Abstract
BACKGROUND:
Colorectal cancer (CRC) is one of the most common cancers worldwide. Microsatellite instability (MSI) is detected in about 15% of all colorectal cancers. CRC with MSI has particular characteristics such as improved survival rates and better prognosis. They also have a distinct sensitivity to the action of chemotherapy.
AIM:
The aim of the study was to detect microsatellite instability in a cohort of colorectal cancer Egyptian patients using the immunohistochemical expression of mismatch repair proteins (MLH1, MSH2, MSH6 and PMS2).
MATERIAL AND METHODS:
Cases were divided into Microsatellite stable (MSS), Microsatellite unstable low (MSI-L) and Microsatellite unstable high (MSI-H). This Microsatellite stability status was correlated with different clinicopathological parameters.
RESULTS:
There was a statistically significant correlation between the age of cases, tumor site & grade and the microsatellite stability status. There was no statistically significant correlation between the gender of patients, tumor subtype, stage, mucoid change, necrosis, tumor borders, lymphocytic response, lymphovascular emboli and the microsatellite stability status.
CONCLUSION:
Testing for MSI should be done for all colorectal cancer patients, especially those younger than 50 years old, right sided and high-grade CRCs.
Keywords: Colorectal cancer, Immunohistochemistry; Lynch Syndrome, Microsatellite instability, Mismatch repair proteins
Introduction
Globally, more than one million people get colorectal cancer annually [1]. It is the second most common cause of cancer in women (after breast cancer) and the third most common in men (after lung and prostate cancers) [2].
In Egypt, there was a rapid increase in colorectal cancer incidence, where the occurrence was formerly low. Egypt reveals an unusually high rate of colorectal carcinoma under age 40, low prevalence of colorectal polyps in cancer patients and a predominant cancer site in the rectum [3].
Colorectal cancer is a heterogeneous disease, and so far four main molecular pathways have been identified. These four pathways are the Chromosomal Instability (CIN) Pathway, CpG Island Methylator Phenotype (CIMP) pathway, Microsatellite Instability (MSI) pathway and the Serrated pathway [4].
MSI is a kind of genomic instability that arises when mutations occur in nucleotide repeat sequences throughout the genome. These repeat sequences are known as microsatellites, and the discrepancy that arises between these sequences in tumor and germline cells is known as microsatellite instability [5]. MSI arises from defects in the DNA mismatch repair (MMR) system which corrects any errors made by DNA polymerases during the replication of DNA [6].
Lynch Syndrome (LS), previously termed hereditary non-polyposis colorectal cancer, is an inherited condition of defective DNA MMR and predisposes people to a variety of cancers [7]. Colorectal cancer is the most common type of cancer associated with Lynch syndrome [8].
LS is caused by autosomal dominant heterozygous germline mutations in one of the four key MMR genes — the mutL homologue 1 (MLH1) (chromosome 3p21.3), mutS homologue 2(MSH2) (chromosome 2p22–21), mutS homologue 6 (MSH6)(chromosome 2p16) or postmeiotic segregation increased 2 (PMS2) (chromosome 7p22.2) genes [9].
The loss of function occurs due to a germline mutation in one allele of one of the DNA- MMR genes; however, inactivation of the second allele, which is mostly acquired, is needed for the development of cancers [10].
MSI sporadic CRCs were found to be caused primarily by somatically acquired hypermethylation of both alleles of the MLH1 promoter, with resultant loss of MLH1 protein expression, which was closely associated with the presence of the oncogenic BRAFV600E mutation [11].
The DNA-MMR enzymes work in pairs (dimers- MLH1/PMS2 and MSH2/MSH6), and formation of the complex is important for their stability. When a MLH1 function is lost, immunoreactivity for PMS2 disappears. The same happens with MSH6; when the MSH2 function is lost. However, when the PMS2 orMSH6 function is lost, MLH1 and MSH2 find other MMR partners and hence appear partially preserved on immunohistochemistry [12].
