Abstract
AIM:
The purpose of this study was to investigate the usefulness of the neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR) Mean Platelet Volume (MPV) and Red Cell Distribution Width (RDW) in the differential diagnosis and follow-up of patients with Bells Palsy.
MATERIAL AND METHODS:
Twenty-eight patients diagnosed with Bells Palsy and 28 control patients were included in the study. Serum samples were analysed retrospectively on the initial presentation and the seventh day of admission.
RESULTS:
On admission, the NLR was 1.7±1.2. The mean absolute neutrophil count was 6100 ± 900/mm^3 in Bells Palsy Group. NLR was 0.9 ± 0.2. The mean absolute neutrophil count was 4400 ± 1100/mm^3 in control group. Statistically, significant changes were not observed in NLR, PLR, MPV and RDW measurements in Bells Palsy group between House-Brackman Staging.
CONCLUSION:
Statistically significant changes in the neutrophil count and NLR were determined in the measurements between Bells Palsy and control group (p = 0.013, p = 0.016 respectively) on admission. A grade of the disease and NLR measurements had no statistically significant connection. RDW value was investigated for the first time in the literature for Bells Palsy patients.
Keywords: Facial paralysis, inflammatory marker, neutrophil, neutrophil-to-lymphocyte ratio
Introduction
Bells palsy (idiopathic facial paralysis) is the most common cause of unilateral peripheral facial paralysis. The incidence of the disease is 11-40/100,000 in the literature [1]. Vascular causes, autoimmune diseases and inflammation of the nerve sheath can be the etiological factors of the disease [1].
The neutrophil to lymphocyte ratio (NLR) is a useful marker that demonstrates the general inflammation condition. The presence of an elevated NLR was demonstrated in a follow-up of inflammatory diseases like larynx cancer, chronic hepatitis, Behcet’s disease, Celiac disease and ulcerative colitis [2-4].
Platelet to lymphocyte ratio (PLR) can be used in the follow-up of inflammation and cancer diseases like peripheral vascular system disorders, coronary artery disease, gynecologic and hepatobiliary system malignancies [5, 6].
Mean Platelet Volume (MPV) and Red Cell Distribution Width (RDW) are markers that can be used in general inflamatory and peripheric thrombotic disease in the literature [7]. There are no investigations about Bells Palsy and RDW measures in literature.
In the present study, we aimed to evaluate the NLR, PLR, MPV and RDW differences between the differential diagnosis and follow-ups of Bells Palsy patients. We aimed to investigate the whole markers that can be investigated from the simple blood test in Bells Palsy. RDW value was investigated for the first time in the literature for Bells Palsy patients.
Material and Methods
Patients admitted to the Sakarya Akyazi General Hospital between February 2013 and December 2015 with complaints about acute peripheral Bells Palsy were evaluated retrospectively. The study population comprised 28 patients diagnosed with Bells Palsy and 28 control patients.
Patients underwent general examination, with a neurological and otorhinolaryngological examination. Blood biochemistry and whole blood analysis were performed. Temporal Magnetic Resonance (MR) was performed on all members of the study population. The disease is staged using House-Brackman Staging system. 12 patients were Grade 2, ten patients were Grade 3, three patients were Grade 4 and three patients were Grade.
The exclusion criteria comprised the presence of an acute/chronic ear infection history, chronic liver failure, chronic inflammatory immunological disorders, neurological diseases, diabetes mellitus, hypertension, chronic renal failure, acute coronary artery disease, active connective tissue disorder, vasculitis, inflammatory bowel disease and. Patients using drugs such as antidiabetic drugs, steroids, chemotherapy drugs, immunomodulatory drugs, antihistaminic drugs, sedative drugs and analgesics were also excluded. Peripheral venous sampling for whole blood analysis and blood biochemistry was performed between 08:00 and 10:00. Whole blood analyses were performed using the same device (ABOTT CELL DYN 3700). Whole blood analyses were performed on admission and the seventh day of the first control after a period of fasting lasting at least eight hours. Systemic neurological and otorhinolaryngological examinations were performed.
Whole blood analysis results were evaluated retrospectively. The NLR was calculated by dividing the neutrophil count by the lymphocyte count per microlitre (NLR = neutrophils (×10^3 per μl) ÷ lymphocytes (×10^3 per μl)). PLR ratio was calculated by dividing platelet count by the lymphocyte count per microlitre.
