Abstract
BACKGROUND:
Cerebral palsy is the most common cause of motor disability in children with a prevalence of 2-10/1,000 live births in the developing areas.
AIM:
The epidemiology, clinical picture, and associated comorbidities in CP have been extensively studied in high-resource countries, but in low-resource areas, including Africa, those studies are still lacking.
METHODS:
Cerebral palsy cases were prospectively recruited from every physiotherapy centre in Bani-Mazar city, Egypt, in a cross-sectional study from May 2015 to November 2015.
RESULTS:
Two hundred cases were enrolled with a prevalence of 1 per 1000 live births. Within the study population, 72.5% were the spastic type, 16% were dyskinetic, 7% were ataxic, and 4.5% were hypotonic. The most common comorbidities were cognitive impairment and epilepsy affecting 77% and 38%, respectively.
CONCLUSION:
Cerebral palsy in developing countries has a higher prevalence and different clinical profile regarding severity and associated disability. The perinatal and high-quality neonatal care together with physical therapy and rehabilitation programs is still lacking in developing countries.
Keywords: Cerebral palsy, prevalence, subtypes, comorbidity, Egypt
Introduction
Cerebral palsy (CP) is a term that has been formally defined as a group of permanent disorders of the development of movement and posture, causing activity limitation, which is attributed to non-progressive disturbances that occurred in the developing fetal or infant brain. The motor disorders of cerebral palsy are often accompanied by disturbances of sensation, perception, cognition, communication and behaviour, by epilepsy and by secondary musculoskeletal problems [1].
Prevalence estimates range from 1.5-3/1,000 in western countries, with much higher and wider range, 2-10/1,000 live births, in the developing areas [2] Durkin et al. 2016.
In an Egyptian study by El-Tallawy et al., 2014 [3] it was 2.04 per 1000 live births. The variation in prevalence and clinical picture depends mainly on study design, populations, and diagnostic criteria.
The epidemiology, clinical picture, and associated comorbidities in CP have been extensively studied in high-resource countries, but in low-resource areas, including Africa, those studies are still lacking. CP has many risk factors. The most common are low birth weight and perinatal hypoxia accounting for almost 50% and 10-20%, respectively [4].
This study aims at identifying the prevalence and the disability profile and associated comorbidities of CP cases in a prospective cross-sectional study from referral centres of physiotherapy and rehabilitation in Bani-Mazer district, Elminia Governorate, Egypt.
Subjects and Methods
All documented CP cases, according to the consensus definition by Bax et al., 2005 [5], from Bani-Mazar city and related nearby villages have been included in this study which was conducted from May 2015 to November 2015.
Inclusion criteria
The age range was from 3 months to 18 years, with disease onset within the first year of life. Cases were recruited from every physiotherapy and rehabilitation centre.
Exclusion criteria
Cases with developmental regression, malignancy and peripheral central nervous system affection were excluded.
For evaluation
1. The Gross Motor Function Classification System (GMFCS): Is a classification system developed for children with CP. Initially, children with CP were divided into five levels by considering their independency in gross motor functions such as sitting, walking, mobilisation and transfer activities and the tools-equipment.
Motor function is classified based on walking ability. Children classified as GMFCS Levels I or II were categorised as ‘walks independently’, Level III as ‘walks with handheld mobility device’, Levels IV as ‘limited walking ability’, and level V as wheel-chair bound. As motor functions of children differ according to age, functions have been defined as below 2-year old, between 2 and four years old, between 4 and six years old, between 6 and 12 years old, and above 12-year-old for each level.
GMFC classification system: LEVEL I - Walks without Limitations, LEVEL II - Walks with Limitations, LEVEL III - Walks Using a Hand-Held Mobility Device, LEVEL IV - Self-Mobility with Limitations; May Use Powered Mobility, LEVEL V - Transported in a Manual Wheelchair.
2. A clinical evaluation carried out including history taking and thorough neurological examination. Associated impairments have been documented by reviewing available formal documents including the history of true recurrent seizures, cognitive assessment, visual acuity, and hearing evaluation. For hearing impairment, an official audiometry result was reviewed.