Testing tumors for MSI by immunohistochemistry for MMR proteins and/or by molecular-based methods is routinely performed for patients diagnosed with colorectal carcinoma, primarily to screen for Lynch syndrome. Up to 15% of all colorectal carcinomas demonstrate MSI, more frequently secondary to acquired methylation of MLH1 (sporadic cases) than caused by a germline mutation (Lynch syndrome) [5].
From a clinical point of view, MSI-high (MSI-H) tumors as compared with microsatellite stable (MSS) ones, are diagnosed at a younger age, with a predominance in the right colon, frequently raised from sessile serrated adenoma and are diagnosed at an earlier stage [13].
Mucinous cells, signet-ring cells and poorly differentiated cells are uncommon histologic types and are commonly observed in cancers with MSI-H [14]. Patients with MSI-positive tumors tend to have a better prognosis and are less likely to be associated with distant metastasis [15, 16].
The aim of the study was to detect microsatellite instability in a cohort of colorectal cancer Egyptian patients using the immunohistochemical expression of mismatch repair proteins (MLH1, MSH2, MSH6 and PMS2).
Material and Methods
Fifty-two cases of colorectal cancer were retrieved from the pathology department, Ahmed Maher teaching hospital, Cairo, Egypt during the period from January 2012 to December 2015. Demographic and clinical data of the patients were collected from the hospital files.
Five thick sections were cut from Formalin-fixed paraffin embedded tissue blocks and stained with Hematoxylin and eosin for routine histopathological examination and determination of tumor type, grade, stage of the tumor and reporting co-findings such as; areas of tumor necrosis, presence or absence of lymphovascular invasion and lymphocytic response. Mucinous carcinoma was diagnosed if > 50% of the lesion was composed of mucin. Signet ring cell carcinoma was diagnosed if > 50% of tumor cells showed prominent intracytoplasmic mucin. Otherwise gland forming tumors without specific morphology were diagnosed as adenocarcinoma, NOS.
Immunohistochemical staining was performed using immunostainer (Shandon Sequenza) using the labeled streptavidin biotin method with the following reagents: Diva Decloaker, pretreatment antigen – retrieval, (Biocare Medical Catalog number: DV2004 LX, MX), Hydrogen peroxide block (Lab Vision, USA, Catalog number: TA-060-HP), Ultravision large volume detection system (Lab Vision, USA, Catalog number: TP-060- HL) including Ultra V Block, Biotinylated goat anti -polyvalent plus (link) & Streptavidin peroxidase plus (label) and DAB plus substrate system (Lab Vision, USA, Catalog number: TA-060-HDX) including DAB plus chromogen & DAB plus substrate.
The primary antibodies were PMS-2: a mouse polyclonal antibody (Biocare Medical Catalog number: PM 344 AA), MLH-1: a mouse monoclonal antibody (Biocare Medical Catalog number: PM 220 AA), MSH-6: a mouse monoclonal antibody (Biocare Medical Catalog number: PM 265 AA) and MSH-2: a mouse monoclonal antibody (Biocare Medical Catalog number: PM 219 AA).
Non-neoplastic colonic mucosa, stromal cells, infiltrating lymphocytes or the centres of lymphoid follicles, were used as positive internal controls. Sections of the same tissue were used following the same procedure, but the PBS was used instead of the primary antibody were used as negative internal controls.
Cases were categorised into positive (nuclear staining within tumor cells) and negative (complete absence of nuclear staining within tumor cells with concurrent internal positive controls) [17].
Then cases were interpreted as Microsatellite stable (MSS) when all the four antibodies show positive nuclear staining of the tumor cells, as Microsatellite unstable low (MSI-L) when one antibody shows negative nuclear staining of the tumor cells (Fig. 1) and as Microsatellite unstable high (MSI-H) when two antibodies or more show negative nuclear staining of the tumor cells (Fig. 2) [18].
Figure 1.