Statistical analysis was performed using SPSS, version 19.0 for Windows (IBM, Armonk, NY). Descriptive data were expressed as means and standard deviation. The Kolmogorov-Smirnov test was used for a normality test. Mann-Whitney-U tests were used to evaluate differences between the groups. The Wilcoxon test was used to evaluate differences between whole blood count parameters on admission, and on the seventh day of admission, within groups Pearson, correlation analysis was used to evaluate differences between House-Brackman Staging system and parameters. For each test, a P value of 0.05 or less was treated as statistically significant.
Results
Twenty-eight patients were included in the study (16 males (57%), 12 females (43%). The mean age was 29.5 ± 10.5 (18–62).
Socio-demographic data and the entire blood results of the Bells Palsy population are demonstrated in Table 1. On admission, the NLR was 1.7 ± 1.2 (Fig. 1). The mean absolute neutrophil count was 6100 ± 900/mm^3, and the mean absolute lymphocyte count was 3800 ± 1200/mm^3. PLR was 129.4 ± 15.4; MPV was 8.2 ± 0.4 fL and RDW was 12.9 ± 0.6 on admission. On the seventh day of admission, the NLR was 1.5 ± 0.9. The mean absolute neutrophil count was 5900 ± 900/mm^3, and the mean absolute lymphocyte count was 3000 ± 800/mm^3. PLR was 135.2 ± 19, MPV was 8.4 ± 0.3 fL and RDW was 12.2 ± 0.4 (Table 1).
Table 1.
Haemogram parameters of patients with Bells Palsy
| Admission (Mean ± Std. Dev.) | 1st Week (Mean ± Std. Dev.) | P | |
|---|---|---|---|
| Haemoglobin (g/dl) | 12.4 ± 0.7 | 12.9 ± 0.6 | 0.12 |
| Haematocrit (%) | 37.8 ± 4.2 | 37.0 ± 4.2 | 0.66 |
| Neutrophil (K/μl) | 6.1 ± 0.9 | 5.9 ± 0.9 | 0.304 |
| Lymphocyte (K/μl) | 3.8 ± 1.2 | 3.0 ± 0.8 | 0.376 |
| NLR | 1.7 ± 1.2 | 1.5 ± 0.9 | 0.07 |
| PLR | 129.4 ± 15.4 | 135.2 ± 19 | 0.07 |
| MPV (fL) | 8.2 ± 0.4 | 8.4 ± 0.3 | 0.6 |
| RDW (%) | 12.9 ± 0.6 | 12.2 ± 0.4 | 0.7 |
Mean ± Std. Dev: Mean ± Standard Deviation, WBC: White Blood Cell, NLR: Neutrophil to Lymphocyte Ratio, PLR: Platelet to Lymphocyte Ratio, MPV: Mean Platelet Volume, RDW; Red Cell Distribution.
Figure 1.
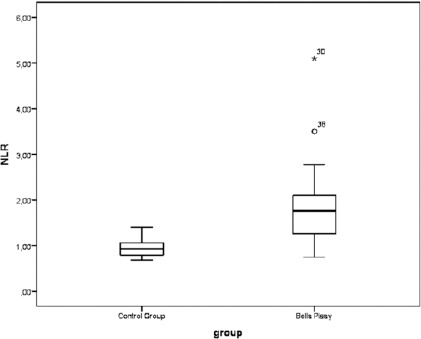
NLR and Bells Palsy can be seen on Figure (NLR: Neutrophil to Lymphocyte Ratio)
On admission, NLR was 0.9 ± 0.2, the mean absolute neutrophil count was 4400 ± 1100/mm^3, and the mean absolute lymphocyte count was 3900 ± 1200/mm^3. PLR was 139.4 ± 19.4; MPV was 8.4 ± 0.4 fL and RDW was 12.5 ± 0.6 on a control group. On the seventh day of admission, NLR was 1.1 ± 0.1, the mean absolute neutrophil count was 4100 ± 300/mm^3, and the mean absolute lymphocyte count was 4000 ± 600/mm^3. PLR was 134.2 ± 12, MPV was 8.1 ± 0.3 fL and RDW was 12.1 ± 0.4 (Table 2).
Table 2.