A formal written consent has been taken from parents or care givers for all cases. The study was approved by the local ethical committee.
Data was analysed using IBM SPSS Statistics 22.
Results
The total population under the age of 18 years old, at the area of study, was 198,776 (32%of total population). The number of CP cases in this population who receive physical therapy services was 200 representing the prevalence of 1 per 1000 live births.
The demographic data of the cases (number = 200) has been presented in Table 1.
Table 1.
Demographics of cases (Number = 200)
| Variables | Number | % | ||
|---|---|---|---|---|
| Gender | Male | 110 | 55 | |
| Female | 90 | 45 | ||
| Residence | Rural | 162 | 81 | |
| Urbane | 38 | 19 | ||
| Parents consanguinity | Positive | 96 | 48 | |
| Negative | 104 | 52 | ||
| Type of delivery | Normal | 74 | 37 | |
| Cesarean | 126 | 63 | ||
| Gestational age | Pre-term | 32 | 16 | |
| Full-term | 163 | 81.5 | ||
| Unknown | 5 | 2.5 | ||
| Birth weight | Extremely LBW | 7 | 3.5 | |
| Very LBW | 10 | 5 | ||
| LBW | 36 | 18 | ||
| Normal birth weight | 85 | 42.5 | ||
| High birth weight | 13 | 6.5 | ||
| Uncertain | 49 | 24.5 | ||
| Chronological Age | 3month < 2year | 76 | 38 | |
| 2year < 4year | 60 | 30 | ||
| 4year < 6year | 34 | 17 | ||
| 6year < 12year | 30 | 15 | ||
LBW = low birth weight
Cases with CP has been divided according to type into spastic (72.5%), dyskinetic (16%), ataxic (7%), and hypotonic (4.5%). Spastic cases have been further categorised according to the distribution of spasticity to diplegic, quadriplegic, and hemiplegic (Table 2).
Table 2.
Types of CP and distribution of spasticity
| Clinical variables | Number (%) | Total |
|---|---|---|
| Sub types of CP | ||
| Spastic | 145 (72.5%) | 200 |
| Dyskinetic | 32 (16%) | |
| Ataxic | 14 (7%) | |
| Hypotonic | 9 (4.5%) | |
| Distribution of spasticity | ||
| Diplegic | 70 (48.27%) | 145 |
| Quadriplegic | 44 (30.3%) | |
| Hemiplegic | 31 (21.4%) |
CP = cerebral palsy.
The distribution of motor impairment according to GMFCS across age groups has been presented in Table 3 and Figure 1.
Table 3.
Motor impairment according to GMFCS
| Level | ||||||
|---|---|---|---|---|---|---|
| Age range | I | II | III | IV | V | Total |
| 0-2y | 6 | 15 | 22 | 19 | 15 | 76 |
| 2-4y | 4 | 20 | 19 | 7 | 8 | 60 |
| 4-6y | 6 | 5 | 10 | 7 | 6 | 34 |
| 6-12y | 3 | 5 | 15 | 6 | 2 | 30 |
| Total | 19 | 45 | 66 | 39 | 31 | 200 |
GMFCS = Gross Motor Classification System.
Figure 1.
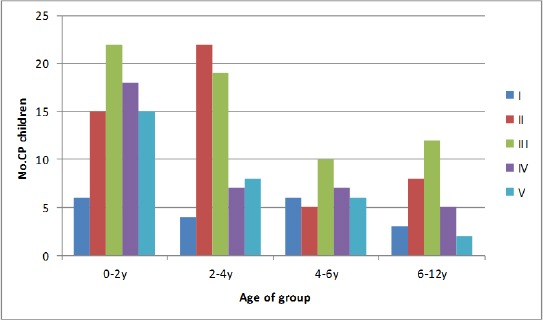
Level of impairment according to Gross Motor Functional Classification system (Birth-12 year)
Cases with hemiplegic type fall mostly in the range from level I through level III on GMFCS scale, while cases with quadriplegia are more disabled on level III through level V. There is a statistically significant difference between spastic subtypes regarding GMFCS score (Table 4).