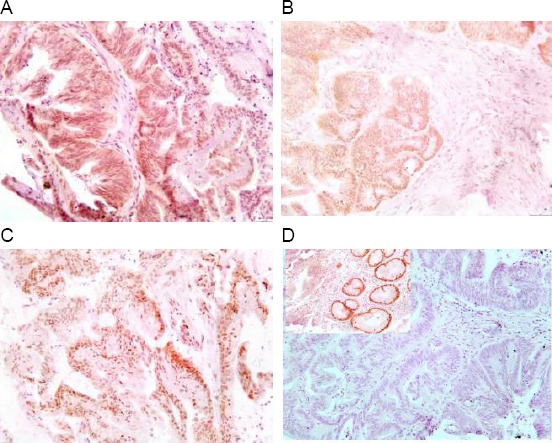
Immunohistochemical staining pattern of an MSI-L colorectal carcinoma with intact staining of MLH1 (A), PMS2 (B) and MSH2 (C) and isolated loss of MSH6 (D) with positive internal control (Upper left of D)
Figure 2.
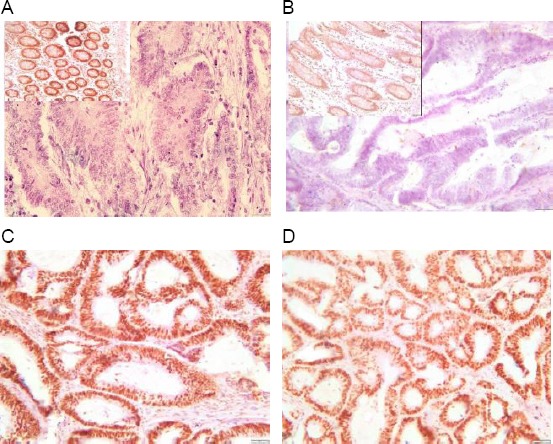
Immunohistochemical staining pattern of an MSI-H colorectal carcinoma with loss of both MLH1 (A) & PMS2 (B) with positive internal control (Upper left of A, B) and intact staining of MSH2 (C) & MSH6 (D)
Statistical analyses were performed using Statistical Package for Social Science (SPSS 17.0 for Windows; SPSS Inc, Chicago, IL, 2010). Chi-Square test was used to examine the variable. P- value is significant when ≤ 0.05.
Results
The patients’ age ranged from 27 to 87 years with a mean age of 55.86 ± 13.11 years with a male: female ratio ~1:1. 82.7% of cases were adenocarcinomas, NOS, 9.6% were a mucinous carcinoma, and 7.7% were signet ring cell carcinoma.
Both MLH-1 and PMS-2 were positive in 61.5% of cases, both were lost in 30.8%, and PMS-2 loss without MLH-1 loss was seen in 7.7%. Both of MSH-2 and MSH-6 were positive in 94.2% of cases, while the loss of MSH-6 without MSH-2 loss was seen in 5.8%. Accordingly; 57.7% of cases were MSS, 11.5% were MSI-L, and 30.8% were MSI-H.
On the correlation of the stability status with the clinicopathological parameters, there was a statistically significant correlation between patients’ age and microsatellite stability status; older age (≥ 50 years), was associated with MSS status while younger age (< 50 years), was associated with MSI-H status. Also, MSI-L status was seen only in older age (P value = 0.034). Also, there was a statistically significant correlation between tumor site and microsatellite stability status, where the left and transverse colon tumors tend to be MSS, while right colon tumors tend to be MSI-H (P-value = 0.014).
A significant relationship was found between tumor grade and microsatellite stability status after adding the MSS to MSI-L cases; MSS and MSI-L tumors tend to be low grade, while MSI-H tumors tend to be high grade. (P value= 0.025).
An insignificant correlation between the microsatellite stability status and patients’ sex, tumor subtype, tumor borders, tumor necrosis, lymphocytic response, lymphovascular emboli, T stage or lymph node status was found (Table 1).
Table 1.