Haemogram parameters of control patients
| Admission (Mean ± Std. Dev.) | 1st Week (Mean ± Std. Dev.) | P | |
|---|---|---|---|
| Haemoglobin (g/dl) | 13.5 ± 0.4 | 13.2 ± 0.9 | 0.08 |
| Haematocrit (%) | 37.8 ± 4.4 | 38 ± 3.4 | 0.92 |
| Neutrophil (K/μl) | 4.4 ± 1.1 | 4.1 ± 0.3 | 0.3 |
| Lymphocyte (K/μl) | 3.9 ± 1.2 | 4 ± 0.6 | 0.4 |
| NLR | 0.9 ± 0.2 | 1.1 ± 0.1 | 0.2 |
| PLR | 139.4 ± 19.4 | 134.2 ± 12 | 0.07 |
| MPV (fL) | 8.4 ± 0.4 | 8.1 ± 0.3 | 0.6 |
| RDW (%) | 12.5 ± 0.6 | 12.1 ± 0.4 | 0.7 |
Mean ± Std. Dev: Mean ± Standard Deviation, WBC: White Blood Cell, NLR: Neutrophil to Lymphocyte Ratio, PLR: Platelet to Lymphocyte Ratio, MPV: Mean Platelet Volume, RDW; Red Cell Distribution.
Statistically significant changes between the neutrophil count, the lymphocyte count, NLR, PLR, MPV and RDW were not determined using the measurements made in the one week interval between examinations of the Bells Palsy group (p = 0.3, p = 0.37, p = 0.2, p = 0.07, p = 0.6, and p = 0.7, respectively) (Table 2).
Statistically, significant changes in the neutrophil count and NLR were determined in the measurements between Bells Palsy and control group (p = 0.013, p = 0.016 respectively) on admission (Table 3). There is still statistically significant difference on 7. Day of admission (p = 0.016, p = 0.03 respectively).
Table 3.
Comparison of haemogram parameters between patients with patients and control group
| Admission (Mean ± Std. Dev.) | P | 1st Week (Mean ± Std. Dev.) | P | |||
|---|---|---|---|---|---|---|
| Bells Palsy | Control | Bells Palsy | Control | |||
| Haemoglobin | 12.4 ± 0.7 | 13.5 ± 0.4 | 0.203 | 12.9 ± 0.6 | 13.2 ± 0.9 | 0.635 |
| Haematocrit | 37.8 ± 4.2 | 37.8 ± 4.4 | 0.677 | 37 ± 4.2 | 38 ± 3.4 | 0.828 |
| Neutrophil (K/μl) | 6.1 ± 0.9 | 4.4 ± 1.1 | 0.013 | 5.9 ± 0.9 | 4.1 ± 0.3 | 0.016 |
| Lymphocyte (K/μl) | 3.8 ± 1.2 | 3.9 ± 1.2 | 0.42 | 3 ± 0.8 | 4 ± 0.6 | 0.3 |
| NLR | 1.7 ± 1.2 | 0.9 ± 0.2 | 0.0016 | 1.5 ± 0.9 | 11.1 ± 0.1 | 0.03 |
| PLR | 129.4 ± 15.4 | 139.4 ± 19.4 | 0.65 | 135.2 ± 19 | 134.2 ± 12 | 0.8 |
| MPV (fl) | 8.2 ± 0.4 | 8.4 ± 0.4 | 0.67 | 8.4 ± 0.3 | 8.1 ± 0.3 | 0.6 |
| RDW (%) | 12.9 ± 0.6 | 12.5 ± 0.6 | 0.8 | 12.2 ± 0.4 | 12.1 ± 0.4 | 0.7 |
Mean ± Std. Dev: Mean ± Standard Deviation, WBC: White Blood Cell, NLR: Neutrophil to Lymphocyte Ratio, PLR: Platelet to Lymphocyte Ratio, MPV: Mean Platelet Volume, RDW; Red Cell Distribution.
Significant changes were not observed in haemoglobin and haematocrit values, lymphocyte counts, PLR, MPV and RDW measurements between Bells Palsy and control Group (p = 0.2, p = 0.67, p = 0.42, p = 0.65, p = 0.67, p = 0.8 respectively) on first admission. There is still no significant change on 7, day of admission between groups.
Table 4.