Table 4.
GMFCS according to type of spasticity
| GMFCS | Spasticity type | χ2 | |||
|---|---|---|---|---|---|
| Hemiplegic | Diplegic | Quadriplegic | Total | ||
| I | 10 | 5 | 0 | 15 | P < 0.001 |
| II | 10 | 23 | 4 | 37 | |
| III | 10 | 24 | 14 | 48 | |
| IV | 1 | 11 | 14 | 26 | |
| V | 1 | 6 | 12 | 19 | |
| Total | 32 | 69 | 44 | 145 | |
GMFCS = Gross Motor Classification System.
Associated impairment has been documented using available formal reports. Cognitive impairment was the most common as it affected 77% of cases (Table 5).
Table 5.
Associated impairments
| Disorder | Number (%) |
|---|---|
| Cognitive impairment | 145 (77%) |
| Epilepsy | 76 (38%) |
| Visual impairment | 9 (4.5 %) |
| Hearing impairment | 5 (2.5 %) |
It has been found that both cognitive impairment and epilepsy were found in a higher percentage of children with a higher score on GMFCS (Table 6, 7).
Table 6.
Distribution of cases with cognitive impairment according to GMFCS score
| Cognition | Total | P value | |||
|---|---|---|---|---|---|
| Normal | Affected | ||||
| GMFCS | I | 11 | 8 (42.1%) | 19 | |
| II | 18 | 28 (60.8%) | 46 | <0.001 | |
| III | 12 | 56 (82.3%) | 68 | ||
| IV | 4 | 36 (90%) | 40 | ||
| V | 1 | 26 (96.2%) | 27 | ||
| Total | 46 | 154 (77%) | 200 | ||
GMFCS = Gross Motor Classification System.
Table 7.
Distribution of cases with epilepsy according to GMFCS score
| Epilepsy | Total | P value | |||
|---|---|---|---|---|---|
| Normal | Affected | ||||
| GMFCS | I | 16 | 3 (15.7%) | 19 | 0.01 |
| II | 34 | 12 (26%) | 46 | ||
| III | 38 | 30 (44.1%) | 68 | ||
| IV | 25 | 15 (37.5%) | 40 | ||
| V | 11 | 16 (59.2%) | 27 | ||
| Total | 124 | 76 (38%) | 200 | ||
GMFCS = Gross Motor Classification System.
Binary logistic regression models were tested for the relation between GMFCS and both cognitive impairment and epilepsy. It was founded that each grade higher on GMFCS was associated with 2.5 and 1.5 fold increased the risk for occurrence of cognitive impairment and epilepsy, respectively.
Discussion
The current study was conducted to establish a data base about children with CP receiving physical therapy services in general or health insurance hospitals as well as private centres in Bani-Mazar district Elminia governorate.
There was a little higher male to female ratio (1.22), almost similar to 1.3 reported by Johnson, 2002 [6] in Europe.
The urban to the rural ratio of cases was 1to 4 which is expected as the antenatal care and the medical services for neonates in general, and those with high-risk factors for CP in particular, are of low calibre and capacity.
According to our sample, there were no cases above 12-year-old receiving physiotherapy service. This finding could be related to the extreme sides of the disability spectrum, being either mild and almost completely rehabilitated, or severe and neglected at home due to logistic issues (difficult transportation of grown-up patients, negative attitude towards severe cases in the low socio-economic population, or financial issues).
The total number of CP cases was 200 cases, representing 1 per 1000 live birth. Prevalence of CP occurs at a rate of 2-2.5 per 1000 live births in developed countries [5]. Also in Egypt; El-Tallawy [7] reported a prevalence of 2.03and 3.6 per1000 live birth in Al-Kharga District and Al-Quseir city [3], respectively. The lower prevalence can be attributed to multiple factors. First of all, the different methodology as our study includes only those cases under the age of 18 year receiving physiotherapy services. Also, our cases are those with a disability severe enough to push the care givers to seek medical services. Second, the prevalence of CP has dynamic properties related to attitude and quality of health care and neonatal mortality [4].