Descriptive Statistics of clinicopathological parameters and microsatellite stability status of CRC cases
| MSS n (%) | MSI-L n (%) | MSI-H n (%) | Total n (%) | P value | ||
|---|---|---|---|---|---|---|
| Age | < 50 | 9 (17.3) | 0 (0) | 9 (17.3) | 18 (34.6) | 0.034 |
| ≥ 50 | 21 (40.4) | 6 (11.5) | 7 (13.5) | 34 (65.4) | ||
| Gender | Male | 16 (30.8) | 2 (3.8) | 9 (17.3) | 27 (51.9) | 0.614 |
| Female | 14 (26.9) | 4 (7.7) | 7 (13.5) | 25 (48.1) | ||
| Tumor site | Lt colon | 22 (42.3) | 5 (9.6) | 5 (9.6) | 32 (61.5) | 0.014 |
| Rt colon | 6 (11.5) | 1 (1.9) | 11 (21.2) | 18 (34.6) | ||
| Transverse | 2 (3.8) | 0 (0) | 0 (0) | 2 (3.8) | ||
| Tumor type | Adenocarcinoma. | 25 (48.1) | 6 (11.5) | 12 (23.1) | 43 (82.7) | 0.729 |
| Mucinous ca | 3 (5.8) | 0 (0) | 2 (3.8) | 5 (9.6) | ||
| Signet ring ca | 2 (3.8) | 0 (0) | 2 (3.8) | 4 (7.7) | ||
| Tumor grade | Low | 29 (55.8) | 8 (15.4) | 37 (71.2) | 0.025 | |
| High | 7 (13.5) | 8 (15.4) | 15 (28.8) | |||
| Mucoid change | Present | 10 (19.2) | 2 (3.8) | 5 (9.6) | 17 (32.7) | 0.989 |
| Absent | 20 (38.5) | 4 (7.7) | 11 (21.2) | 35 (67.3) | ||
| Tumor necrosis | Present | 13 (25) | 2 (3.8) | 8 (15.4) | 23 (44.2) | 0.773 |
| Absent | 17 (32.7) | 4 (7.7) | 8 (15.4) | 29 (55.8) | ||
| Tumor border | Infiltrative | 18 (34.6) | 3 (5.8) | 11 (21.2) | 32 (61.5) | 0.698 |
| Pushing | 12 (23.1) | 3 (5.8) | 5 (9.6) | 20 (38.5) | ||
| Lymphocytic response | Mild | 20 (38.5) | 3 (5.8) | 11 (21.2) | 34 (65.4) | 0.789 |
| Moderate | 8 (15.4) | 3 (5.8) | 4 (7.7) | 15 (28.8) | ||
| Marked | 2 (3.8) | 0 (0) | 1 (1.9) | 3 (5.8) | ||
| Vascular emboli | Present | 17 (32.7) | 2 (3.8) | 7 (13.5) | 26 (50) | 0.484 |
| Absent | 13 (25) | 4 (7.7) | 9 (17.3) | 26 (50) | ||
| T stage | T1+T2 | 2 (3.8) | 1 (1.9) | 3 (5.8) | 6 (11.5) | 0.434 |
| T3+T4 | 28 (53.8) | 5 (9.6) | 13 (25) | 46 (88.5) | ||
| N stage | N0 | 10 (19.2) | 5 (9.6) | 8 (15.4) | 23 (44.2) | 0.078 |
| N1 | 14 (26.9) | 0 (0) | 3 (5.8) | 17 (32.7) | ||
| N2 | 6 (11.5) | 1 (1.9) | 5 (9.6) | 12 (23.1) | ||
Discussion
Colorectal cancer is a common malignancy. It is the fourth most common cause of cancer death after lung, stomach and liver cancer [2]. It is not uncommon among Egyptian patients and rates are higher in patients under 40 years of age [19].
CRC shows a significant heterogeneity in both prognosis and response to therapy, even within the same pathological stage. This clinical heterogeneity may be in part linked to genetic alterations occurring during the pathogenesis [20].
MSI represents a molecular hallmark of Lynch syndrome. Nevertheless, the majority of cases with MSI are sporadic, more often due to an epigenetic inactivation of hMLH1 [21].
Concerning the microsatellite stability status of the cases in this study, a significant relation was found between the microsatellite stability status and patients’ age, where older age is associated MSS status, and the younger age is associated with MSI-H status. This result is the same as that of Huang et al. [22] andof Yuan et al. [17]. Also, Jenkins et al. [23] and Greenson et al. [24] found that age under 50 years is a strong predictor of MSI.
Table 2.