The distribution of NLR and mean PLR by the patient groups divided according to House-Brackmann Grading of Paralysis
| Paralysis grade | Number of patients | NLR | PLR | MPV | RDW |
|---|---|---|---|---|---|
| 2 | 12 | 1.7 ± 1.2 | 129.4 ± 15.4 | 8.2 ± 0.4 | 12.9 ± 0.6 |
| 3 | 10 | 1.6 ± 1.3 | 130.4 ± 15.2 | 8.1 ± 0.4 | 12.8 ± 0.5 |
Mean ± Std. Dev: Mean ± Standard Deviation, WBC: White Blood Cell, NLR: Neutrophil to Lymphocyte Ratio, PLR: Platelet to Lymphocyte Ratio, MPV: Mean Platelet Volume, RDW; Red Cell Distribution.
Significant changes were not observed in NLR, PLR, MPV and RDW measurements in Bells Palsy group between House-Brackman Staging (p = 0.84, p = 0.79, p = 0.63, p = 0.64 respectively). A grade of the disease and NLR measurements had no statistically significant connection.
Figure 2.
Normality scores of NLR in Bells Palsy patients
Discussion
The most important outcome of the present study was that the NLR and neutrophil count was significantly higher in patients with Bells Palsy than in control Group; this ratio was higher in the Bells Palsy than in the control group in the first week after admission. The second most important result of this study was the demonstration of statistically not significant changes on PLR; MPV; RDW measurements. The third important result of the study is there is no significant change in measures between House-Brackman classifications of Bells palsy. Therefore the NLR value does not change in the different grades of disease.
There were several causative aetiological factors for Bells Palsy. Viral infections and inflammation, vascular neuritis, peripheral vascular diseases, thromboembolism and microvascular circulatory impairment, immunological diseaseswere known factors [1]. Viruses were the most common aetiological factors, but it is not enough to determine the whole aetiology. From the microvascular circulatory impairment point of view, neuritis was associated with vascular inflammation.
Whole blood analysis is associated with the general condition of the patient. Higher neutrophil counts can be associated with inflammatory conditions. Lower lymphocyte count can be associated with higher organic stress [3]. The NLR is an inflammatory marker that has been studied in recent years, for the differential diagnosis and follows up of certain diseases. The NLR was evaluated in, acute hearing loss, Bell’s palsy and vertigo in the practice of Otorhinolaryngology [8, 9]. The NLR as an inflammatory marker was shown to be associated with the prognosis of cancer of the body [10].
The presence of an elevated NLR in Bells Palsy was demonstrated via the literature search [11-13]. NLR ratio was higher in 656 patients Bells Palsy group in a study [12]. NLR was also higher in Bells patients and another study by Ozler et al. NLR measurements were correlated with the disease in this study [13]. Atan et al. (2015) revealed that NLR was higher in 99 Bells palsy group, but the NLR was not correlated with House-Brackman grade [14]. Eryılmaz et al. (2015) revealed that NLR was higher in paediatric Bells palsy group, but the NLR was not correlated with House-Brackman grade [11]. There was no consensus on literature about the correlation of NLR and grade of the disease in the literature.
MPV is a parameter indicating platelet volume. It can be correlated with microvascular thrombotic diseases. MPV correlation with stroke is revealed in the literature [15]. In this perspective, MPV can be associated with microvascular obstruction. Correlation with MPV levels and Bells palsy was not documented in the literature [12]. In our study MPV was no statistically significantly higher in Bells patients.
RDW is a parameter indicating height and heterogeneity of the erythrocyte volume [16]. This parameter can increase in anaemia, myelodysplastic syndromes, haemolytic diseases and cause microvascular thrombotic diseases [16]. Correlation of RDW and migraine is posted in the literature [17]. Also, there are studies about the relation of RDW with coronary diseases in the literature [18, 19]. There is no actual study about RDW and Bells palsy in the literature. In our study RDW was no statistically significantly higher in Bells palsy patients.
PLR is also a parameter used as NLR widely in the literature nowadays. There are studies that have been done with the values PLR in the myocardial infarct [20]. PLR is posted in gastric and oesophagal cancer in the literature [21]. PLR is associated with sudden hearing loss in the literature [8]. There are few investigations about Bells Palsy and PLR in literature. In our study PLR was no statistically significantly higher in Bells palsy patients.
The limitations of our study were the retrospective design of the study, the limited number of cases and the limited time available for the second evaluation. Further prospective studies to evaluate the association between the NLR and both the diagnosis and prognosis of Bells palsy are needed.
In the present study, the NLR and neutrophil count were significantly higher in patients with Bells Palsy than in those with a control group. There is statistically significant change between NLR and Grade of the disease. Our study strengthened the inflammation theory as an aetiological factor for Bells Palsy rather than ischemic theory. Factors can be associated with peripheral vascular diseases and thromboembolism such as PLR, MPV, and RDW was normal in our study regarding control group. We aimed to investigate the whole blood parameters in Bells Palsy to evaluate the mean and connection with Bells Palsy herein.