According to our study, premature delivery and LBW accounted for 16% and 26.5%, respectively. These figures are low in comparison to international figures (78%) reported in developed countries. This difference is expected regarding high mortality rate of premature and LBW and a higher incidence of perinatal hypoxic events [8].
Our results on the types of CP and distribution of spasticity are similar to those reported in developing countries as reported by Kakooza-Mwesige et al. 2015 [9]. Spastic CP is the most common type, worldwide. Similarly, most of our patients (72.5%) were spastic. Dyskinetic CP (16%) is higher than the figures in western countries (6%) [10].
The higher ratio in our area can be explained by the lack of awareness of families to the impact of neonatal hyperbilirubinemia and the reluctance to seek medical advice. The ataxic cases constituted almost 7% of total number which is in consistency with local and international figures [3, 7, 9].
Spastic quadriplegic CP is the most severe form affecting 44 patients (22%) of all CP cases and (30.3%) of spastic CP patients.
Epilepsy and mental sub normality were found in 38% and 77%, respectively. These figures are similar to those reported in Africa [3, 9] but higher than reported in developed areas [10]. The difference can be explained by the greater proportion of cases with extensive bilateral brain injury in diplegic and quadriplegic cases which are more vulnerable to develop symptomatic epilepsy. Also, the definition of mental sub normality in our study was based on available formal IQ test in contrast to the study by El-tallawy et al.,(2014) [3], where the IQ test was available for only 24 cases. It is worth mentioning that epilepsy was related to the level of GMFCS. This finding was also addressed by Hundozi-Hysenaj and Boshnjaku-Dallku, (2008) [11]. Regarding cognitive profile, GMFCS level was a detrimental factor which is consistent with Dalvand et al., (2012) [12], who stated that GMFCS could be considered as a gross proxy for evaluating the cognitive deficit.
The gross motor function severity varied significantly across spastic subtypes (Table 4). GMFCS I through III was found to compress mainly children with diplegia whose gross and fine function is more homogeneous than in children with hemiplegia.
Regarding GMFCS, 9.5% of cases were at level I, 18.5% at level IV, and 15.5% at level V. In the study conducted by Kakooza-Mwesige et al. 2015 [9], the percentage of cases at level I is very similar to little higher percentage at level IV and V. on the other hand, in a Swedish study, 32% of cases were at level I, 15% at level IV, and 16% at level V [13]. The difference mainly lies with level I which is the mildest form. This reflects the effect of public awareness, the different etiologies, the level of neonatal care, the importance of early detection, and the quality of rehabilitation programs.
In conclusion, our study revealed a prevalence of 1 per 1000 live births from the age of 3 months to 18 years. Two-thirds of our study are ambulant, evidence for the paramount importance of early detection and intervention. The most common subtype is spastic CP, while the most common comorbidity is cognitive impairment followed by epilepsy. GMFCS is a useful tool for assessment, and it may offer a good predictor for epilepsy and cognitive impairment. In comparison to international figures, it seems that the perinatal and high-quality neonatal care is still lacking in developing countries. Also, being of a low-resource population, the accessibility to physical therapy and rehabilitation programs is to be revisited.