Summary of other studies findings regarding clinicopathological parameters related to MSI-H
| Parameters related to MSI-H | |
|---|---|
| Faghani et al. [25] | Left colon |
| Frey et al. [26] | Right colon and high grade |
| Greenson et al. [24] | Young age, right colon, high grade and mucinous differentiation |
| Huang et al. [22] | Young age, right colon, low grade and mucinous differentiation |
| Jenkins et al. [23] | Young age, right colon, high grade and mucinous differentiation |
| Joel et al. [28] | Low grade |
| Raut et al. [13] | High grade and mucinous differentiation |
| Yearsley et al. [27] | High grade and mucinous differentiation |
| Yuan et al. [17] | Young age |
On the contrary, Faghani et al. [25] found that there was no a statistically significant correlation between MSI and patients’ age, this can be explained by that their study was designed to determine the correlation between MSI and sporadic cases only.
A significant relationship was found between the microsatellite stability status and tumor site where the left and transverse colon tumors tend to be MSS while right colon tumors tend to be MSI-H. Also, Huang et al. [22] and Frey et al. [26] found the same. Jenkins et al. [23] and Greenson et al. [24] found that right location was also a strong predictor of MSI.
On the contrary, Faghani et al. [25] found that 81.8% of total MSI-H had distal tumors. This is may be due to studyng only sporadic cases and analysis of MSI frequencies by testing the BAT-26 and BAT-25 markers.
Also, a significant relation was found between microsatellite stability status and the tumor grade, where MSS and MSI-L tumors tend to be low grade, while MSI-H tumors tend to be high grade. This is the same as Raut et al. [13], Yearsley et al. [27] and Frey et al. [26] results. Jenkins et al. [23] and Greenson et al. [24] found that poor or undifferentiated histology is a strong predictor of microsatellite instability.
On the contrary, Joel et al. [28] found that the presence of well-differentiated tumors were important markers for microsatellite instability. Also, Huang et al. [22] found that MSI-H tumors were more likely to show less local aggressiveness and lower differentiation. These results may be due to the difference in the genetic and hereditary background among their patients and the Egyptian patients included in this study and use of molecular analyses besides the immunohistochemical tests.
Although no significant relationship could be found in our study between the microsatellite stability status and tumor subtype or mucin presence, a great production of mucin with extracellular accumulation often correlates with MSI [29]. This discrepancy is due to the small number of mucinous and signet ring cell carcinoma cases in our study. Yearsley et al. [28] found the percentage of mucin differed significantly between MSI-H and MSI-L or MSS. Also, Raut et al. [13] and Huang et al. [22] found that MSI is associated with a mucinous histology. Jenkins et al. [23] and Greenson et al. [24] found that signet ring or focal signet ring differentiation and mucinous or focal mucinous differentiation were statistically significant predictors of microsatellite instability.
In conclusion, our study showed a statistically significant correlation between patients’ age, tumor site and grade and the microsatellite stability status. It is recommended that testing for MSI is done for all colorectal cancer patients younger than 50 years old, right sided and high-grade tumors. Further studies on a larger number of patients should be done to study the relation between MSI and other pathological parameters.