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.McCaul JA, Cascarini L, Godden D, Coombes D, Brennan PA, Kerawala CJ. Evidence-based management of Bell’s palsy. Br J Oral Maxillofac Surg. 2014;52(5):387–91. doi: 10.1016/j.bjoms.2014.03.001. https://doi.org/10.1016/j.bjoms.2014.03.001. PMid: 24685475. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Razik A, Mousa N, Besheer TA, Eissa M, Elhelaly R, Arafa M, El-Wakeel N, Eldars W. Neutrophil to lymphocyte ratio as a reliable marker to predict insulin resistance and fibrosis stage in chronic hepatitis C virus infection. Acta Gastroenterol Belg. 2015;78(4):386–92. PMid: 26712048. [PubMed] [Google Scholar]
- 3.Alan S, Tuna S, Turkanoglu EB. The relation of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and mean platelet volume with the presence and severity of Behcet’s syndrome. Kaohsiung J Med Sci. 2015;31(12):626–31. doi: 10.1016/j.kjms.2015.10.010. https://doi.org/10.1016/j.kjms.2015.10.010. PMid: 26709224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demir AK, Demirtas A, Kaya SU, Tastan I, Butun I, Sagcan M, et al. The relationship between the neutrophil-lymphocyte ratio and disease Activity in patients with ulcerative colitis. Kaohsiung J Med Sci. 2015;31(11):585–90. doi: 10.1016/j.kjms.2015.10.001. https://doi.org/10.1016/j.kjms.2015.10.001. PMid: 26678939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toprak C, Tabakci MM, Simsek Z, Arslantas U, Durmus HI, et al. Platelet/lymphocyte ratio was associated with impaired myocardial perfusion and both in-hospital and long-term adverse outcome in patients with ST-segment elevation acute myocardial infarction undergoing primary coronary intervention. Postepy Kardiol Interwencyjnej. 2015;11(4):288–97. doi: 10.5114/pwki.2015.55599. https://doi.org/10.5114/pwki.2015.55599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aldemir MN, Turkeli M, Simsek M, Yildirim N, Bilen Y, et al. Prognostic Value of Baseline Neutrophil-Lymphocyte and Platelet-Lymphocyte Ratios in Local and Advanced Gastric Cancer Patients. Asian Pac J Cancer Prev. 2015;16(14):5933–7. doi: 10.7314/apjcp.2015.16.14.5933. https://doi.org/10.7314/APJCP.2015.16.14.5933. [DOI] [PubMed] [Google Scholar]
- 7.Danese E, Lippi G, Montagnana M. Red blood cell distribution width andcardiovascular diseases. J Thorac Dis. 2015;7(10):402–11. doi: 10.3978/j.issn.2072-1439.2015.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seo YJ, Park YA, Bong JP, Park DJ, Park SY. Predictive value of neutrophil to lymphocyte ratio in first-time and recurrent idiopathic sudden sensorineural hearing loss. Auris Nasus Larynx. 2015;42(6):438–42. doi: 10.1016/j.anl.2015.04.011. https://doi.org/10.1016/j.anl.2015.04.011. PMid: 26013767. [DOI] [PubMed] [Google Scholar]
- 9.Chung JH, Lim J, Jeong JH, Kim KR, Park CW, Lee SH. The significance of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in vestibular neuritis. Laryngoscope. 2015;125(7):257–61. doi: 10.1002/lary.25204. https://doi.org/10.1002/lary.25204. PMid: 25677212. [DOI] [PubMed] [Google Scholar]
- 10.Kumar R, Geuna E, Michalarea V, Guardascione M, Naumann U, Lorente D, Kaye SB, de Bono JS. The neutrophil–lymphocyte ratio and its utilisation for the management of cancer patients in early clinical trials. British journal of cancer. 2015;112(7):1157–65. doi: 10.1038/bjc.2015.67. https://doi.org/10.1038/bjc.2015.67. PMid: 25719834. PMCid: PMC4385959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eryilmaz A, Basal Y, Tosun A, Kurt Omurlu I, Basak S. The neutrophil to lymphocyte ratios of our pediatric patients with Bell’s palsy. Int J Pediat Otorhinolaryngol. 2015;79(12):2374–7. doi: 10.1016/j.ijporl.2015.10.047. https://doi.org/10.1016/j.ijporl.2015.10.047. PMid: 26602556. [DOI] [PubMed] [Google Scholar]