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Graham HK, Rosenbaum P, Paneth N, Dan B, Lin JP, Damiano DL, Becher JG, Gaebler-Spira D, Colver A, Reddihough DS, Crompton KE, Lieber RL. Cerebral palsy. Nat Rev Dis Primers. 2016;2:15082. doi: 10.1038/nrdp.2015.82. https://doi.org/10.1038/nrdp.2015.82. PMid: 27188686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durkin MS, Benedict RE, Christensen D, Dubois LA, Fitzgerald RT, Kirby RS, Maenner MJ, Van Naarden Braun K, Wingate MS, Yeargin-Allsopp M. Prevalence of Cerebral Palsy among 8-Year-Old Children in 2010 and Preliminary Evidence of Trends in Its Relationship to Low Birthweight. Paediatr Perinat Epidemiol. 2016;30(5):496–510. doi: 10.1111/ppe.12299. https://doi.org/10.1111/ppe.12299. PMid: 27215680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Tallawy HN, Farghaly WM, Shehata GA. Cerebral palsy in Al-Quseir City, Egypt: prevalence, subtypes, and risk factors. Neuropsychiatric Disease and Treatment. 2014;10:1267–1272. doi: 10.2147/NDT.S59599. PMid: 25045270. PMCid: PMC4099193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Surveillance of Cerebral Palsy in Europe (SCPE) Dev Med Child Neurol. 2000;42(12):816–24. doi: 10.1017/s0012162200001511. PMid: 11132255. [DOI] [PubMed] [Google Scholar]
- 5.Bax M. Proposal definition and classification of cerebral palsy. Dev Med Child Neurol. 2005;47:571–6. doi: 10.1017/s001216220500112x. https://doi.org/10.1017/S001216220500112X. PMid: 16108461. [DOI] [PubMed] [Google Scholar]
- 6.Johnson A. Prevalence and Characteristics of Children with Cerebral Palsy in Europe. Developmental Medicine Child Neurology. 2002;44:633–640. https://doi.org/10.1017/S0012162201002675. [PubMed] [Google Scholar]
- 7.El Tallawy HN, Farghaly WM, Rageh TA, Shehata GA, Metwaly NA, Abo Elfto N, et al. Epidemiology of major neurological disorders project in Al Kharga District, New Valley, Egypt. Neuroepidemiol. 2010;35:291–7. doi: 10.1159/000320240. https://doi.org/10.1159/000320240. PMid: 20948236. [DOI] [PubMed] [Google Scholar]
- 8.Bearden DR, Monokwane B, Khurana E, Baier J, Baranov E, Westmoreland K, Mazhani L, Steenhoff AP. Pediatric Cerebral Palsy in Botswana: Etiology, Outcomes, and Comorbidities. Pediatr Neurol. 2016;59:23–9. doi: 10.1016/j.pediatrneurol.2016.03.002. https://doi.org/10.1016/j.pediatrneurol.2016.03.002. PMid: 27114082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kakooza-Mwesige A, Forssberg H, Eliasson AC, Tumwine JK. Cerebral palsy in children in Kampala, Uganda: clinical subtypes, motor function and co-morbidities. BMC Res Notes. 2015;23(8):166. doi: 10.1186/s13104-015-1125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guro L. Andersena, Lorentz M. Irgensb, Ivar Haagaasa, Jon S. Skranesc, Alf E. Mebergd. Torstein Vike Cerebral palsy in Norway: Prevalence, subtypes and severity. Eur J Paediatr Neurol. 2008;12(1):4–13. doi: 10.1016/j.ejpn.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Hundozi-Hysenaj H, Boshnjaku-Dallku I. Epilepsy in children with cerebral palsy. Journal of Pediatric Neurology. 2008;6(1):43–6. https://doi.org/10.1055/s-0035-1557423. [Google Scholar]
- 12.Dalvand H, Dehghan L, Hadian MR, Feizy A, Hosseini SA. Relationship between gross motor and intellectual function in children with cerebral palsy: a cross-sectional study. Arch Phys Med Rehabil. 2012;93(3):480–4. doi: 10.1016/j.apmr.2011.10.019. https://doi.org/10.1016/j.apmr.2011.10.019. PMid: 22265344. [DOI] [PubMed] [Google Scholar]
- 13.Himmelmann K, Beckung E, Hagberg G, Uvebrant P. Gross and fine motor function and accompanying impairments in cerebral palsy. Dev Med Child Neurol. 2006;48:417–23. doi: 10.1017/S0012162206000922. https://doi.org/10.1017/S0012162206000922. PMid: 16700930. [DOI] [PubMed] [Google Scholar]


