Kochwa S. Low ionic antiglobulin tests. Transfusion. 1987;27:125–33. doi: 10.1046/j.1537-2995.1987.27287150182.x. [DOI] [PubMed] [Google Scholar]
- 27.Swarup D, Dhot PS, Kotwal J, Verma AK. Comparative study of blood cross matching using conventional tube and gel method. Med J Armed Forces India. 2008;64:129–30. doi: 10.1016/S0377-1237(08)80054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lange J, Selleng K, Heddle NM, Traore A, Greinacher A. Coombs’ crossmatch after negative antibody screening – A retrospective observational study comparing the tube test and the microcolumn technology. Vox Sang. 2010;98(3 Pt 1):e269–75. doi: 10.1111/j.1423-0410.2009.01278.x. [DOI] [PubMed] [Google Scholar]