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Cunningham D, Atkin W, Lenz HJ, et al. Colorectal cancer. Lancet. 2010;375:1030–1047. doi: 10.1016/S0140-6736(10)60353-4. https://doi.org/10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61(2):69–90. doi: 10.3322/caac.20107. https://doi.org/10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Lo AC, Soliman A, Khaled HM, et al. Lifestyle, Occupational, and Reproductive Factors and Risk of Colorectal Cancer. Dis Colon Rectum. 2010;53(5):830–837. doi: 10.1007/DCR.0b013e3181d320b1. https://doi.org/10.1007/DCR.0b013e3181d320b1. PMid: 20389219. PMCid: PMC3223860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Worthley DL, Leggett BA. Colorectal cancer: molecular features and clinical opportunities. Clin Biochem Rev. 2010;31(2):31–8. PMid: 20498827. PMCid: PMC2874430. [PMC free article] [PubMed] [Google Scholar]
- 5.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138(6):2073–87. doi: 10.1053/j.gastro.2009.12.064. https://doi.org/10.1053/j.gastro.2009.12.064. PMid: 20420947. PMCid: PMC3037515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vilar E, Gruber SB. Microsatellite instability in colorectal cancer e the stable evidence. Nat Rev Clin Oncol. 2010;7(3):153–62. doi: 10.1038/nrclinonc.2009.237. https://doi.org/10.1038/nrclinonc.2009.237. PMid: 20142816. PMCid: PMC3427139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch HT, Snyder CL, Shaw TG, et al. Milestones of Lynch syndrome 1895–2015. Natural reviews Cancer. 2015;15:181–194. doi: 10.1038/nrc3878. https://doi.org/10.1038/nrc3878. PMid: 25673086. [DOI] [PubMed] [Google Scholar]
- 8.Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304–2310. doi: 10.1001/jama.2011.743. https://doi.org/10.1001/jama.2011.743. PMid: 21642682. [DOI] [PubMed] [Google Scholar]
- 9.Heald B, Church J, Plesec T, et al. Detecting and managing hereditary colorectal cancer syndromes in your practice. Cleveland clinic journal of medicine. 2012;79(11):787–796. doi: 10.3949/ccjm.79a.11165. https://doi.org/10.3949/ccjm.79a.11165. PMid: 23125329. [DOI] [PubMed] [Google Scholar]
- 10.Riddell R, Jain D, Bernstein CN, et al. Gastrointestinal pathology and its clinical implications. Philadelphia: Lippincott Williams & Wilkins; 2014. pp. 1327–1523. [Google Scholar]
- 11.Deng G, Bell I, Crawley S, et al. BRAF mutation is frequently present in sporadic colorectal cancer with methylated hMLH1, but not in hereditary nonpolyposis colorectal cancer. Clin Cancer Res. 2004;10:191–195. doi: 10.1158/1078-0432.ccr-1118-3. https://doi.org/10.1158/1078-0432.CCR-1118-3. PMid: 14734469. [DOI] [PubMed] [Google Scholar]
- 12.Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol. 2006;7(5):335–346. doi: 10.1038/nrm1907. https://doi.org/10.1038/nrm1907. PMid: 16612326. [DOI] [PubMed] [Google Scholar]
- 13.Raut CP, Pawlik TM, Rodriguez-Bigas MA. Clinicopathologic features in colorectal cancer patients with microsatellite instability. Mutat Res. 2004;568(2):275–82. doi: 10.1016/j.mrfmmm.2004.05.025. https://doi.org/10.1016/j.mrfmmm.2004.05.025. PMid: 15542114. [DOI] [PubMed] [Google Scholar]
- 14.Jung SH, Kim SH, Kim JH. Prognostic impact of microsatellite instability in colorectal cancer presenting with mucinous, signet -Ring, and poorly differentiated cells. Ann Coloproctol. 2016;32(2):58–65. doi: 10.3393/ac.2016.32.2.58. https://doi.org/10.3393/ac.2016.32.2.58. PMid: 27218096. PMCid: PMC4865466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guastadisegni C, Colafranceschi M, Ottini L, et al. Microsatellite instability as a marker of prognosis and response to therapy: a meta analysis of colorectal cancer survival data. Eur J Cancer. 2010;46(15):2788–98. doi: 10.1016/j.ejca.2010.05.009. https://doi.org/10.1016/j.ejca.2010.05.009. PMid: 20627535. [DOI] [PubMed] [Google Scholar]
- 16.Malesci A, Laghi L, Bianchi P, et al. Reduced likelihood of metastases in patients with microsatellite-unstable colorectal cancer. Clin Cancer Res. 2007;13(13):3831–9. doi: 10.1158/1078-0432.CCR-07-0366. https://doi.org/10.1158/1078-0432.CCR-07-0366. PMid: 17606714. [DOI] [PubMed] [Google Scholar]