- 12.Kum RO, Yurtsever Kum N, Ozcan M, Yilmaz YF, Gungor V, Unal A, Ciliz DS. Elevated neutrophil-to-lymphocyte ratio in Bell’s palsy and its correlation with facial nerve enhancement on MRI. Otolaryngol Head Neck Surg. 2015;152(1):130–5. doi: 10.1177/0194599814555841. https://doi.org/10.1177/0194599814555841. PMid: 25347990. [DOI] [PubMed] [Google Scholar]
- 13.Ozler GS, Gunak G. Neutrophil-lymphocyte ratio: a new predictive and prognostic factor in patients with Bell palsy. J Craniofac Surg. 2014;25(3):944–5. doi: 10.1097/SCS.0000000000000722. https://doi.org/10.1097/SCS.0000000000000722. PMid: 24657977. [DOI] [PubMed] [Google Scholar]
- 14.Atan D, Ikinciogulları A, Koseoglu S, Ozcan KM, Metin Ensari MA, et al. New Predictive Parameters of Bell’s Palsy: Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio. Balkan Med J. 2015;32(2):167–70. doi: 10.5152/balkanmedj.2015.15456. https://doi.org/10.5152/balkanmedj.2015.15456. PMid: 26167340. PMCid: PMC4432696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Xiao Y, Lin Z, Xiao X, He C, et al. The Role of Circulating Platelets Microparticles and Platelet Parameters in Acute Ischemic Stroke Patients. J Stroke Cerebrovasc Dis. 2015;24(10):2313–20. doi: 10.1016/j.jstrokecerebrovasdis.2015.06.018. https://doi.org/10.1016/j.jstrokecerebrovasdis.2015.06.018. PMid: 26169549. PMCid: PMC4592794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52(2):86–105. doi: 10.3109/10408363.2014.992064. https://doi.org/10.3109/10408363.2014.992064. PMid: 25535770. [DOI] [PubMed] [Google Scholar]
- 17.Celikbilek A, Zararsiz G, Atalay T, Tanik N. Red cell distribution width in migraine. Int J Lab Hematol. 2013;35(6):620–8. doi: 10.1111/ijlh.12100. https://doi.org/10.1111/ijlh.12100. PMid: 23650958. [DOI] [PubMed] [Google Scholar]
- 18.Poglajen G, Sever M, Cernelsck P, Haddad F, Vrtovec B. Increased red cell distribution width is associated with poor stem cell mobilization in patients with advanced chronic heart failure. Biomarkers. 2015;20(6-7):365–70. doi: 10.3109/1354750X.2015.1094137. https://doi.org/10.3109/1354750X.2015.1094137. PMid: 26472500. [DOI] [PubMed] [Google Scholar]
- 19.Kurtul A, Yarlioglues M, Murat SN, Demircelik MB, Acikgoz SK, Ergun G, Duran M, Cetin M, Ornek E. Red cell distribution width predicts contrast-induced nephropathy in patients undergoing percutaneous coronary intervention for acute coronary syndrome. Angiology. 2015;66(5):433–40. doi: 10.1177/0003319714535238. https://doi.org/10.1177/0003319714535238. PMid: 24834929. [DOI] [PubMed] [Google Scholar]
- 20.Toprak C, Tabakci MM, Simsek Z, Arslantas U, Durmus HI, Ocal L, Demirel M, Ozturkeri B, Ozal E, Kargin R. Platelet/lymphocyte ratio was associated with impaired myocardial perfusion and both in-hospital and long-term adverse outcome in patients with ST-segment elevation acute myocardial infarction undergoing primary coronary intervention. Postepy Kardiol Interwen. 2015;11(4):288–97. doi: 10.5114/pwki.2015.55599. https://doi.org/10.5114/pwki.2015.55599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunaldi M, Goksu S, Erdem D, Gunduz S, Okuturlar Y, Tiken E, Kahraman S, Inan YO, Genc TB, Yildirim M. Prognostic impact of platelet/lymphocyte and neutrophil/lymphocyte ratios in patients with gastric cancer: a multicenter study. Int J Clin Exp Med. 2015;8(4):5937–42. [PMC free article] [PubMed] [Google Scholar]