- 17.Yuan L, Chi Y, Chen W, et al. Immunohistochemistry and microsatellite instability analysis in molecular subtyping of colorectal carcinoma based on mismatch repair competency. Int J Clin Exp Med. 2015;8(11):20988–21000. PMid: 26885030. PMCid: PMC4723875. [PMC free article] [PubMed] [Google Scholar]
- 18.Rustgi AK. The genetics of hereditary colon cancer. Genes Dev. 2016;21:2525–2538. doi: 10.1101/gad.1593107. https://doi.org/10.1101/gad.1593107. PMid: 17938238. [DOI] [PubMed] [Google Scholar]
- 19.Gadoa A, Ebeidb B, Abdelmohsen A, et al. Colorectal cancer in Egypt is commoner in young people: Is this cause for alarm? Alexandria Journal of Medicine. 2014;50(3):197–201. https://doi.org/10.1016/j.ajme.2013.03.003. [Google Scholar]
- 20.Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008;135(4):1079–99. doi: 10.1053/j.gastro.2008.07.076. https://doi.org/10.1053/j.gastro.2008.07.076. PMid: 18773902. PMCid: PMC2866182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunningham JM, Christensen ER, Tester DJ, et al. Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. Cancer Res. 1998;58(15):3455–60. PMid: 9699680. [PubMed] [Google Scholar]
- 22.Huang Y, Yuan Y, Wei-ting GE, et al. Comparative features of colorectal and gastric cancers with microsatellite instability in Chinese patients. Biomed & Biotechnol. 2010;11(9):647–653. doi: 10.1631/jzus.B1000198. https://doi.org/10.1631/jzus.b1000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkins MA, Hayashi S, O’shea A, et al. Pathology features in Bethesda Guidelines predict colorectal cancer microsatellite instability: A population-based study. Gastroenterol. 2007;133:48–56. doi: 10.1053/j.gastro.2007.04.044. https://doi.org/10.1053/j.gastro.2007.04.044. PMid: 17631130. PMCid: PMC2933045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenson JK, Huang SC, Herron C, et al. Pathologic Predictors of Microsatellite Instability in Colorectal Cancer. Am J Surg Pathol. 2009;33(1):126–133. doi: 10.1097/PAS.0b013e31817ec2b1. https://doi.org/10.1097/PAS.0b013e31817ec2b1. PMid: 18830122. PMCid: PMC3500028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faghani M, Asl SF, Ghanaei FM, et al. The Correlation between Microsatellite Instability and the Features of Sporadic Colorectal Cancer in the North Part of Iran. Gastroenterology Research and Practice. 2012;2012:1–6. doi: 10.1155/2012/756263. https://doi.org/10.1155/2012/756263. PMid: 23213329. PMCid: PMC3507153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frey DM, Droeser RA, Viehl CT, et al. High frequency of tumor-infiltrating FOXP3+regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patient. International Journal of Cancer. 2010;126(11):2635–2643. doi: 10.1002/ijc.24989. https://doi.org/10.1002/ijc.24989. [DOI] [PubMed] [Google Scholar]
- 27.Yearsley M, Hampel H, Lehman A, et al. Histologic features distinguish microsatellite-high from microsatellite-low and microsatellite-stable colorectal carcinomas, but do not differentiate germline mutations from methylation of the MLH1 promoter. Human pathology. 2006;37(7):831–838. doi: 10.1016/j.humpath.2006.02.009. https://doi.org/10.1016/j.humpath.2006.02.009. PMid: 16784982. [DOI] [PubMed] [Google Scholar]
- 28.Joel KG, Joseph DB, Ofer BZ, et al. Phenotype of Microsatellite Unstable Colorectal Carcinomas: Well-Differentiated and Focally Mucinous tumors and the absence of dirty necrosis correlate with microsatellite instability. American Journal of Surgical Pathology. 2003;27(5):563–57. doi: 10.1097/00000478-200305000-00001. https://doi.org/10.1097/00000478-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Setaffy L, Langner C. Microsatellite instability in colorectal cancer: clinicopathological significance. Pol J Pathol. 2015;66(3):203–18. doi: 10.5114/pjp.2015.54953. https://doi.org/10.5114/pjp.2015.54953. PMid: 26619098. [DOI] [PubMed] [Google Scholar]


